Abstract
Novel NaLaMgWO6:Tb3+,Eu3+ phosphors with tunable color emissions were prepared by a solid-state reaction. The crystal structure, composition, and luminescent properties of the obtained phosphors were investigated by X-ray diffraction (XRD), scanning electron microscope (SEM), and fluorescence spectrophotometer. The results reveal that NaLaMgWO6 crystallizes in a monoclinic double perovskite structure with C2/m space group. Tb3+ and Eu3+ single-doped phosphors show the intense green and red emissions at 545 and 617 nm, corresponding to the 5D4 → 7F5 transition of Tb3+ and the 5D0 → 7F2 transition of Eu3+, respectively, while for Tb3+ and Eu3+ co-doped phosphors, a tunable emission color from green to red is obtained by variation of the ratio of Eu3+ to Tb3+. The emission spectra and luminescence decay lifetimes verify the occurrence of energy transfer from Tb3+ to Eu3+ in NaLaMgWO6: Tb3+, Eu3+ phosphor, and the energy transfer mechanism is dominated by the dipole–quadrupole interaction. Furthermore, the thermal stability of NaLaMgWO6:Tb3+, Eu3+ phosphor was discussed from 305 to 515 K. The results exhibit the phosphors possess excellent thermal stability, and the activation energy is calculated to be 0.302 eV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphor-converted white light emitting diodes (pc-LEDs) have been used in numerous fields, such as indoor and outdoor lighting, screen display, and safety warning, because of their environmental friendliness, long lifetime, high brightness, and reliability. Nowadays, the most common way to achieve white LEDs is to combine a blue LED chip (InGaN) with yellow-emitting phosphors (Y3Al5O12:Ce3+) [1, 2]. However, the deficiency of red-emitting component leads to poor color-rendering index and low stability of color temperature that restrict its applications. To circumvent these drawbacks, ultraviolet (UV) LED chips can be combined with tricolor (blue, green, and red) phosphors to obtain high-quality white LEDs. Nevertheless, they still suffer from some problems, such as low luminous efficiency and poor color stability because of the strong reabsorption of blue light by the red and green phosphors. Using single-phase phosphors with tunable emission can effectively avoid the above problems [3, 4]. Therefore, it is important and urgent to explore single-phase tunable color phosphors with high luminescent efficiency under UV.
On the basis of energy transfer from the sensitizer to the activator, the combination of multiple activator ions in single-phase host is assumed to be a feasible method to realize tunable color emission [5]. In the rare earth family, the Eu3+ and Tb3+ ions are well considered as a promising red and green emitting activator for their characteristic 5D0 → 7F2 transition and 5D4 → 7F5 transitions, respectively. Meanwhile, when Tb3+ and Eu3+ ions are co-doped in a single-phase host, Tb3+ ion as a good sensitizer can transfer its energy to activator Eu3+ with the phonon spectrum. By adjusting the Eu3+/Tb3+ ratio, color-tunable emission can be realized in Tb3+ and Eu3+ co-doped phosphors [6, 7]. Consequently, plenty of researches on Tb3+ and Eu3+ ion co-doped phosphors have been proposed to obtain abundant emitting colors, such as CaGd2(WO4)4:Tb3+, Eu3+ [6], KBaGd(WO4)3:Tb3+, Eu3+ [7], Sr3La(PO4)3:Tb3+, Eu3+ [8], and Na2MgSiO4:Tb3+, Eu3+ [9]. In general, the luminescence properties of rare earth ions (Tb3+ and Eu3+) depend on the host crystal structure, so it is important to select an appropriate host to obtain efficient phosphors. Recently, double perovskite structure compounds with the general formula AA’BB’O6 (where A = K, Na, Li; A′ = Gd, La; B = Ca, Mg; B′ = W, Mo) being host doped with a variety of activators have been studied extensively due to their excellent luminescence characteristics and chemical and physical properties [10,11,12,13]. AA’BB’O6 compound exhibits the diversity of crystal structure with the different ion types of A/A’ and B/B’. Among them, the representative compound NaLaMgWO6 possesses a monoclinic structure with the space group of C2/m. It has double ordering structural characteristics: the layered A site cation ordering (Na+/La3+) and rock salt B site cation ordering (Mg2+/W6+) [14]. The structure provides lots of crystal field to co-dope numerous rare earth ions, which is beneficial to tune the luminescent characteristics. Furthermore, NaLaMgWO6 host shows broad and strong charge transfer band in the UV, and the energy could effectively transfer to rare earth activators, resulting in the enhancement of luminous efficiency. As a result, rare earth ion-doping NaLaMgWO6 phosphors have been widely studied, especially Eu3+ single-doped NaLaMgWO6:Eu3+ red-emitting phosphors, as the suitable candidate for high-performance white LEDs [13,14,15,16]. However, no report is available on the luminescence properties and the energy transfer between Tb3+ and Eu3+ in NaLaMgWO6:Tb3+, Eu3+ phosphors.
In this paper, a series of Tb3+ and Eu3+ co-doped NaLaMgWO6 phosphors were prepared by the solid-state reaction technique and their crystal structure, photoluminescence properties, and thermal stability were systematically discussed. Under excitation at 273 nm, NaLaMgWO6:Tb3+, Eu3+ phosphors generate tunable color emission from green to red by tuning the ratio of Eu3+ to Tb3+. Evidence of energy transfer from Tb3+ to Eu3+ was illustrated via the emission spectrum and decay curve measurements.
2 Experimental section
The phosphor samples NaLa1-x-yMgWO6:xTb3+,yEu3+ (0 ≤ x ≤ 0.15; 0 ≤ y ≤ 0.8) were prepared via the high-temperature solid-state technique. Firstly, stoichiometric amounts of raw materials Na2CO3 (A.R), MgCO3 (A.R), La2O3 (99.95%), Eu2O3 (99.9%), Tb4O7 (99.9%) and WO3 (99.9%) were weighed and mixed thoroughly in a planetary ball mill. The ball-milled mixtures were put into a Petri dish and dried in a drying oven at 80 °C. Then, the homogeneous powders were calcined at 1100 °C for 4 h and cooled to room temperature with the furnace. Finally, the obtained samples were ground again for further characterizations.
The X-ray diffraction (XRD) patterns of the phosphors were collected using an X-ray diffractometer (D8 Advance, Bruker, Germany) with Cu Kα (λ = 0.15418 nm) radiation at 40 kV and 40 mA. The morphology and composition analysis of the samples were obtained via a field emission scanning electron microscopy (Apreo + HiVac, FEI, America) equipped with energy-dispersive spectrometer (EDS). The photoluminescence spectra were characterized using a fluorescent spectrometer (F-7000, Hitachi, Japan), and TAP-02 high-temperature fluorescent controller was equipped to the spectrometer to measure the temperature-dependent emission spectra. The fluorescence decay curves were collected with a FluoroLog-3 fluorescence spectrophotometer (Jobin–Yvon, Horiba, France).
3 Results and discussion
3.1 Phase analysis
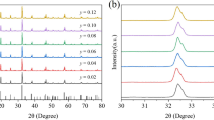
Figure 1 shows the XRD patterns of NaLa0.9-yMgWO6:0.1Tb3+, yEu3+ (0 ≤ x ≤ 0.8) phosphors. It is clearly seen that all the diffraction peaks are consistent with (NaLa)(MgW)O6 standard diffraction card (JCPDS 37-0243). This result indicates that all studied phosphors have the same monoclinic double perovskite structure without any other impurity phase. In other words, Tb3+ and Eu3+ have been successfully incorporated into the host lattice without any structural changes. Furthermore, as shown in Fig. 1b, as the Eu3+ doping concentration increases, the diffraction peaks of NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors show a regularly shift toward higher angle which may be due to the replacement of smaller Eu3+ ion (r = 1.120 Å, CN = 9) for La3+ ion (r = 1.216 Å, CN = 9) [17].
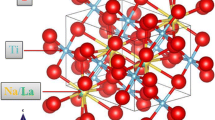
To further explore the crystal structure and confirm that the Tb3+ and Eu3+ ions are successfully introduced into the NaLaMgWO6 host, the Rietveld refinement of three representative samples NaLaMgWO6, NaLa0.9MgWO6:0.1Tb3+, and NaLa0.4MgWO6:0.1Tb3+,0.5Eu3+ were performed by the Rietica software. According to the report on the structure evolution of NaLaMgWO6 by Liu et al. [18], the un-doped NaLaMgWO6 crystallizes in monoclinic structure with two possible space groups named P21 and C2/m, and the space group of C2/m has been proved to be more appropriate for the crystal structure of NaLaMgWO6. Therefore, the space group C2/m was adopted as starting model for Rietveld refinement of above three samples, and the refined results are exhibited in Fig. 2. The red solid lines, crosses, blue bars, and green lines stand for calculated patterns, experimental data, Bragg positions, and differences between experimental data and calculated patterns, respectively. The refined Rp and Rwp for NaLaMgWO6, NaLa0.9MgWO6:0.1Tb3+ and NaLa0.4MgWO6:0.1Tb3+,0.5Eu3+ are 5.308/5.683/6.138 (Rp) and 7.109/8.929/9.677 (Rwp), respectively, which indicates that un-doped and doped NaLaMgWO6 samples are good agreement with the space group C2/m. Moreover, the refined unite cell parameters are displayed in Table 1. It is obvious that the lattice parameters (a, b, c) and unite cell volume (V) of NaLa0.9MgWO6:0.1Tb3+ and NaLa0.4MgWO6:0.1Tb3+, 0.5Eu3+ are smaller than those of un-doped NaLaMgWO6 host. The lattice shrinkage confirms further the successful substitution of smaller Tb3+/Eu3+ ions for La3+ ions, and the substitution has no obvious effect on the structure of NaLaMgWO6.
3.2 SEM and EDX study
In order to study the composition and element distribution of NaLa0.4MgWO6:0.1Tb3+, 0.5Eu3+ phosphor, the elemental mapping and EDX spectrum are shown in Fig. 3. The sample contains spherical-like particles with good dispersion and uniform particle size. The particle size is in the range of 0.5–2 μm. In addition, EDX spectrum of the sample suggests that the sample is composed of Eu, Tb, Na, La, Mg, W, and O elements, and the elements of Tb, Eu, Na, La, Mg, and W are uniformly distributed throughout the whole particle. The results further manifest that Eu3+ and Tb3+ ions are incorporated into NaLaMgWO6 host, and the NaLaMgWO6:Tb3+,Eu3+ phosphor can be achieved.
3.3 Photoluminescence properties
Figure 4a displays the excitation and emission spectra of Tb3+ single-doped NaLa0.9MgWO6 phosphors by monitoring excitation and emission wavelength at 273 nm and 545 nm, respectively. The excitation spectrum contains a strong broad band ranging from 250 to 350 nm and a series of weak narrow peaks in the range of 350–450 nm. The broad band located at 273 nm is ascribed to the spin-forbidden and spin-allowed 4 f 8 → 4 f 75d transitions of Tb3+ ions. The weak peaks are located at 357 nm, 368 nm, and 378 nm, corresponding to 7F6 → 5D2, 7F6 → 5L10 , and 7F6 → 5D3 transitions of Tb3+ ions, respectively [19, 20]. Under the excitation of 273 nm, The emission spectrum of NaLa0.9MgWO6:0.1Tb3+ comprises of four emission peaks at 486 nm, 545 nm, 585 nm and 623 nm, which correspond to 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+ ions, respectively [21]. Moreover, the emission intensity of NaLa1-xMgWO6:xTb3+ phosphors is related to Tb3+ doping concentration. As shown in Fig. 4b, with the increase of Tb3+ doping content, the emission intensity of NaLa1-xMgWO6:xTb3+ phosphor increases initially and reaches maximum at x = 0.1, then exhibit a decrement tendency due to the effect of concentration quenching.
The Fig. 5 demonstrates the excitation and emission spectra of Eu3+ single-doped NaLaMgWO6 phosphors. The excitation spectrum of NaLaMgWO6:Eu3+ (Fig. 5a) consists of a broad band located at 313 nm with several line peaks covering from 350 to 500 nm. The former broad band is attributed to the charge transfer band (CTB) of O2− → W6+ in WO42− and the CTB of O2− → Eu3+ transition. The latter narrow peaks are assigned to the typical 4-4f transitions of Eu3+ions. Figure 5b presents the emission spectra of NaLaMgWO6:Eu3+ phosphors under excitation at 313 nm and 397 nm. The emission spectra have similar emission profile, which show the Eu3+ 4–4f transition peaks centered at 536 nm (5D1 → 7F1), 580 nm (5D0 → 7F0), 592 nm (5D0 → 7F1), 617 nm (5D0 → 7F2), 653 nm (5D0 → 7F3), and 695 nm (5D0 → 7F4) [15, 16]. Furthermore, the intensity of electric dipole transition (5D0 → 7F2, 617 nm) is much higher than that of magnetic dipole transition (5D0 → 7F1, 592 nm) for both emission spectra, indicating that the Eu3+ ions are placed to the non-centrosymmetric lattice sites in this double perovskite NaLaMgWO6.
Figure 6a presents the excitation of Eu3+ and the emission spectra of Tb3+. It can be seen that only small spectral overlap in the range of 475–510 nm could be found. Energy transfer between Tb3+ to Eu3+ generally increase with the spectral overlap. However, some literatures [7, 8] have proved that the energy transfer from the Tb3+ to Eu3+ can still occur through assisted the phonons spectrum even if little spectral overlap. Figure 6b shows the excitation spectra of NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors by monitoring with 617 nm. The excitation spectra are almost identical to that of NaLaMgWO6: Eu3+ phosphors without any excitation peaks of Tb3+. This is due to that the excitation peaks of Tb3+ overlap with those of Eu3+ and the spectrum intensity of Tb3+ is weaker than that of Eu3+.
Figure 6c depicts the emission spectra of NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors by exciting with 273 nm. At y = 0.1, the emission spectrum of NaLa0.8MgWO6:0.1Tb3+,0.1Eu3+ phosphor contains both the typical 5D4 → 7F6,5,4,3 transitions of Tb3+ ions and the 5D0,1 → 7F0,1,2,3,4 transitions of Eu3+ ions. In addition, with the increase of Eu3+ doping content, the emission intensity of Tb3+ decreases monotonically, while the intensity of Eu3+ increases initially then reduces, and its maximum is observed when y = 0.5 (Fig. 6d). It demonstrates the presence of energy transfer from Tb3+ to Eu3+ and indicates that the color tuning of the emission from green to red could be realized by precisely changing the ratio of Eu3+/Tb3+ ions concentration. The corresponding CIE chromaticity coordinates and CIE chromaticity diagram of NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors are estimated and exhibited in Fig. 7. The emission color of NaLa0.9MgWO6:0.1Tb3+ phosphor is green with CIE coordinates of (0.293, 0.586) (Fig. 7a). With raising Eu3+ concentration, the emission hue changes from green (0.293, 0.586) to red (0.648, 0.351).
3.4 Decay curves and energy transfer mechanism
To explore the energy transfer process from Tb3+ to Eu3+, the luminescence decay curves of NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors excited at 273 nm and monitored at 545 nm are measured and presented in Fig. 8.
It can be seen that the decay curves can be fitted with the following biexponential function:
where I0 and I(t) represent the luminescence intensities at time t=0 and t, respectively. τ1 and τ2 refer to the decay lifetimes for the exponential components. A1 and A2 are the fitting coefficient. The average lifetime can be derived from the following equation [22]:
From the Eq. (2), the average decay lifetimes for NaLa0.9−yMgWO6:0.1Tb3+,yEu3+ phosphors are determined to be 0.435, 0.261, 0.184, 0.134, 0.0727, and 0.0521 ms for y = 0, 0.1, 0.2, 0.3, 0.4, and 0.5, respectively. In general, the decay lifetime of the sensitizer will be shorten due to the influence of energy transfer from the sensitizer to the activator. As shown in Fig. 8, the decay lifetime of Tb3+ decreases monotonically with the increase of Eu3+ doping concentration, which strongly indicates that Tb3+ ion can act as a good sensitizer, transferring its energy to activator Eu3+ in the NaLa0.9-yMgWO6:0.1Tb3+,yEu3+ phosphors. On the basis of the obtained decay lifetimes, the energy transfer efficiency (ηET) from Tb3+ to Eu3+ can be determined by the following formula [6, 7]:
where τs and τso refer to the lifetimes of the sensitizer Tb3+ with and without the activator Eu3+. According to the Eq. (3), the ηET values of NaLa0.9−yMgWO6:0.1Tb3+,yEu3+ (y = 0, 0.1, 0.2, 0.3, 0.4, 0.5) phosphors are estimated and exhibited in Fig. 8b. The energy transfer efficiency ηET increases with the increase of the Eu3+ content and reaches the maximum of 88.11% at y = 0.5, suggesting that the energy of Tb3+ can be efficiently transfer to Eu3+ in NaLaMgWO6:Tb3+,Eu3+phosphors.
General speaking, the energy transfer from sensitizers to activators in host is associated with exchange and multipolar interaction [23]. The prerequisite of exchange interaction mechanism is that the critical distance (Rc) between the sensitizer and activator is less than about 0.5 nm. As suggested by Blasse, the critical distance (Rc) between Tb3+ and Eu3+ in NaLaMgWO6 host can be estimated using the following formula [24]:
where V is the volume of the unit cell, N is the number of the La3+ lattice sites in the unit cell, and χc is the total concentration of Tb3+ and Eu3+. For NaLa0.4MgWO6:0.1Tb3+,0.5Eu3+ phosphors, V = 473.949 Å, N = 4, and χc = 0.6. Therefore, the critical distance (Rc) is estimated to be 7.23 Å. This value is longer than 5 Å for the exchange interaction, indicating the multipolar interaction play a leading role in the mechanism of energy transfer between Tb3+ and Eu3+.
According to Dexter’s energy transfer formula of multipolar interaction, the ratio of the luminescence quantum efficiency of the sensitizer in the absence of activation to the efficiency of the sensitizer in the presence of activation can be defined as [23, 25]:
where η0 and η are the quantum efficiencies of Tb3+ without and with Eu3+, respectively. C is the sum content of Tb3+ and Eu3+, and n = 6, 8, 10, which correspond to dipole–dipole (d–d), dipole–quadrupole (d–q) and quadrupole–quadrupole (q–q) interaction, respectively. Experimentally, the value of η0/η can be estimated approximately by the ratio of luminescence intensities (I0/I) based on Reisfeld’s approximation [3, 26]. Figure 9 shows the relationships of I0/I versus C n/3 for NaLaMgWO6:Tb3+,Eu3+ phosphors. It is obvious that the optimal linear relationship is observed when n = 8, indicating that the energy transfer from Tb3+ to Eu3+ is realized via dipole–quadrupole interaction.
3.5 Thermal quenching properties
The thermal quenching temperature of the phosphors is a vital parameter for their practical application in high power white LEDs. The temperature-dependent emission spectra for NaLa0.4MgWO6:0.1Tb3+, 0.5Eu3+ phosphors excited by 273 nm was measured and shown in Fig. 10a. The emission intensity declined gradually with the increase of the temperature, and the emission intensity at 455 K drops to 64.2% of the initial intensity at 305 K. It implies that NaLa0.4MgWO6:0.1Tb3+, 0.5Eu3+ possesses excellent thermal stability. The thermal quenching is associated with the temperature of the electron–phonon interaction, affecting the ground state as well as the excited states [27]. The thermal quenching mechanism of NaLa0.4MgWO6:0.1Tb3+,0.5Eu3+ phosphors can be analyzed by calculating the activation energy according to the Arrhenius equation [5]:
where I(T) and I0 refer to the emission intensity at test temperatures and initial emission intensity, respectively. k is the Boltzmann constant (8.629 × 10–5 eV/K), c is the constant, and ΔE corresponds to the activation energy. The equation can be converted to the natural logarithm [28]:
Figure 10b presents the plot of ln[(I0/IT) − 1] versus 1/kT. The experimental data can be linear fitted with a slope of − 0.302. Hence, based on the Arrhenius Eq. (7), the activation energy ΔE is approximately 0.302 eV for NaLa0.4MgWO6:0.1Tb3+, 0.5Eu3+ phosphors.
4 Conclusions
In conclusion, Novel NaLaMgWO6:Tb3+,Eu3+ phosphors with tunable emission color were successfully prepared by a solid-state reaction. X-ray diffraction study shows that NaLaMgWO6:Tb3+,Eu3+ phosphors have the monoclinic crystal structure with the C2/m space group, and Rietveld refinement demonstrates the effective replacement of La3+ by Tb3+ and Eu3+ in the host has no influence on the crystallinity of NaLaMgWO6. Photoluminescence investigation reveals that with the increase of the ratio of Eu3+ to Tb3+, the emission color of NaLaMgWO6:Tb3+,Eu3+ phosphors can be tuned from green to red upon the excitation of ultraviolet light, and the corresponding CIE coordinates vary from (0.293, 0.586) to (0.648, 0.351). The energy transfer from Tb3+ to Eu3+ in NaLaMgWO6 host is responsible for their tunable color emission, and the mechanism of energy transfer is confirmed to be the dipole–quadrupole interaction. Moreover, the thermal stability of NaLaMgWO6:Tb3+,Eu3+ phosphors was also investigated and ΔE value is estimated to be 0.302 eV.
References
D.D. Xu, W. Zhou, Z. Zhang, X.X. Ma, Z.G. Xia, Luminescence property and energy transfer behavior of apatite-type Ca4La6(SiO4)4(PO4)2O2:Tb3+, Eu3+phosphor. Mater. Res. Bull. 108, 101–105 (2018)
C. Liang, H.P. You, Y.B. Fu, X.M. Teng, K. Liu, J.H. He, Luminescence properties of a tunable blue-green-emitting Ca10(PO4)6S:Ce3+, Tb3+ phosphors for UV-excited white LEDs. Optik 131, 335–342 (2017)
P.A.Nascimento, A.J. Silva, I.S. Carvalho, M.V. Rezende, Luminescence varied by selective excitation in Eu3+, Tb3+-doped LiSrPO4 phosphors for W-LEDs applications, Opt. Mater. 96, 109369 (2019)
Y.M. Feng, J.P. Huang, C.M. Li, G. Hu, J. Liu, X.B. Yu, Structure energy transfer and tunable photoluminescence of Sr7Zr(PO4)6:Tb3+, Eu3+ phosphors for near-UV white LEDs. J. Alloys Compd. 706, 478–484 (2017)
B. Li, X.Y. Huang, H. Guo, Y.J. Zeng, Energy transfer and tunable photoluminescence of LaBWO6:Tb3+, Eu3+ phosphors for near-UV white LEDs. Dyes Pigments 150, 67–72 (2018)
X.Y. Huang, B. Li, P. Du, H. Guo, R.P. Cao, J.S. Yu, K. Wang, Realizing highly efficient multicolor tunable emissions from Tb3+ and Eu3+ co-doped CaGd2(WO4)4 phosphors via energy transfer by single ultraviolet excitation for lighting and display applications. Dyes Pigments 151, 202–210 (2018)
M.J. Song, W. Zhao, W.G. Ran, J.P. Xue, Y.L. Liu, Multicolor tunable luminescence and energy transfer mechanism in a novel single-phase KBaGd(WO4)3:Tb3+, Eu3+ phosphor for NUV. J. Alloys Compd. 803, 1063–1074 (2019)
X. Zhang, J. Zhang, Y. Chen, M. Gong, Energy transfer and multicolor tunable emission in single-phase Tb3+, Eu3+ co-doped Sr3La(PO4)3 phosphors. Ceram. Int. 42, 13919–13924 (2016)
X.M. Zhu, Z.F. Zhou, Photoluminescence and energy transfer mechanism of a novel tunable color phosphor Na2MgSiO4:Tb3+, Eu3+. J. Lumin. 188, 589–594 (2017)
Y.J. Liang, M.N. Hyeon, W.G. Ran, S.H. Park, C.C. Byung, J.H. Jeong, K.H. Kim, The design and synthesis of new double perovskite (Na, Li)YMg(W, Mo)O6:Eu3+ red phosphors for white light-emitting diodes. J. Alloys Compd. 716, 56–64 (2017)
A.R. Sharits, J.F. Khoury, P.M. Woodward, Evaluating NaREMgWO6 (RE=La, Gd, Y) doubly ordered double perovskites as Eu3+ Phosphor Hosts. Inorg. Chem. 55, 12383–12390 (2016)
B. Han, Y.Z. Dai, J. Zhang, B.K. Liu, H.Z. Shi, Photoluminescence properties of a double perovskite tungstate based redemitting phosphor NaLaMgWO6:Sm3+. Ceram. Int. 44, 3734–3740 (2018)
W.G. Ran, H.M. Noh, B.K. Moon, S.H. Park, J.H. Jeong, J.H. Kim, G.Z. Liu, J.S. Shi, Crystal structure, electronic structure and photoluminescence properties of KLaMgWO6:Eu3+ phosphors. J. Lumin. 197, 270–276 (2018)
L. Zhang, Z. Lu, P.D. Han, L.X. Wang, Q.T. Zhang, Synthesis and photoluminescence of Eu3+-activated double perovskite NaGdMg(W, Mo)O6: a potential red phosphor for solid state lighting. J. Mater. Chem. C 1, 54–57 (2013)
L. Zhang, Q. Liu, N. Ding, H. Yang, L.X. Wang, Q.T. Zhang, Dual-channel enhanced luminescence of double perovskite NaGdMgWO6:Eu3+ phosphor based on alternative excitation and delayed quenching. J. Alloys Compd. 642, 45–52 (2015)
Q. Liu, L.X. Wang, L. Zhang, H. Yang, M.X. Yu, Q.T. Zhang, Enhanced luminescence and structure evolution of double perovskite (K, Na)LaMgWO6:Eu3+ phosphor for white LEDs. J. Mater. Sci. 26, 8083–8088 (2015)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Crystallogr. A 32, 751–767 (1976)
Q. Liu, X.B. Li, B. Zhang, L.X. Wang, Q.T. Zhang, L. Zhang, Structure evolution and delayed quenching of the double perovskite NaLaMgWO6:Eu3+ phosphor for white LEDs. Ceram. Int. 42, 15294–15300 (2016)
S. Chahar, R. Devi, M. Dalal, M. Bala, J. Dalal, P. Boora, V.B. Taxak, R. Lather, S.P. Khatkar, Color tunable nanocrystalline SrGd2Al2O7:Tb3+ phosphor for solid state lighting. Ceram. Int. 45, 606–613 (2019)
S.J. Park, B.S. Je, J.W. Jang, M.S. Oh, M.S. Koo, S.J. Yang, H.K. Yang, Green and red emitting YBO3:Ln3+ (Ln=Eu, Tb) phosphors for detection of latent fingerprint. J. Alloys Compd. 789, 367–374 (2019)
D.A. Hakeem, D.H. Kim, S.W. Kim, K. Park, Crystal structure and photoluminescence properties of novel garnet Y2-xLaCaGa3ZrO12:xLn3+ (Ln = Eu and Tb) phosphors. Dyes Pigments 163, 715–724 (2019)
J.X. Bin, H.K. Liu, L.F. Mei, L.M. Liang, H. Gao, H.S. Li, L.B. Liao, Multi-color luminescence evolution and efficient energy transfer of scheelite-type LiCaGd(WO4)3:Ln3+ (Ln = Eu, Dy, Tb) phosphors. Ceram. Int. 45, 1837–1845 (2019)
L.G.Van Uitert, Characterization of energy transfer interactions between rare earth ions. J. Electrochem. Soc. 114, 1048–1053 (1967)
D.L. Dexter, J.H. Schulman, Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 22, 1063–1071 (1954)
D.L. Dexter, A theory of sensitized luminescence in solids. J. Chem. Phys. 12, 2836–2850 (1953)
R. Reisfeld, N. Lieblich-Soffer, Energy transfer from UO22+ to Sm3+ in phosphate glass. J. Solid State Chem. 28, 391–395 (1979)
X.B. Lia, Q. Liu, W.T. Huang, S.H. Chen, L.X. Wang, M.X. Yu, Q.T. Zhang, Structural and luminescent properties of Eu3+-doped double perovskite BaLaMgNbO6 phosphor. Ceram. Int. 44, 1909–1915 (2018)
Q. Liu, L.X. Wang, W.T. Huang, L. Zhang, M.X. Yu, Q.T. Zhang, Enhanced luminescence properties of double perovskite (Ba, Sr)LaMgSbO6:Eu3+ phosphors based on composition modulation. J. Alloys Compd. 717, 156–163 (2017)
Acknowledgements
This research was supported by the National Natural Science Foundation of Shaanxi Province (No. 2018JM5151)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, G., Wang, Y., Wei, Y. et al. Structure, energy transfer, and luminescence properties of NaLaMgWO6: Tb3+,Eu3+ phosphors for solid-state lighting. J Mater Sci: Mater Electron 31, 3835–3844 (2020). https://doi.org/10.1007/s10854-020-02918-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-02918-6