Abstract
Cyclic superheating and cooling were carried out for the undercooled hypereutectic Co80B20, eutectic Co81.5B18.5, and hypoeutectic Co83B17 alloys. For each alloy, there is a critical overheating temperature T ° c at which there is a sharp increase of the mean undercooling, i.e., below (above) T ° c , and the mean undercooling is about 80 °C (200 °C). DSC measurements show that there is a thermal absorption peak in the heating process, the peak temperature of which is nearly equal to the critical overheating temperature, indicating that the temperature-induced liquid–liquid structure transition does occur and should relate highly to nucleation in the undercooled Co-B eutectic melts. The effect of the liquid–liquid structure transition on nucleation was interpreted by the recent nucleation theory that considers the structures of overheated melts, and the composition-dependent overheating temperature was ascribed to the change of local favored structures. The present work provides further evidences for the liquid–liquid structure transition and is helpful for understanding solidification in undercooled melts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A single-component liquid may have more than one isotropic liquid state (i.e., with different lone-range and medium-range ordering structures), and the transition between which is called “liquid–liquid structure transition” (LLST) [1]. In the past few decades, LLST has been paid much attention in the fields of physics, chemistry, and material science, therefore, enriching considerably our understanding of liquid physics and highlighting significantly the importance to explore the potential liquid materials with novel structures and properties. To show the phenomenon of LLST, many experimental and theoretical studies, such as electrical resistivity method [2,3,4], density measurement [5], neutron and X-ray diffraction [6], revised internal friction method [7], differential scanning calorimetry [8,9,10], viscosity measurement [11], and ab initio molecular dynamics simulations [12] were carried out. It was found that the temperature-induced or pressure-induced LLST occurs in many single-component and multiple-components liquids if the temperature is well above the melting temperature or the liquidus temperature.
Nucleation in an undercooled melt is the process with which the formation of new phases begins and is one of the most important fundamental processes in solidification [13, 14]. In general, nucleation can be classified into homogeneous nucleation [15] and heterogeneous nucleation [16]. The latter is an extrinsic process that is dominated by the experimental conditions, whereas the former is an intrinsic process that depends on the properties of the system [17]. By avoiding heterogeneous nucleation, the melt can be solidified at a temperature below its melting temperature or liquidus temperature, i.e., solidification of undercooled melts [18]. Undercooling as is well known is a physical quantity that is sensitive to the structure of liquids, e.g., even very tiny intrinsic un-melted crystals in the liquids can act as the growth nuclei and may reduce considerably undercooling [19, 20]. Since undercooling depends strongly on the liquid structures, the undercooled melt should be an ideal system to study the liquid structures and LLST. Recent work on the undercooled Co83B17 alloy showed that there is a critical overheating temperature of 1390 °C, below (above) which holding the melt obtains a mean undercooling of about 80 °C (190 °C) [21]. Such a significant change of undercooling due to different overheating treatments is ascribed to the structure transitions within the overheated liquid that can be characterized by magnetization. The existence of the critical overheating temperature is suggested to be related to the structure transitions inside the overheated liquid. However, the effect of alloy compositions on the critical overheating temperature is not shown and the effect of structure transitions on the nucleation temperature needs to be analyzed in-depth.
In this work, cyclic superheating and cooling were carried out for the undercooled hypereutectic Co80B20, eutectic Co81.5B18.5, and hypoeutectic Co83B17 alloys. The critical overheating temperature was found to be existent in but different for all the three alloys. DSC measurements show that there is a thermal absorption peak in the heating process above the melting temperature or the liquidus temperature that corresponds to LLST. In other words, LLST does occur when the overheating temperature is above the critical overheating temperature and it should relate highly to nucleation in the undercooled Co-B eutectic melts. The effect of structure transitions on the nucleation temperature was analyzed in-depth by the recent nucleation theory that considers the structure of overheated melts and the alloy composition-dependent overheating temperature was ascribed to the change of local favored structures.
2 Experimental details
The master alloys with nominal compositions of Co80B20, Co81.5B17.5, and Co83B17 (at.%) were prepared by arc-melting the mixture of pure Co (99.99 wt%) and B (99.99 wt%) elements under an argon atmosphere in a water-cooled copper crucible with a nonconsumable tungsten electrode and a titanium getter. Each alloy ingot was melted for five times to ensure the compositional homogeneity. Samples with a mass of about 2 g were cut from the ingot to precede the undercooling experiments by a combination of glass fluxing and cyclic superheating. The sample was placed first into the quartz crucible together with the flux of boron oxide which was dehydrated at 800 °C for 6 h in advance. After that the sample was melted and cyclically superheated and undercooled. The samples were heated to a series of temperatures, e.g., regarding that the melting point of eutectic Co81.5B18.5 alloy is 1133 °C, and the overheating temperatures were set to be 1200, 1300, 1350, 1370, 1380, 1400, 1450, and 1500 °C; the details are shown in Table 1. After heating the sample to the pre-set overheating temperature, the melt was held therein for 5 min and then naturally cooled down to 800 °C. A two-color infrared pyrometer with an accuracy of ±5 °C and a response time of 10 ms was used to measure the thermal history of the samples. The undercooling in each cycle may vary considerably especially in the first several cycles. Afterwards, the melts become uniform and stable with very small fluctuation; thus, undercooling becomes almost constant. The cyclic of melting and solidification was executed until undercooling can be stable for at least 10 cycles (i.e., the deviation of undercooling is within 5 °C). In this case, the undercooled melts are considered to be in a relatively stable state and the mean undercooling is evaluated from the 10 consecutive cycles.
The master alloys were cross-sectioned and polished for structural observation using scanning electron microscope (SEM, TESCAN VEGA3). The phases therein were identified by X-ray diffraction (XRD, DX-2700) utilizing Cu-Kα radiation. The working conditions were 40 kV and 30 mA for the X-ray tube and the scanning rate was set to be 0.02°/s. The thermodynamic behavior of the Co-B alloys was measured by DSC (NETZSCH, STA449C). The samples of DSC experiments with a mass within 35 mg were cut from the original ingot. The sample was placed in a corundum crucible, heated, and cooled in an argon atmosphere. It was heated up first to 1500 °C at a rate of 20 °C/min and then cooled down to the room temperature at the same rate.
3 Results
3.1 Microstructures
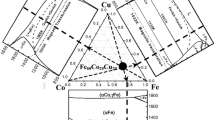
Figure 1 shows the microstructures of the as-cast hypereutectic Co80B20, eutectic Co81.5B18.5, and hypoeutectic Co83B17 alloys. For the hypereutectic Co80B20 alloy, the primary Co3B dendrite was solidified first, and then, the remaining liquid was transformed into lamellar eutectics: α-Co + Co3B (see Fig. 1a). For the eutectic Co81.5B18.5 alloy, the microstructure is lamellar eutectics without any visible primary phases (see Fig. 1b). For the hypoeutectic Co83B17 alloy, primary solidification of α-Co dendrite is followed by the eutectic reaction l→α-Co + Co3B. Such a result for the microstructures is consistent with the Co-B phase diagram [22]. It should be pointed out that α-Co after solidification can be transformed partly into ε-Co by a solid-state transformation as shown in the XRD patterns (Fig. 2), according to which the microstructure should be consisted of α-Co, Co3B, and ε-Co. Although the crystal structures of α-Co (BCC) and ε-Co (HCP) are different, they cannot be distinguished by the contrast in SEM. Regarding that the main aim of the current work is to show LLST and nucleation in undercooled Co-B eutectic alloys, a detailed study of the microstructures of the as-cast and undercooled samples is not present here.
3.2 Dependence of undercooling on the overheating temperature
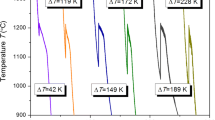
Figure 3 shows the mean undercooling of Co-B eutectic alloys with different overheating temperatures. Undercooling here is defined as the difference between the nucleation temperature (T n ) and the melting temperature (T m ) for the eutectic Co81.5B18.5 or the difference between the nucleation temperature (T n ) and the liquidus temperature (T l ) for the hypereutectic Co80B20 and hypoeutectic Co83B17 alloys. One can see that for all the three alloys, there is a critical overheating temperature T o c at which there is a sharp increase of the mean undercooling, i.e., below T 0 c , the mean undercooling is about 80 °C, whereas above T 0 c , the mean undercooling is about 200 °C. The critical overheating temperature is 1360 °C for the hypereutectic Co80B20 alloy (Fig. 3a), 1380 °C for the eutectic Co81.5B18.5 alloy (Fig. 3b), and 1390 °C for the hypoeutectic Co83B17 alloy (Fig. 3c). It should be pointed out that the mean undercooling here is not obtained for all the cycles, but the cycles when the undercooled melt becomes stable; see Sect. 2.
3.3 LLST in the overheated melts
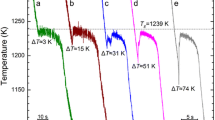
The structure transition in the overheated melt corresponds to the breakage of previous atomic bonds and the formation of new atomic bonds. Because of the different bonding energy between atoms, LLST is inevitably accompanied by the thermal effect. On this basis, a series of DSC measurements were carried out to show the temperature-induced LLST in the overheated Co-B melts (see Fig. 4). For all the hypereutectic Co80B20, eutectic Co81.5B18.5 and hypoeutectic Co83B17 alloys, there is a thermal absorption peak in the heating process above T m or T l but not in the cooling process. The peak temperature is about 1363.4 °C for the hypereutectic Co80B20 alloy (Fig. 4a), 1382.7 °C for the eutectic Co81.5B18.5 alloy (Fig. 4b), and 1392.1 °C for the hypoeutectic Co83B17 alloy (Fig. 4c). The consistency between the peak temperatures in the DSC curve and the critical overheating temperatures (e.g., the temperature difference is less than 4 °C) indicates that the temperature-induced LLST does occur when the overheating temperature is above the critical overheating temperature and nucleation in the undercooled Co-B eutectic melts should relate highly to LLST. To show whether such peak temperatures are heating rate dependent or not, another DSC test, shown in Fig. 4d, with a heating and cooling rate of 10 °C/min was carried out for the eutectic Co81.5B18.5 alloy. One can see that the peak temperature is still at about 1383.4 °C, being consistent with result 1382.7 °C in Fig. 4b. In other words, LLST is not that dependent on the heating and cooling rates.
DSC curves of the Co-B eutectic alloys: a hypereutectic Co80B20 with the heating and cooling rate as 20 °C/min; b eutectic Co81.5B18.5 with the heating and cooling rate as 20 °C/min; c hypoeutectic Co83B17 with the heating and cooling rate as 20 °C/min; and d eutectic Co81.5B18.5 with the heating and cooling rate as 10 °C/min
In all the DSC tests, there are no apparent peaks in the liquid state during the cooling process (see Fig. 4), indicating that LLST in Co-B melts is irreversible. To show this point further, superheating and cooling of eutectic Co81.5B18.5 alloy were carried out with different overheating temperature; see Fig. 5 in which only the cooling histories of the last four cycles are shown. In this case, the undercooling can hold stable after 18 cycles when the overheating temperature is larger than the critical overheating temperature which is in line with the result in Fig. 3b. The large undercooling holds even when the overheating temperature is smaller than the critical overheating temperature (e.g., for 24th and 25th cycles). After that, the undercooling becomes small (e.g., for 26th cycles). If LLST is reversible, the large undercooling cannot be obtained, once the overheating temperature is smaller than the critical overheating temperature. Such a conclusion that LLST is irreversible is consistent with the previous work [20].
4 Discussion
4.1 LLST vs. nucleation
It is generally believed that the atomic bonds of crystals are only partly broken upon melting and there are a lot of short-range ordering domains that correspond to the solid crystal in the melts within a wide temperature range above T m or T l [23]. Thus, the structures of melts are micro-heterogeneous in the continuous heating procedure and many micro-domains (even unsolved particles) exist in the melt [24]. The micro-heterogeneous structure that changes with the overheating temperature is metastable or thermodynamically non-equilibrium. Because different overheating temperatures correspond to different melt states, the structures and properties of many materials are related to the thermal history of their original melts, e.g., the microstructure of amorphous alloys is much more homogeneous if the precursor melts are more overheated [23]. The correlation between the solidified microstructures and the melt thermal history is attributed to different melt microstructures or different liquid states. The overheating temperature as one of the most essential processing parameter has a decisive influence on the liquid state. Thermodynamically, overheating prompts the transition between different liquid states and a critical overheating temperature may correspond to the transition point between the two distinct forms that can be detected.
Zu et al. [24, 25] assumed that LLST is a transition from an inhomogeneous liquid state to a more uniformed liquid state. There are both chemical and topological short-range orders in the liquid alloys with negative excess enthalpy, e.g. ,both the chemical short-range domains with microstructures similar to Co3B and the residual short-range ordering of cube/hexagon solid Co co-exist in the molten Co-B eutectic alloys. These minor domains as the fluctuation of energy dissipate and engender with time and space, but they do not vanish with their statistical equilibrium structures, sizes, and constituents. However, as long as the temperature reaches T 0 c , the inter-atomic bonds in the original domains are broken, the old domains are reduced, and at the same time, new domains form with a relatively more uniformed liquid structure state.
In this study, the melt is encapsulated in the molten glass slag that is close to the levitation melting condition and the impurities in the melt can be removed via electromagnetic stirring and the adhesion of slag. Consequently, the cavity induced heterogeneous nucleation mechanism [26] which is always used to describe the effect of overheating on the undercooling for the nucleation process of a melt on a second solid surface cannot be used. Magnetic field texturing has already shown the possible existence of intrinsic solid nuclei above the melting temperature T m [27]. The clusters exist in the melt and act as the intrinsic growth nuclei, thus leading to nucleation in the cooling process. During solidification, the Co-B melt which did not experience LLST has lots of relatively larger size micro-domains. Through fluctuations in structure and energy, these micro-domains can be easily extended to the critical size of the crystal nucleus at a low undercooling. However, when the melt experienced LLST, it is harder to nucleate because of the smaller and more homogeneous short-range orders, and the melt needs a larger undercooling to nucleate, as shown in Fig. 3.
After analyzing the experimental results of nucleation and growth in 38 elements, Tournier [19, 28,29,30] proposed an explanation by improving the classical nucleation theory. His idea is that the transfer of Δn conduction electrons equalizing the Fermi energies of a particle containing n free electrons and the melt creates an interface electrostatic energy—ɛ v per volume unit that stabilizes tiny crystals above T m . These crystals act as the intrinsic nuclei in the undercooled melts and their presence reduces the critical energy barrier. All these tiny crystal are predicted to be survival up to a critical temperature T m2 = 1.196 T m . Therefore, ɛ v is taken to be part of the contributions to the volume free energy change ΔG v and the total free energy change due to the formation of a nuclei with a radius R is:
where ΔG V = ΔH m θ/V m , σ ls (V m /N A )−1/3 = α ls ΔH m /V m , θ = T/T m − 1. Here, σ ls is the interface energy, V m is the molar volume of liquid, and ΔH m is the enthalpy of fusion at the melting temperature. The critical radius R ls for nucleation can be found if the partial derivation of ΔG ls with respect to R is set to be zero, that is
Integrating Eq. (1) with Eq. (2) yields
If a non-dimensional parameter ɛ ls is introduced to evaluate the contribution by ɛ v , i.e., ɛ v = ɛ ls ΔH/V m , the critical radius R ls is positive when θ < ɛ ls and negative when θ > ɛ ls , indicating that a tiny crystal can be survival up to a second melting temperature T m2 = (1 + ɛ ls )T m .
The nucleation rate I per unit volume and per second in an undercooled melt can be given as a function of the critical activation barrier ΔG * ls (T)/k B T with k B the Boltzmann’s constant:
The solidification can be initiated when one nucleation event occurs in the melt, i.e., Ivt = 1. According to Tournier [19, 28,29,30], the relation between ɛ ls and θ was obtained as
The so-called second melting temperature T m2 can then be calculated by integrating Eqs. (1)–(5) and summarized in Table 2. The consistency between T m2 and T 0 c indicates that the re-melting of tiny crystals that can be survival above to T m2 is highly related to LLST. It should be pointed out that LLST in the liquid may alter the wetting angle due to the abrupt change of surface tension. When LLST occurs, the surface tension changes, thus resulting in the change of f(θ) and the increase of undercooling [10, 31]. Furthermore, the nucleation model of Tournier [19, 28,29,30] is actually proposed for pure elements, and it is plausible to be applied to the current Co-B alloys but at least it can explain satisfactorily the present experimental results.
4.2 Critical overheating temperature vs. alloy compositions
As mentioned above, the melts of Co-B eutectic alloys are mainly consist of microstructure similar to Co3B and the residual short-range ordering of cube/hexagon solid Co within a certain range above T m or T l . When the overheating temperature is up to T 0 c , the kinetic energy of the atoms becomes high enough to overcome the energy barrier, so that the Co–Co or Co-B atomic bonds are continuously broken. During LLST, through the adjustment of atomic bonds and the structural rearrangement, the size of short-range orders becomes smaller, and the liquid structures are completely different to the original ones and become more homogeneous. It should be pointed out that T 0 c is different for different alloys and increases with the composition of Co (Fig. 3). In the liquid, there are locally favored structures [1] such as Co-rich domains and Co3B-rich domains at the temperature not far from T m or T l , the type, amount and size of which change with the composition and temperature as has been evidenced by the magnetization variations of the liquids [21, 32]. Hence, it is reasonable to conclude that the dependence of T 0 c on the alloy composition is due to the change of local favored structures.
5 Conclusions
Cyclic superheating and cooling as well as DSC measurements were carried out for the hypereutectic Co80B20, eutectic Co81.5B18.5 and hypoeutectic Co83B17 alloys. Our main conclusions are as follows.
-
1.
For each alloy, there is a critical overheating temperature T 0 c at which there is a sharp increase of the mean undercooling, i.e., below (above) T 0 c ; the mean undercooling is about 80 °C (200 °C). The critical overheating temperature is 1360 °C for the hypereutectic Co80B20 alloy, 1380 °C for the eutectic Co81.5B18.5 alloy, and 1390 °C for the hypoeutectic Co83B17 alloy.
-
2.
DSC measurements show that there is a thermal absorption peak in the heating process above the melting temperature or the liquidus temperature, the peak temperature of which is nearly equal to the overheating temperature, indicating that the temperature-induced LLST does occur when the overheating temperature is above the critical overheating temperature and it should relate highly to nucleation in the undercooled Co-B eutectic melts. LLST is not dependent on the heating and cooling rates and is irreversible.
-
3.
The effect of LLST on nucleation is interpreted by the recent nucleation theory that considers the structure of overheated melts, according to which the second melting temperature T m2 can be calculated and is nearly equal to the critical overheating temperature T 0 c , indicating that the tiny crystals that can be survival above to T m2 is highly related to LLST. This work provides further evidences for LLST and is helpful for our understanding of solidification in undercooled melts.
References
H. Tanaka, General view of a liquid-liquid phase transition. Phys. Rev. E 62, 6968–6976 (2000)
Y. Xi, F.-Q. Zu, X.-F. Li, J. Yu, L.-J. Liu, Q. Li, Z.-H. Chen, High-temperature abnormal behavior of resistivities for Bi-In melts. Phys. Lett. A 329, 221–225 (2004)
X.-F. Li, F.-Q. Zu, H.-F. Ding, J. Yu, L.-J. Liu, Q. Li, Y. Xi, Anomalous change of electrical resistivity with temperature in liquid Pb-Sn alloys. Physica B 358, 126–131 (2005)
X. Li, F. Zu, L. Liu, J. Li, J. Chen, C. Hu, Effect of Sn on reversibility of liquid-liquid transition in Bi-Sb-Sn alloys. J. Alloy. Compd. 453, 508–512 (2008)
H. Geng, G. Zhang, Z. Wang, Y. Deng, H. Qin, Density–temperature properties of Ga-Sb alloy melt. Appl. Phys. A 98, 227–232 (2010)
D. Le Coq, A. Bytchkov, V. Honkimäki, B. Beuneu, E. Bychkov, Neutron and X-ray diffraction studies of TeCl4 and TeBr 4 liquids. J. Non-Cryst. Solids 354, 259–262 (2008)
F.Q. Zu, Z.G. Zhu, B. Zhang, Y. Feng, J.P. Shui, Post-melting anomaly of Pb-Bi alloys observed by internal friction technique. J. Phys.: Condens. Matter 13, 11435 (2001)
J. Chen, Influence of a Liquid Structural Change on the Solidification of the Alloy CuSn30. Met. Mater. Int. 14, 569–574 (2008)
C. Zhou, L. Hu, Q. Sun, J. Qin, X. Bian, Y. Yue, Indication of liquid-liquid phase transition in CuZr-based melts. Appl. Phys. Lett. 103, 171904 (2013)
X. Qiu, J. Li, J. Wang, T. Guo, H. Kou, E. Beaugnon, Effect of liquid-liquid structure transition on the nucleation in undercooled Co-Sn eutectic alloy. Mater. Chem. Phys. 170, 261–265 (2016)
L. Wang, X. Bian, J. Liu, Discontinuous structural phase transition of liquid metal and alloys (1). Phys. Lett. A 326, 429–435 (2004)
G. Zhao, C. Liu, Z. Zhu, Ab initio molecular-dynamics simulations of the structural properties of liquid In 20 Sn 80 in the temperature range 798-1193 K. Phys. Rev. B 73, 024201 (2006)
M. Krivilyov, T. Volkmann, J. Gao, J. Fransaer, Multiscale analysis of the effect of competitive nucleation on phase selection in rapid solidification of rare-earth ternary magnetic materials. Acta Mater. 60, 112–122 (2012)
M. Gäumann, R. Trivedi, W. Kurz, Nucleation ahead of the advancing interface in directional solidification. Mater. Sci. Eng., A 226, 763–769 (1997)
Z. Wang, F. Wang, Y. Peng, Z. Zheng, Y. Han, Imaging the homogeneous nucleation during the melting of superheated colloidal crystals. Science 338, 87–90 (2012)
H.-T. Li, Y. Wang, Z. Fan, Mechanisms of enhanced heterogeneous nucleation during solidification in binary Al-Mg alloys. Acta Mater. 60, 1528–1537 (2012)
S. Klein, D. Holland-Moritz, D.M. Herlach, Crystal nucleation in undercooled liquid zirconium. Phys. Rev. B 80, 212202 (2009)
D.M. Herlach, Solidification from undercooled melts. Mater. Sci. Eng., A 226, 348–356 (1997)
R.F. Tournier, Presence of intrinsic growth nuclei in overheated and undercooled liquid elements. Phys. B 392, 79–91 (2007)
J.H. Perepezko, G. Wilde, Melt undercooling and nucleation kinetics. Curr. Opin. Solid State Mater. Sci. 20, 3–12 (2016)
J. Wang, Y. He, J. Li, R. Hu, H. Kou, E. Beaugnon, Overheating dependent undercooling in a hypoeutectic Co-B alloy. Mater. Chem. Phys. 149–150, 17–20 (2015)
P.K. Liao, K.E. Spear, The B-Co (Boron-Cobalt) system. Bull. Alloy Phase Diagr. 9, 452–457 (1988)
U. Dahlborg, M. Calvo-Dahlborg, P.S. Popel, V.E. Sidorov, Structure and properties of some glass-forming liquid alloys. Eur. Phys. J. B 14, 639–648 (2000)
F.-Q. Zu, J. Chen, X.-F. Li, L.-N. Mao, Y.-C. Liu, A new viewpoint to the mechanism for the effects of melt overheating on solidification of Pb-Bi alloys. J. Mater. Res. 24, 2378–2384 (2011)
F.-Q. Zu, Temperature-induced liquid-liquid transition in metallic melts: a brief review on the new physical phenomenon. Metals 5, 395–417 (2015)
B. Yang, J.H. Perepezko, J.W. Schmelzer, Y. Gao, C. Schick, Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 140, 104513 (2014)
R.F. Tournier, E. Beaugnon, Texturing by cooling a metallic melt in a magnetic field. Sci. Technol. Adv. Mater. 10, 014501 (2009)
R.F. Tournier, Crystallization of supercooled liquid elements induced by superclusters containing magic atom numbers. Metals 4, 359–387 (2014)
R.F. Tournier, Crystal growth nucleation and Fermi energy equalization of intrinsic spherical nuclei in glass-forming melts. Sci. Technol. Adv. Mater. 10, 014607 (2009)
R.F. Tournier, Expected properties of gold melt containing intrinsic nuclei. In: Proceedings of the 6th International Conference on Electromagnetic Processing of Materials, EPM 2009, 2009, pp. 304–307
X. Li, F. Zhang, F. Zu, X. Lv, Z. Zhao, D. Yang, Effect of liquid-liquid structure transition on solidification and wettability of Sn-0.7Cu solder. J. Alloy. Compd. 505, 472–475 (2010)
J. Wang, J. Li, R. Hu, H. Kou, E. Beaugnon, Evidence for the structure transition in a liquid Co-Sn alloy by in situ magnetization measurement. Mater. Lett. 145, 261–263 (2015)
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 51690163), the fund of the State Key Laboratory of Solidification Processing in NWPU (No. 103-QP-2014), the Fundamental Research Funds for the Central Universities (No. 3102015ZY085), and the Program of Introducing Talents of Discipline to Universities (No. B08040). The author Yixuan HE gratefully acknowledges financial support from the China Scholarship Council (CSC).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, Y., Li, J., Wang, J. et al. Liquid–liquid structure transition and nucleation in undercooled Co-B eutectic alloys. Appl. Phys. A 123, 391 (2017). https://doi.org/10.1007/s00339-017-0984-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0984-4