Abstract
In this study, we investigated the change in biodegradability of biodegradable polymer films by deep-ultraviolet laser irradiation with different pulse durations. Measurements of water absorption and mass change as well as microscopic observation revealed that the femtosecond laser irradiation significantly accelerated the degradation rate of the biodegradable polymer films, whereas the nanosecond laser irradiation did not induce a comparable degree of change. Analyses with X-ray photoelectron spectroscopy and X-ray diffraction indicate that the difference in the biodegradability following laser irradiation with different pulse durations is attributable to the difference in chemical structure for amorphous polymers including PLGA, while the difference in chemical structure as well as crystallinity affects the biodegradability for crystalline polymer including PLLA. The obtained results suggest that deep-ultraviolet laser processing enables the fabrication of a tissue scaffold with a desirable degradation rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lasers have been widely used for processing of biomaterials because of their abilities to process complex-shaped surfaces even after molding without using a toxic chemical component [1]. Recently, laser processing of biodegradable polymers, which are promising materials for a tissue scaffold owing to its biodegradability into low-toxic components in a human body, has been attracting growing interest. The fabrication of a scaffold in desirable shape and size [2], as well as the modification of the surface nano/micro-morphology [3,4,5], chemical structure [6, 7], and wettability [8] has been studied with laser processing of biodegradable polymers. Study on laser processing of biodegradable polymers is not only for subtractive processing, but also for additive processing. Brandi, et al. reported pioneering and detailed works on photocuring of biodegradable polymers for tissue scaffolding using deep-ultraviolet (UV) laser [9,10,11,12].
The morphological and chemical properties of a scaffold surface play an important role in cell behaviors including cell growth, differentiation, and proliferation. Because a large number of biodegradable polymers show large linear absorption at UV wavelengths, the effect of UV nanosecond laser irradiation on physical and chemical properties of biodegradable polymer has been investigated previously [13, 14]. Castillejo et al. [13] reported that fibroblast cells on chitosan-based scaffold preferentially grow and proliferate on the area irradiated with deep-UV nanosecond laser. Yao et al. [14] reported that the ablated grooves on poly(lactic-co-glycolic acid) (PLGA) formed by UV nanosecond laser irradiation provide a guidance effect on the direction of neurite elongation.
Ultrashort pulsed lasers realized the precise processing of biodegradable polymers with minimized heat affected zone because of the shorter interaction time compared to electron–phonon relaxation time (τ ep ~ 10 ps). Aguilar et al. [15] reported that the ablated crater formed by femtosecond laser irradiation to poly(ɛ-caprolactone) (PCL) and poly(glycolic acid) (PGA) shows less thermal modification than that formed by nanosecond laser irradiation. Hendricks et al. [16] demonstrated the fabrication of biodegradable polymer-based stent by femtosecond laser irradiation to poly(l-lactic acid) (PLLA) tube.
Recent studies on laser processing of biodegradable polymers revealed that biodegradability is dependent on laser parameters [17,18,19]. Biodegradability is a key property of biodegradable tissue scaffolds because it affects sustainability as well as provides a space for cell proliferation [20]. Farkas et al. [17] reported that the biodegradability of poly(propylene fumarate) fabricated by deep-UV nanosecond laser stereolithography is dependent on laser fluences and repetition rates. We previously demonstrated that PLGA film irradiated with femtosecond laser pulses at a wavelength of 400 nm showed faster water absorption and larger mass reduction during degradation than 800 nm-laser-irradiated PLGA film [18]. In addition, the results of X-ray photoelectron spectroscopy (XPS) analysis of PLGA films following femtosecond laser irradiation indicated that the significant chemical bonds dissociation via multi-photon absorption occurred following 400 nm-laser irradiation. Based on the results, the acceleration of degradation could be attributed to the decrease in molecular weight induced by chemical bonds dissociation via multi-photon absorption. Considering the previous study, deep-UV femtosecond laser irradiation would cause more significant chemical bonds dissociation and acceleration of degradation than the cases with femtosecond laser irradiation at wavelengths of 800 nm and 400 nm due to the less required photon numbers to induce chemical bonds dissociation via multi-photon absorption.
In this study, biodegradability of PLGA and PLLA films are investigated by comparing results with deep-UV femtosecond laser and those with nanosecond laser. The effect of laser irradiation on the chemical structure and the crystallinity are studied to discuss the interaction of high-intensity pulsed laser and biodegradable polymers for understanding the underlying mechanism of alteration of biodegradability by laser irradiation.
2 Experimental
PLGA films (lactic to glycolic acid ratio of 50:50 mol%) and PLLA films were purchased from BMG Inc. (Kyoto, Japan). Weight average molecular weight (M w), number average molecular weight (M n), glass transition temperature (T g) of the PLGA and PLLA films are summarized in Table 1, that were provided by BMG Inc. The thickness of the PLGA and PLLA films were 1 and 0.3 mm, respectively.
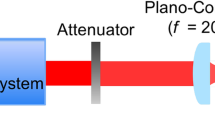
Linearly polarized femtosecond laser pulses of 100 fs pulse duration at 800 nm in central wavelength at a repetition rate of 1 kHz were generated from a Ti:sapphire chirped pulse amplification (CPA) laser system (Libra, Coherent, Inc., California, USA). Laser pulses at 266 nm in central wavelength (third harmonics generation, THG) were obtained by sum-frequency mixing of the fundamental and second harmonic waves. Femtosecond laser-irradiated films for the experiments were prepared by scanning focused femtosecond laser pulses onto a surface of PLGA or PLLA film at normal incidence using a plano-convex lens (focal length 200 mm) in air. The spot diameter of the femtosecond laser pulses was 300 μm. In the experiment with nanosecond laser, laser pulses at 266 nm (forth harmonics generation, FHG) from Q-switched Nd:YAG laser (Brilliant, Quantel, Les Ulis Cedex, France) were used. Pulse duration and repetition rate were 5 ns and 10 Hz, respectively. The beam profiles of the femtosecond laser pulse and nanosecond laser pulses are both a Gaussian. The laser spot size is a value of full width at half maximum (FWHM).
For the evaluation on the modification of the chemical structure and the crystallinity of PLGA and PLLA surfaces, we analyzed with X-ray photoelectron spectroscopy (XPS, JPS-9010, JEOL, Tokyo, Japan) and with X-ray diffraction (XRD, D8 DISCOVER, BRUKER, Massachusetts, USA), respectively, before and after laser irradiation. The laser fluence was 75 mJ/cm2 for the both cases of the femtosecond laser and the nanosecond laser. The femtosecond laser pulses were scanned on a surface of films with the scanning speed of 60 μm/s so as to obtain sufficient area for the evaluations. Nanosecond laser-irradiated films for the analyses were prepared by irradiating 5000 ns laser pulses with spot diameter of 6 mm on a film surface without scanning to equalize the number of laser pulses per area to the case of femtosecond laser irradiation. The laser parameters were determined based on the minimum values for the surface modification with nanosecond laser pulses. The parameters for femtosecond laser irradiation were set to perform the comparative study with nanosecond laser.
For the evaluation on the biodegradability of PLGA and PLLA films following laser irradiation, we measured the mass change as well as observed the morphological transition. Films of 5 × 5 mm2 were used. Each film for the degradation tests was prepared by scanning focused laser pulses on one side of the film. The femtosecond laser pulses were scanned on a surface of films with the scanning speed of 60 μm/s. To equalize the number of laser pulses per unit area for both cases with femtosecond and nanosecond laser irradiation, the spot diameter and the scanning speed of nanosecond laser pulses were set to 3 mm and 6 μm/s, respectively. The laser fluence of femtosecond laser pulses was 75 mJ/cm2, while that of nanosecond laser pulses was 100 mJ/cm2 for the evaluation on the biodegradation. The laser fluences were chosen to obtain obvious morphological transition which can be confirmed by visual observation for the both cases. The experimental scheme for degradation test is similar to that described in our previous study [18]. Films were weighed following the laser irradiation to determine the initial mass (m ini) of each film. Films were fully immersed in phosphate-buffered saline (PBS) in vials. The films were removed from the vials every 2 days and weighed immediately to obtain the wet mass (m wet). Films were then dried in vacuum for 6 days and weighed to obtain the dry mass (m dry). The water absorption and the mass remaining of the films were calculated by following equations.
Scanning electron microscopy (SEM) of the films was also performed.
3 Results and discussion
3.1 Structural properties
To investigate the modification of the chemical structure of PLGA and PLLA surfaces with or without laser irradiation, XPS analysis was performed. Figure 1 shows the C1s narrow scan XPS spectra of PLGA and PLLA surfaces. Each spectrum consists of three peaks at bonding energies of 285, 287, and 289 eV, which are attributable to the carbon in the alkyl group (–CH3), neighboring carbon in the C–O bond, and carbon in the ester bond (O–C=O), respectively [6]. As shown in Fig. 1, the peak at 285 eV significantly reduced in height for both cases with femtosecond and nanosecond laser irradiation. This indicates that C–H bonds were dissociated by laser irradiation. Since the bond energy of C–H bond (4.3 eV) is less than photon energy at 266 nm (4.65 eV), the dissociation of C–H bonds are attributable to linear absorption for both cases with femtosecond and nanosecond laser irradiation. Although the peak at 289 eV remained relatively unchanged following nanosecond laser irradiation, the peak significantly decreased following femtosecond laser irradiation. Since the bond energy of C=O bond (7.5 eV) is higher than photon energy at 266 nm (4.65 eV), two-photon absorption is required to induce C=O bonds dissociation. The dissociation of C=O bonds is more likely to occur by femtosecond laser irradiation. The peak at 287 eV showed no significant change following nanosecond laser irradiation, indicating that the quantity of C–O bonds would be unchanged following nanosecond laser irradiation. However, C–O bonds are theoretically dissociated by nanosecond laser irradiation at 266 nm because the bond energy of C–O bond (3.8 eV) is less than photon energy at 266 nm (4.65 eV). Further study is required to elucidate the precise mechanism of C–O bonds dissociation.
XRD analysis was performed to investigate the modification of the crystallinity of PLGA and PLLA surfaces with or without laser irradiation. Figure 2 shows the XRD spectra for PLGA and PLLA surfaces. There was no sharp peak in the XRD spectra of PLGA surfaces. On the other hand, two sharp peaks around 17° and 19° were observed in the XRD spectra of PLLA surfaces. The difference between the spectra of PLGA and PLLA surfaces are attributable to the difference in crystallinity; PLGA is an amorphous polymer while PLLA is a semi-crystalline polymer [21]. There was no significant difference between the XRD spectra of non-irradiated PLGA surface and PLGA surface following laser irradiation, which is probably due to the low crystallinity of the original PLGA (Fig. 2a). As shown in Fig. 2b, peaks in the XRD spectra of PLLA surfaces reduced in height following laser irradiation, which was significant with femtosecond laser irradiation. The results of XPS analysis indicate that chemical bonds dissociation is more likely to occur by femtosecond laser irradiation compared to that by nanosecond laser irradiation. Since a side chain of polymer have higher mobility than a main chain, an increase in side chain by chemical bonds dissociation cause the polymer chains being less ordered, resulting in a decrease in crystallinity [6]. Therefore, the crystallinity of PLLA films is decreased more significantly with femtosecond laser irradiation than with nanosecond laser irradiation.
3.2 Biodegradable properties
We measured the water absorption and change in mass of the PLGA and PLLA films during degradation for the evaluation of biodegradability. As shown in Fig. 3a, the laser-irradiated PLGA films exhibited faster water absorption compared with the non-irradiated PLGA films. Moreover, the water absorption of the femtosecond laser-irradiated PLGA films was greater than that of the nanosecond laser-irradiated PLGA films, especially after an immersion period of 8 days. As shown in Fig. 3b, while the femtosecond laser-irradiated PLGA films exhibited the significant mass loss in the initial 4 days and after 14 days of immersion, the nanosecond laser-irradiated PLGA films exhibited the slight decrease in mass after 14 days of immersion. The biodegradation of biodegradable polymer proceeds via the hydrolysis of ester bonds in the presence of absorbed water. The significant water absorption of the femtosecond laser-irradiated PLGA films would accelerate the hydrolysis, resulting in a faster mass decrease. Comparing with the case of PLGA films, the difference between the degradation rate of the femtosecond laser-irradiated PLLA films and that of the nanosecond laser-irradiated PLLA films was significantly larger (Fig. 4). Previous studies reported that poly(lactic acid) (PLA) shows a slower degradation rate than PLGA due to the higher crystallinity and hydrophobicity of PLA [22,23,24]. Miller et al. [24] reported that the half-lives of the implants consisting of PLA or PLGA (lactic to glycolic acid ratio of 50:50) in rat are 6.1 months and 1 week, respectively. As shown in Fig. 4a, the non-irradiated PLLA films and the nanosecond laser-irradiated PLLA films exhibited the slight water absorption and mass loss, even following 18 days of immersion. On the other hand, the femtosecond laser-irradiated PLLA films exhibited significant water absorption and mass loss even in the initial 6 days of immersion. The comparably rapid increase in water absorption and mass loss continued over 18 days. These results indicate that deep-UV femtosecond laser irradiation accelerate the degradation of hydrophilic polymer as well as hydrophobic polymer.
The morphology transition of the laser-irradiated area was observed. Figure 5 shows SEM images of the laser-irradiated area on the PLGA films. The changes in surface morphology of the PLGA films were observed in the laser-irradiated area (Fig. 5). The femtosecond laser-irradiated area has relatively low surface roughness before degradation. The depth of the femtosecond laser-irradiated area increased with the elapse of time (Fig. 5a). The rapid degradation of the femtosecond laser-irradiated area in 6 days correlates well with the significant mass decrease of PLGA films irradiated with femtosecond laser in the initial 6 days as shown in Fig. 3b. The width of degradation area expands outside beyond the area that was irradiated with femtosecond laser pulses, which is similar to our previous study using femtosecond laser at 400 nm wavelength [18]. The surface roughness of the nanosecond laser-irradiated area increased with the elapse of time. The expansion of the increase in roughness was limited in the nanosecond laser-irradiated area, even after following 6 days of immersion (Fig. 5b). The depth of the nanosecond laser-irradiated area showed no significant change over 6 days of immersion, which is consistent with the slight decrease in mass of PLGA films irradiated with nanosecond laser in the initial 6 days as shown in Fig. 3b.
3.3 Discussion on biodegradation rate after laser irradiation
There are two possible explanations for the difference between the degradation rates of the films treated with different pulse durations: (i) a difference in chemical structure and (ii) a difference in crystallinity following laser irradiation [19, 25, 26].
Considering the former case, chemical bonds dissociation results in the acceleration of hydrolysis since the degradation products, including hydrophilic carboxylic acid, increase the degree of water absorption [27]. In addition, the decrease in molecular weight owing to chemical bonds dissociation accelerates the diffusion of polymer chains into the water, resulting in a faster mass decrease [28]. As shown in Fig. 1, the chemical bonds dissociation via multi-photon ionization with the femtosecond laser-irradiated films is much significant compared to that with the nanosecond laser-irradiated films. Therefore, the femtosecond laser-irradiated films exhibited a higher rate of the water absorption and mass decrease compared to the nanosecond laser-irradiated films. The water absorption and mass decrease of the femtosecond laser-irradiated PLGA films with 266 nm shown in this study was faster than that of the femtosecond laser-irradiated PLGA films with 800 or 400 nm, which was reported in our previous study [18]. The difference between the degradation rates of the PLGA films treated with different laser wavelengths can also be explained by the difference in chemical structure following laser irradiation. Since the bond energy of C–O bond is 3.8 eV, the C–O bonds dissociation requires three-photon absorption, two-photon absorption, and single-photon absorption for the cases with 800, 400, and 266 nm, respectively. Since C–H bonds and C=O bonds would also be dissociated most significantly with 266 nm-laser irradiation, the degradation rate of the PLGA films irradiated with 266 nm was fastest among the PLGA films irradiated with the different wavelengths.
As for the latter case, from the point of view of the difference in crystallinity, since the polymer chains in amorphous site are highly disordered, the water diffusion into amorphous site is generally faster than that of crystalline site, resulting in a faster degradation of amorphous site. The results of XRD analysis of PLLA films show that the decrease in crystallinity is more significant following femtosecond laser irradiation compared to the case with nanosecond laser irradiation (Fig. 2b). The significant decrease in crystallinity of PLLA films by femtosecond laser irradiation would accelerate the degradation. Since the crystallinity of PLGA films was relatively constant for both cases with femtosecond and nanosecond laser irradiation, the effect of the crystallinity modification on the degradation rate of the PLGA films would be negligible.
According to the discussion above, the difference between the degradation rates of amorphous polymer films, including PLGA, may be attributable to the difference in chemical structure following laser irradiation. In crystalline polymer, including PLLA, the difference between the degradation rates is probably attributable to not only the difference in chemical structure, but also the difference in crystallinity following laser irradiation.
4 Conclusion
We demonstrated that pulse durations of deep-UV laser affect the biodegradability of the biodegradable polymers following laser irradiation. The results of the degradation tests show that the degradation rate of the femtosecond laser-irradiated films was much faster than that of the nanosecond laser-irradiated films. Based on the XPS and XRD results, the difference in the biodegradability following laser irradiation is attributable to the difference in chemical structure for amorphous polymers, including PLGA, while not only the difference in chemical structure, but also the difference in crystallinity affects the biodegradability for the case of crystalline polymers, including PLLA. Since deep-UV femtosecond laser processing significantly accelerates degradation, it enables us to utilize slow-biodegradable polymers including PLLA for a tissue scaffold with a desirable degradation rate.
References
S. Chen, V.V. Kancharla, Y. Lu, Int. J. Mater. Prod. Technol. 18, 457 (2003)
P. Danilevicius, L. Georgiadi, C.J. Pateman, F. Claeyssens, M. Chatzinikolaidou, M. Farsari, Appl. Surf. Sci. 336, 2 (2015)
I. Michaljaničová, P. Slepička, J. Heitz, R.A. Barb, P. Sajdl, V. Švorčík, Appl. Surf. Sci. 339, 144 (2015)
S. Yada, M. Terakawa, Opt. Express 23, 5694 (2015)
V. Dinca, P. Alloncle, P. Delaporte, V. Ion, L. Rusen, M. Filipescu, C. Mustaciosu, C. Luculescu, M. Dinescu, Appl. Surf. Sci. 352, 82 (2015)
S.T. Hsu, H. Tan, Y.L. Yao, Polym. Degrad. Stab. 97, 88 (2012)
B.D. Stępak, A.J. Antończak, K. Szustakiewicz, P.E. Kozioł, K.M. Abramski, Polym. Degrad. Stab. 110, 156 (2014)
P. Slepička, I. Michaljaničová, P. Sajdl, P. Fitl, V. Švorčík, Appl. Surf. Sci. 283, 438 (2013)
S. Beke, F. Anjum, H. Tsushima, L. Ceseracciu, E. Chieregatti, A. Diaspro, A. Athanassiou, F. Brandi, J.R. Soc, Interface 9, 3017 (2012)
S. Beke, F. Anjum, L. Ceseracciu, I. Romano, A. Athanassiou, A. Diaspro, F. Brandi, Laser Phys. 23, 035602 (2013)
S. Beke, B. Farkas, I. Romano, F. Brandi, Opt. Mater. Express 4, 2032 (2014)
B. Farkas, S. Dante, F. Brandi, Nanotechnology 28, 034001 (2017)
M. Castillejo, E. Rebollar, M. Oujja, M. Sanz, A. Selimis, M. Sigletou, S. Psycharakis, A. Ranella, C. Fotakis, Appl. Surf. Sci. 258, 8919 (2012)
L. Yao, S. Wang, W. Cui, R. Sherlock, C. O’Connell, G. Damodaran, A. Gorman, A. Windebank, A. Pandit, Acta Biomater. 5, 580 (2009)
C.A. Aguilar, Y. Lu, S. Mao, S. Chen, Biomaterials 26, 7642 (2005)
F. Hendricks, R. Patel, V.V. Matylitsky, Proc. SPIE 9355, 935502 (2015)
B. Farkas, I. Romano, L. Ceseracciu, A. Diaspro, F. Brandi, S. Beke, Mater. Sci. Eng. C 55, 14 (2015)
A. Shibata, S. Yada, M. Terakawa, Sci. Rep. 6, 27884 (2016)
S.T. Hsu, H. Tan, Y.L. Yao, J. Manuf. Sci. Eng. 136, 011005 (2013)
B. Tesfamariam, Pharmacol. Res. 107, 163 (2016)
P.X. Ma, R. Zhang, J. Biomed. Mater. Res. 56, 469 (2001)
S.H. Oh, J.H. Lee, Biomed. Mater. 8, 014101 (2013)
F. Alexis, Polym. Int. 54, 36 (2005)
R.A. Miller, J.M. Brady, D.E. Cutright, J. Biomed. Mater. Res. 11, 711 (1977)
E. Ikada, J. Photopolym. Sci. Technol. 10, 265 (1997)
H. Tsuji, Y. Echizen, Y. Nishimura, J. Polym. Environ. 14, 239 (2006)
Y. Zhu, H. Jiang, S.H. Ye, T. Yoshizumi, W.R. Wagner, Biomaterials 53, 484 (2015)
R.N. Shirazi, F. Aldabbagh, A. Erxleben, Y. Rochev, P. McHugh, Acta Biomater. 10, 4695 (2014)
Acknowledgements
This work was supported in part by KAKENHI Grant-in-Aid for Young Scientists (A) No. 26702019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, A., Machida, M., Kondo, N. et al. Biodegradability of poly(lactic-co-glycolic acid) and poly(l-lactic acid) after deep-ultraviolet femtosecond and nanosecond laser irradiation. Appl. Phys. A 123, 438 (2017). https://doi.org/10.1007/s00339-017-1055-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1055-6