Abstract
The Ni–38 wt% Si alloy whose eutectic products are two stoichiometric intermetallic compounds (i.e., NiSi and NiSi2) was undercooled by the melt fluxing technique. After in situ observations of the recalescence processes using a high-speed camera and by electron back-scattering diffraction analysis of the solidification microstructures, the crystal growth velocities, phase selection, and microstructure evolutions were studied. Due to a growth-controlled mechanism, the primary phase changes from the NiSi to the NiSi2 phase at a critical undercooling ΔT ≈ 48 K. Even in the absence of the driving force of chemical superheating, the transition from regular eutectics to anomalous eutectics happens. The reason is that the single-phase dendrite of NiSi2 phase solidifies firstly and then the NiSi phase grows epitaxially to form an uncoupled eutectic-dendrite at high undercooling. The present work provides further experimental evidences for the dual origins of anomalous eutectics (e.g., uncoupled eutectic-dendrite growth during the recalescence stage and coupled lamellar eutectic growth at low undercooling during the post-recalescence stage) and is helpful for understanding of non-equilibrium phenomena in undercooled melts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutectic alloys are of major practical importance ascribing to their good casting, wetting, and mechanical properties [1]. Due to different solidification conditions, physical properties, and volume fractions of eutectic phases, an extensive variety of eutectic morphologies exhibit [2–7]. Taking the case of undercooling as an example, a well-known non-equilibrium phenomenon is the transition from regular eutectics to anomalous eutectics (e.g., in the Ag–Cu [8, 9], Co–Si [10, 11], Co–Sn [12–14], and Ni–Sn [15–18] alloys), the mechanism of which has been studied extensively [12–21].
In the undercooled Ag–Cu eutectic alloy, two types of eutectic microstructures, i.e., “lamellar” and “anomalous” eutectics, were found by Powell and Hogan [19]. The anomalous eutectics were suggested to be formed by a repeated nucleation of Cu-rich solid-solution phase ahead of the growing matrix of Ag-rich solid-solution phase. In the undercooled Ni–Sn eutectic alloy, Kattamis and Flemings [17] found that the anomalous eutectics were of a “dendrite-like” morphology. After successively polishing and examining the microstructures of parallel sections, both phases in the anomalous eutectics were found to be interconnected along a polyhedral network. Accordingly, they suggested that the initial “dendrite-like” microstructure should be a precipitation of supersaturated single α-Ni solid-solution phase, subsequent decomposition of which during the post-recalescence stage forms the anomalous eutectics with α-Ni and β-Ni3Sn phases. In contrast, Jones [20] found neither dendritic branching nor continuous network of both phases in the undercooled Ag–Cu eutectic alloy and attributed the formation of anomalous eutectics to uncoupled eutectic growth. Such mechanism was followed by Li et al. [12] who ascribed uncoupled eutectic growth to extremely diverse linear kinetic coefficients of eutectic phases. Wei et al. [21], however, proposed that the two eutectic phases nucleated independently and grew cooperatively, i.e., coupled eutectic-dendrite growth should be responsible for the formation of anomalous eutectics.
According to their experimental results in the undercooled Ni–Si, Co–Sb, and Ni–Al–Ti eutectic alloys, Goetzinger et al. [23] suggested that the fragmentation of primary eutectic lamellae with the reduction of interfacial energy as the driving force leads to the formation of anomalous eutectics. Based on the in situ observations of the recalescence processes in which a double recalescence was found during spasmodic growth, Clopet et al. [9] ascribed the origin of anomalous eutectics in the undercooled Ag–Cu alloy to re-melting of eutectic-dendrites. Li et al. [22] supposed that the anomalous eutectics are formed by both coupled and uncoupled eutectic growth in the undercooled Ni–Sn eutectic alloy. At low undercooling, anomalous eutectics are formed by fragmentation and ripening of lamellar eutectics. At high undercooling, uncoupled eutectic growth (or re-melting of the primary single dendritic phase and subsequent crystallization of the second phase from the inter-dendritic liquid) gives rise to the anomalous eutectics. Such a mechanism of dual origins was proved further by in situ observations and electron back-scattering diffraction (EBSD) analysis of undercooled Ni–Sn eutectic alloy [15].
In the very recent work of Wei et al. [4], the re-melted fraction of primary eutectics was analyzed systematically according to the eutectic-dendrite growth theory and was suggested to be an indicator of the tendency for the formation of anomalous eutectics. After a comparison among three representative binary eutectic alloys (i.e., Ag–39.9 at.% Cu, Ni–19.6 at.% P, and Pd–16.0 at.% P alloys whose eutectic products are solid solution–solid solution, solid solution–stoichiometric intermetallic compound, and stoichiometric intermetallic compound–stoichiometric intermetallic compound, respectively), it was concluded that anomalous eutectics are formed by re-melting of primary solid during solidification of undercooled eutectic alloy melts. The dominative driving force for re-melting should be the chemical superheating regardless of whether the primary solid consists of a single-phase or eutectic microstructure. The reduction of interfacial energy plays a role when superheating-induced re-melting takes place and promotes the fragmentation during the post-recalescence stage. The recent work above [4, 9, 15, 22, 23] shows that re-melting is the primary mechanism for the formation of anomalous eutectics. However, whether the dominative driving force for re-melting is the reduction of interfacial energy because solids attempts to minimize its surface areas via heat and solute diffusions [23] or the chemical superheating [4] because the non-equilibrium supersaturated primary solid evolves to the equilibrium state by excluding redundant solute is still an open problem.
In the present work, rapid solidification of undercooled Ni–38 wt% Si alloy whose eutectic products are two stoichiometric intermetallic compounds (i.e., NiSi and NiSi2) was carried out. The reasons for choosing this alloy system are as follows: (I) The composition of stoichiometric intermetallic does not change with undercooling, thus eliminating the chemical superheating effect and leaving the reduction of interfacial energy as the only driving force for re-melting [4]. (II) According to the Ni–Si phase diagram [24], there are no phase transformations except for the eutectic solidification. Consequently, the primary eutectic microstructure during the recalescence stage and/or re-melting process during the post-recalescence stage could be retained to the room temperature. After in situ observations using a high-speed camera and by EBSD analysis of the solidification microstructures, phase selection between the NiSi and NiSi2 phases was found to be due to a growth-controlled mechanism. The present work shows that the transition from regular eutectics to anomalous eutectics happens even in the absence of the driving force of chemical superheating and provides further experimental evidences for the dual origins of anomalous eutectics [22], e.g., uncoupled eutectic-dendrite growth during the recalescence stage and coupled lamellar eutectic growth during the post-recalescence stage.
Experimental
A high-purity ingot with a weight of 50 g of Ni–38 wt% Si eutectic alloy was prepared by the pure nickel (99.99 %) and silicon (99.999 %). The master alloy was re-melted for at least three times to ensure chemical homogeneity in the arc furnace facility under a high-purity Ar atmosphere. The final mass loss was found to be within 0.3 %.
The melt fluxing technique was applied to undercool the Ni–38 wt% Si eutectic alloy. A quartz glass crucible, which was rinsed previously in an alcoholic solution by an ultrasonic cleaning machine, was chosen as the container. The sample with a weight of about 2 g was cut from the master alloy and put into the quartz glass crucible with a certain quantity of glass flux of boron oxide. The melt fluxing of boron oxide was used to restrain heterogeneous nucleation within the melts by removing impurities and surface oxides away from the sample and isolating the melts from the surface of the crucible. The quartz glass crucible was located within the high-frequency induction coil of a chamber which was evacuated firstly to a pressure of 3 × 10−3 Pa and then backfilled by a high-purity Ar gas to a pressure of 5 × 10−2 MPa.
The sample was heated firstly to 500 K for about 30 min, which is higher than its melting point of boron oxide but lower than the eutectic temperature of Ni–38 wt% Si alloy 1239 K. Then, the sample covered by the melt fluxing was overheated to 100–250 K above the eutectic temperature and held for about 20 min. Each sample was cyclically superheated and cooled down until a desired undercooling was obtained. The cooling history was recorded by a one-color pyrometer with a response time of 1 ms. The recalescence process was captured using a high-speed camera (OLYMPUSI-Speed 3 MONO). The frame rate was set to be 400–1500 fps according to the duration of recalescence. With the help of software (i-SPEED Software Suite), the average crystal growth velocities were measured by dividing the total traveling distance of the growth front by the solidification time.Footnote 1 The as-solidified sample was mounted in Bakelite and carefully polished. After etching in a solution of 10 mL H2O2, 10 mL HCl, and 50 mL H2O, the sample was observed by an optical microscopy (Olympus GX71). The grain orientations were measured using a scanning electron microscope (TESCAN VEGA 3 LMU) with an EBSD analysis system after polishing the sample with the SiO2 colloidal suspension in a vibratory polisher.
Results
Cooling histories
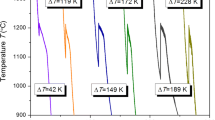
Figure 1 shows the cooling histories of samples with different undercooling ΔT ≈ 3 K, ΔT ≈ 15 K, ΔT ≈ 31 K, ΔT ≈ 51 K, and ΔT ≈ 74 K. For ΔT ≈ 3 K, the sample solidifies under a near-equilibrium condition and only one thermal plateau that corresponds to eutectic solidification is available. For ΔT ≈ 15 K, the superheated melt cools down to 1224 K and then heats up to the eutectic temperature of 1239 K. After a thermal plateau of about 21 s, the temperature decreases monotonously. When the undercooling becomes much larger, e.g., ΔT ≈ 31 K, ΔT ≈ 51 K and ΔT ≈ 74 K, two recalescence events are available in the cooling history. For all the three undercooling, the second recalescence seems to occur within the first recalescence process. Regarding that the onset temperature of second recalescence is higher than that of the first one, the undercooling ΔT in the present work is defined as the difference between the onset temperature of first recalescence and the eutectic temperature. The measured cooling curves (Fig. 1) suggest possibly that two rapid solidification processes that correspond to the two recalescence events happen in relative high undercooled Ni–38 wt% Si eutectic alloys. It should be pointed out that the critical undercooling for the onset of two recalescence events is ΔT ≈ 20 K (Fig. 3).
Recalescence behaviors and crystal growth velocities
Figure 2 shows a typical series of high-speed video camera images of the recalescence processes for ΔT ≈ 51 K. The initial (nucleation) points for the first and the second recalescence processes are marked by solid circles. Although the second recalescence seems to occur within the first recalescence process according to the cooling history (Fig. 1), growth of the recalescence front during the first recalescence process has completed before the onset of second recalescence (Fig. 2). For each recalescence process, the recalescence front migrates across the sample surface from one side to the other side, and the solidification microstructures show directional characters at low and high undercooling (see the following section), indicating that it is reasonable to obtain the crystal growth velocity quantitatively by tracking the loci of the recalescence front recorded in the video images at different moments [15]. The determined crystal growth velocities of two recalescence processes are summarized in Fig. 3. For the first recalescence process, an abrupt increase happens at ΔT ≈ 40 K, whereas for the second recalescence process, the growth velocity increases monotonously with undercooling and changes from a power law to a linear law [26] at ΔT ≈ 40 K. The regions I–V are defined according to the solidification microstructures in which the cases of a-e marked by the open circles are shown in the next section, i.e., Fig. 4.
High-speed video camera images showing the recalescence fronts on the surface of Ni–38 wt% Si eutectic alloy with an undercooling of ΔT ≈ 51 K: a The first recalescence, b the second recalescence. The dark part and light part correspond to the undercooled liquid and recalescing solid, respectively. The initial (nucleation) points for the first and the second recalescence processes are marked by solid circles. With the help of software (i-SPEED Software Suite), the average crystal growth velocities for the first and the second recalescence processes are determined as 40.03 and 20.56 mm/s, respectively
Crystal growth velocity versus undercooling of solidification of Ni–38 wt% Si eutectic alloys. The solid squares and solid circles correspond to the first and the second recalescence processes, respectively. The regions I–V are defined according to the microstructures in which the cases of a–e marked by the open circles are presented in Fig. 4. The solid line and the dotted line are drew qualitatively for the growth of the NiSi and NiSi2 phases, according to which the critical undercooling for the transition of primary phase from the NiSi to the NiSi2 phase is found to be at the critical undercooling ΔT ≈ 48 K
Typical optical micrographs corresponding to the regions I–V defined in Fig. 3: a ΔT ≈ 3 K, b ΔT ≈ 15 K, c ΔT ≈ 31 K, d ΔT ≈ 51 K, e ΔT ≈ 74 K
Microstructures and EBSD analysis
Figure 4 shows the optical micrographs of samples with ΔT ≈ 3 K, ΔT ≈ 15 K, ΔT ≈ 31 K, ΔT ≈ 51 K, and ΔT ≈ 74 K that correspond to a–e in the regions I–V of Fig. 3, respectively. For ΔT ≈ 3 K (or region I), the microstructure is a typical thin regular lamellar eutectics. For ΔT ≈ 15 K (or region II), a primary directional granular structure of NiSi phase is surrounded by the thin regular lamellar eutectics. For the region III, e.g., at ΔT ≈ 31 K, the primary NiSi phase is surrounded by a coarse eutectic structure, and the rest is the thin regular lamellar eutectics. For ΔT ≈ 51 K (or region IV), strip-shaped NiSi structures and dendritic NiSi2 phase are found together with the thin regular lamellar eutectics. At the maximal undercooling obtained in the present work ΔT ≈ 74 K (or region V), NiSi and NiSi2 eutectic-dendrites are prevailed. In some regions, the eutectic-dendrites are fragmented into anomalous eutectics. It should be pointed out that all the microstructures consist solely of the NiSi and NiSi2 phases according to the XRD and EDS results. In order to show the mechanism of microstructure evolutions, EBSD analysis was carried out, and the results are shown as follows.
The EBSD orientation maps and the {100} pole figures (PFs) of the Ni–38 wt% Si eutectic alloy solidified at ΔT ≈ 3 K are shown in Fig. 5a–d. The NiSi (Fig. 5a) and NiSi2 (Fig. 5c) phases have almost one single color. From the PFs, only one main orientation can be found for the NiSi (Fig. 5b) and the NiSi2 (Fig. 5d) phase, being consistent with cooperative growth of lamellar eutectics. Figure 6 shows the EBSD orientation maps and the {100} PFs for the case of ΔT ≈ 15 K. Three main orientations are found for the NiSi phase, even though the differences between the orientations are not that large. Because the granular structure and the surrounding regular lamellar of NiSi phases share the same orientation, the lamellar eutectics should be formed by an epitaxial growth mechanism. Correspondingly, the regular lamellar of NiSi2 also has three main orientations. Although only one recalescence event can be found in the cooling history (Fig. 1), two transformation processes can be distinguished from the solidification microstructures, i.e., the primary crystal growth of NiSi phase and the second lamellar eutectic growth of NiSi and NiSi2 phases.Footnote 2 Furthermore, the granular structure of NiSi might be formed by an initial strip-shaped structure which is fragmented during the post-recalescence stage.
Figure 7 shows the EBSD orientation maps and the {100} PFs for the case of ΔT ≈ 31 K. For the NiSi phase, only one main orientation is available, indicating that both the coarse eutectic structure and the thin regular lamellar eutectics are formed by an epitaxial growth mechanism. For the NiSi2 phase, however, three main orientations are found, the orientation differences between which are rather large. Furthermore, the green grain shares one concentrated pole with the blue and the pink grain in the PF (Fig. 7d), indicating the orientation relationships for cooperative growth could be diversified. An explanation of such phenomenon is however out of the scope of the present work. In the case of ΔT ≈ 31 K, two recalescence events are found in the cooling history (Fig. 1a). It is quite reasonable to speculate that the first (second) recalescence corresponds to the formation of primary granular structure of NiSi phase and subsequent growth of NiSi phase in the coarse eutectic structure (epitaxial growth of NiSi2 phase in the coarse eutectic structure). The thin lamellar eutectic is solidified during the post-recalescence stage.Footnote 3
EBSD analysis of primary granular NiSi structure surrounding successively by a coarse eutectic structure and a thin lamellar eutectic of the Ni–38 wt% Si eutectic alloy with an undercooling of ΔT ≈ 31 K. a EBSD orientation map and b {100} pole figure of NiSi phase. c EBSD orientation map and d {100} pole figure of NiSi2 phase
Figure 8 shows the EBSD orientation maps and the {100} PFs for the case of ΔT ≈ 51 K. For both the NiSi and the NiSi2 phases, only one main orientation can be found. In this case, the coarse strip-shaped NiSi structure and the NiSi2 dendrite coexist, both of which could be the primary phase that is crystalized during the first recalescence stage. Integrating with the experimental results of ΔT ≈ 74 K (Figs. 9, 10), however, the NiSi2 dendrite should be the primary phase that forms in the first recalescence stage and then the coarse strip-shaped NiSi structure is crystallized epitaxially during the second recalescence stage. The remained liquid solidifies finally as the thin regular lamellar eutectics during the post-recalescence stage.
For the case of ΔT ≈ 74 K, the EBSD orientation maps and the {100} PFs are shown in Figs. 9 and 10. Besides some random orientations, only one main orientation can be found for both the NiSi and the NiSi2 phases. For the eutectic-dendrite region, the NiSi2 phase is of a well-developed dendritic microstructure, while the NiSi phase grows epitaxially to form a eutectic-dendrite. This kind of eutectic-dendrite grows by not a coupled but an uncoupled mechanism in which the primary NiSi2 dendrite is formed during the first recalescence stage, and the NiSi phase is solidified during the second recalescence stage.Footnote 4 In the inter-eutectic-dendrite region, fine eutectic structures can be observed. For the region with fragmentation, the characters of orientations are similar except that the random orientations become more. For the NiSi2 phase, the dendrites are re-melting into strip-shaped and coarse granular structures. For the NiSi phase, fragmentation is also prevailed. For the NiSi2 and NiSi phases with the interfacial energy as the only driving force for re-melting, the re-melting process cannot take place so adequately that the fragmentation does not have the time and the chance to be moved far away from their origins and rotated with large angles (Figs. 9a, c, 10a, c). Consequently, the intermediate fragmentation process can be retained to show the mechanism and the origin of anomalous eutectics.
Discussion
Mechanism of phase selection
In the undercooled Ni–38 wt% Si eutectic alloy, the primary phase changes from the NiSi to the NiSi2 phase with the increase of undercooling, i.e., phase selection occurs. In a similar Co–61.8 at.% Si eutectic alloy in which CoSi is a non-stoichiometric intermetallic compound and CoSi2 is a stoichiometric intermetallic compound, a transition from the CoSi to the CoSi2 phase occurs [10, 11, 25]. Li et al. [25] expected that the two intermetallic compounds have a similar short-range diffusion-controlled growth mechanism at the solid/liquid interface because the CoSi and CoSi2 phases share the same simple cubic lattice structure. Since the melting temperature of CoSi phase (1460 °C) is much larger than that of CoSi2 phase (1326 °C) and the interface energies are roughly proportional to the melting temperature, the interfacial energy of CoSi phase should be higher than that of CoSi2 phase. Regarding that the critical nucleation barrier is proportional to the cube of the interfacial energy, nucleation of CoSi2 phase is preferred at high undercooling. Yao et al. [10] found that the CoSi2 phase has a lower nucleation rate than that of the CoSi phase but grows faster over a wide range of undercooling. Recently, Zhang et al. [11] calculated the critical work of nucleation and found that the CoSi2 phase has lower critical work of nucleation when the undercooling is larger than the critical undercooling where the transition of primary phase happens. All the previous work [10, 11, 25] shows that phase selection in undercooled Co–61.8 at.% Si eutectic alloys are nucleation controlled.
Actually, phase selection is quite a common non-equilibrium phenomenon in undercooled melts and the mechanisms can be classified into two categories, i.e., nucleation controlled and growth controlled [27, 29, 30]. In the present work, the NiSi phase is of an orthogonality primitive lattice structure (oP8) with a space group Pnma, and the NiSi2 pase is of a face-centered cubic lattice structure (cF12) with a space group Fm3 m. According to the Ni–Si phase diagram [24], the melting temperature of the NiSi phase is 1265 K, but the melting temperature of the NiSi2 phase cannot be obtained directly because of the peritectic transformation L + Si → NiSi2. According to the thermodynamic assessment of the Ni–Si system [31], the melting temperatures of the NiSi and NiSi2 phases are calculated to be 1258 and 1282 K, respectively. If the lattice structure difference between the NiSi and NiSi2 phase is omitted and the viewpoint of Li et al. [27] is followed, the difference between the melting temperatures 24 K is so small that the nucleation-controlled mechanism should not play a dominative role in phase selection.
In the present work, the crystal growth velocities are measured for both the first and the second recalescence processes (Fig. 3). At low undercooling, the primary phase is the NiSi phase, and the growth velocity of the first recalescence is larger than that of the second recalescence. Taking the case of ΔT ≈ 31 K as an example, the NiSi and NiSi2 phases are crystallized in the first and the second recalescence processes, respectively. The growth velocity of NiSi phase is larger than that of the NiSi2 phase. At high undercooling, the primary phase is the NiSi2 phase, and the growth velocity of the first recalescence is larger than that of the second recalescence. Taking the case of ΔT ≈ 74 K as an example, the NiSi2 and NiSi phases are crystallized in the first and the second recalescence processes, respectively. The growth velocity of NiSi2 phase is larger than that of NiSi phase. At the intermediate undercooling, the increase of growth velocity in the second recalescence is much faster than that in the first recalescence, and the growth velocities of the first and the second recalescence processes could coincide nearly with each other (Fig. 3). In other words, phase selection between the NiSi and the NiSi2 phase is growth controlled. In order to show the growth-controlled mechanism more clearly, a solid line and a dotted line are drawn qualitatively for the growth of the NiSi and NiSi2 phases in Fig. 3, and the critical undercooling for the transition of primary phase is found to be at ΔT ≈ 48 K. For phase selection and metastable phase formation in undercooled Ni–Si alloys with different eutectic compositions, special attention should be paid to the work by Mullis et al. [32–35].
Driving force for re-melting
As already mentioned in the introduction, re-melting has been regarded to be the primary mechanism for the formation of anomalous eutectics. For the driving force of re-melting, Goetzinger et al. [23] proposed that it is the reduction of interfacial energy. Such viewpoint is an extension of the grain refinement theory of dendrite of single solid-solution phase in which a mushy zone exists during the post-recalescence stage [36–38]. In other words, minimization of surface areas happens by heat and solute diffusions in liquid. For regular lamellar eutectic where there is no mushy zone, re-melting cannot happen. In this case, coarsening happens [39], but this process should be quite slow because minimization of surface areas happens by solute diffusion in solid. For undercooled melts, the negative thermal gradient ahead of the eutectic front can make the interface unstable to eutectic cells or eutectic-dendrite [8]. In this case, the mushy zone forms and thus re-melting happens. For uncoupled eutectic-dendrite growth at high undercooling (e.g., Figs. 9, 10), re-melting of the primary phase is similar to that of single-phase dendrite [36–38]. Solidification (and re-melting) of the second phase then forms the coarse anomalous eutectics.
In contrast, Wei et al. [4] proposed that the chemical superheating should be the dominative driving force and the reduction of interfacial energy should be the secondary one. In the case of Pd–16.0 at.% P alloy whose eutectic products are two stoichiometric intermetallic compounds, there is no chemical superheating and thus no anomalous eutectics. For the present Ni–38 wt% Si eutectic alloy whose products are also two stoichiometric intermetallic compounds, anomalous eutectics are formed even in the absence of chemical superheating. Such contradictory results are actually understandable. For the Pd–16.0 at.% P alloy, lamellar eutectic growth happens at high undercooling; please see Fig. 11 in Ref. [4]. There is therefore no mushy zone during the post-recalescence stage and no re-melting by reduction of interfacial energy. For the present Ni–38 wt% Si eutectic alloy, single-phase dendrite of NiSi2 phase is solidified firstly and then the NiSi phase grows epitaxially to form an uncoupled eutectic-dendrite at high undercooling (Fig. 9). In this case, re-melting happens with the reduction of interfacial energy as the only driving force (Fig. 10). Combining the present work with those by Wei et al. [4] and Goetzinger et al. [23], one can see that the dominative driving force for re-melting could be either the chemical superheating, or the reduction of interfacial energy, or both. For both the cases of coupled growth with mushy zone (e.g., coupled eutectic-dendrite) and uncoupled growth (e.g., primary single-phase dendrite or uncoupled eutectic-dendrite), one cannot distinguish absolutely which driving force is the dominative one if both driving forces are present. It should be pointed out that Wei et al. [4] themselves also did not exclude re-melting of primary single-phase dendrite (please see Fig. 5 in Ref. [4]), even though their work is based on the theory of coupled eutectic-dendrite growth.
One can see that there are three kinds of anomalous eutectic, i.e., chemical superheating-induced, reduction of interfacial energy-induced, and chemical superheating-induced and reduction of interfacial energy-induced anomalous eutectics according to the driving forces. Form origins, there are two kinds of anomalous eutectic: uncoupled eutectic-dendrite growth-formed and coupled lamellar eutectic growth-formed anomalous eutectics. For uncoupled eutectic-dendrite growth, the driving force is the reduction of interfacial energy and the chemical superheating or only the reduction of interfacial energy if the supersaturated primary solid cannot form during rapid solidification. For coupled lamellar eutectic growth without the mushy zone, the chemical superheating is the only driving force. In the present work, lamellar/granular eutectic is available in the inter-eutectic-dendrite region (Figs. 9a, c and 10a, c), re-melting of which could also form the second kind of anomalous eutectic from origins, if there is the driving force of chemical superheating. Therefore, the dual origins of anomalous eutectics [22] are also supported by the present work.
Conclusions
Rapid solidification of undercooled Ni–38 wt% Si alloy whose eutectic products are two stoichiometric intermetallic compounds was observed in situ using a high-speed camera. The crystal growth velocities were measured, and the as-solidified microstructures were analyzed by EBSD. Our main conclusions are as follows:
-
1.
When ΔT < 20 K, there is only one recalescence event, whereas when ΔT > 20 K, there are two recalescence events. For the former, the recalescence corresponds to either eutectic solidification or solidification of primary NiSi phase. For the latter, the first and second recalescence processes correspond with crystallization of NiSi and NiSi2 phase at low undercooling (NiSi2 and NiSi phases at high undercooling), respectively. Phase selection between the NiSi and the NiSi2 phases is growth controlled, and the critical undercooling for the transition is ΔT ≈ 48 K.
-
2.
With the increase of undercooling, the microstructure changes in sequence from thin lamellar eutectics, primary granular structure of NiSi phase surrounded by thin regular lamellar eutectics, primary NiSi phase surrounded successively by coarse eutectics and thin regular lamellar eutectics, dendritic NiSi2 phase surrounded by strip-shaped NiSi phase, and thin regular lamellar eutectics to uncoupled eutectic-dendrites and anomalous eutectics.
-
3.
EBSD analysis shows that the NiSi and the NiSi2 phases share only one or some main orientations and the random orientations are limited even when anomalous eutectics form at high undercooling, indicating that the primary eutectic microstructure during the recalescence stage and/or re-melting process during the post-recalescence stage can be retained to the room temperature.
-
4.
The transition from regular eutectics to anomalous eutectics happens even in the absence of the driving force of chemical superheating because the single-phase dendrite of NiSi2 phase is solidified firstly and then the NiSi phase grows epitaxially to from an uncoupled eutectic-dendrite at high undercooling. The dual origins of anomalous eutectics [22], e.g., uncoupled eutectic-dendrite growth during the recalescence stage and coupled lamellar eutectic growth at low undercooling during the post-recalescence stage, are supported by the present experimental results.
Notes
It should be mentioned that in recent work by Binder et al. [25], the high-speed video data with the help of POV-Ray were fitted assuming a reasonable overall shape of the growing phase (e.g., a spherical envelope) to obtain the growth velocity. Such a method should be more reliable than but is not adopted by the current work.
This is similar to the work by Li et al [27], in which the Co–61.8 at.% Si eutectic alloy was undercooled by both an electromagnetic levitator and an electrostatic levitator. At low undercooling, only a single recalescence event can be found but the microstructure consists of primary CoSi phase and CoSi–CoSi2 eutectics. In subsequent work by Zhang et al. [11], the recalescence behaviors of undercooled Co–61.8 at.% Si eutectic alloy are much more complex; please see their Fig. 1. All these results indicate that the transformation process and the recalescence behaviors may not follow the one-to-one relationship.
It should be noted that for coupled growth, the lamellar spacing of eutectic formed at high undercooling should be smaller than that formed at low undercooling. The present coarse eutectic is formed by uncoupled growth but not coupled growth. Therefore, it is not strange that the lamellar spacing of the present coarse eutectic formed at high undercooling is larger than that of the thin lamellar eutectic formed at low undercooling.
Eutectic-dendrite can be defined as a dendrite on the whole, the solids of which are formed by eutectic solidification. There are two kinds of eutectic-dendrite according to the growth mechanism, i.e., by coupled and uncoupled eutectic growth. For the former, other alloy element should be added to the eutectic alloy or a negative temperature gradient should be improved to the eutectic interface to make the interface unstable to a dendritic morphology. This is the physical basis for the current eutectic-dendrite growth theory [2, 23, 28]. For the latter, the primary dendrite phase is followed by solidification of a second phase.
References
Kurz W, Fisher DJ (1979) Dendrite growth in eutectic alloys: the coupled zone. Int Mater Rev 24:177–204
Li JF, Zhou YH (2005) Eutectic growth in bulk undercooled melts. Acta Mater 53:2351–2359
Li JF, Li XL, Liu L et al (2008) Mechanism of anomalous eutectic formation in the solidification of undercooled Ni–Sn eutectic alloy. J Mater Res 23:2139–2148
Wei XX, Lin X, Xu W et al (2015) Remelting-induced anomalous eutectic formation during solidification of deeply undercooled eutectic alloy melts. Acta Mater 95:44–56
Herlach DM (1994) Non-equilibrium solidification of undercooled metallic melts. Mater Sci Eng Rep R 12:177–272
Liu JM, Zhou YH, Shang BL (1992) Theory and experiments on irregular eutectic growth: investigation on Al–Si eutectic growth. J Mater Sci 27:2067–2074
Wang JT, Kang SB, Kim HW et al (2002) Lamellae deformation and structural evolution in an Al–33 % Cu eutectic alloy during equal-channel angular pressing. J Mater Sci 37:5223–5227
Zhao S, Li JF, Liu L et al (2009) Eutectic growth from cellular to dendritic form in the undercooled Ag–Cu eutectic alloy melt. J Cryst Growth 311:1387–1391
Clopet CR, Cochrane RF, Mullis AM (2013) The origin of anomalous eutectic structures in undercooled Ag–Cu alloy. Acta Mater 61:6894–6902
Yao WJ, Wang N, Wei B (2003) Containerless rapid solidification of highly undercooled Co–Si eutectic alloys. Mater Sci Eng A 344:10–19
Zhang YK, Gao J, Kolbe M et al (2013) Phase selection and microstructure formation in undercooled Co–61.8 at.% Si melts under various containerless processing conditions. Acta Mater 61:4861–4873
Li M, Kuribayashi K (2003) Nucleation-controlled microstructures and anomalous eutectic formation in undercooled Co–Sn and Ni–Si eutectic melts. Metall Mater Trans A 34:2999–3008
Liu L, Wei XX, Huang QS et al (2012) Anomalous eutectic formation in the solidification of undercooled Co–Sn alloys. J Cryst Growth 358:20–28
Liu L, Li JF, Zhou YH (2011) Solidification interface morphology pattern in the undercooled Co–24.0 at.%Sn eutectic melt. Acta Mater 59:5558–5567
Yang C, Gao J, Zhang YK et al (2011) New evidence for the dual origin of anomalous eutectic structures in undercooled Ni–Sn alloys: in situ observations and EBSD characterization. Acta Mater 59:3915–3926
Xing LQ, Zhao DQ, Chen XC (1993) Solidification of undercooled Ni–32.5 wt%Sn eutectic alloy. J Mater Sci 28:2733–2737
Kattamis TZ, Flemings MC (1970) Structure of undercooled Ni–Sn eutectic. Metall Trans 1:1449–1451
Cao YQ, Lin X, Wang ZT et al (2015) Three-dimensional reconstruction of anomalous eutectic in laser remelted Ni–30 wt%Sn alloy. Sci Technol Adv Mater 16:1–11
Powell GL, Hogan LM (1965) Undercooling in silver-copper eutectic alloys. J Inst Metals 93:505–512
Jones BL (1971) Growth mechanisms in undercooled eutectic. Metall Trans 2:2950–2951
Wei BB, Yang GC, Zhou YH (1991) High undercooling and rapid solidification of Ni–32.5%Sn eutectic alloy. Acta Metall Mater 39:1249–1258
Li JF, Jie WQ, Zhao S et al (2007) Structural evidence for the transition from coupled to decoupled growth in the solidification of undercooled Ni–Sn eutectic melt. Metall Mater Trans A 38:1806–1816
Goetzinger R, Barth M, Herlach DM (1998) Mechanism of formation of the anomalous eutectic structure in rapidly solidified Ni–Si, Co–Sb and Ni–Al–Ti alloys. Acta Mater 46:1647–1655
Nash P, Nash A (1987) The Ni–Si (Nickel–Silicon) system. Bull Alloy Phase Diagr 8:6–14
Binder S, Galenko PK, Herlach DM (2014) The effect of fluid flow on the solidification of Ni2B from the undercooled melt. J Appl Phys 115:053511
Wang HF, Liu F, Chen Z et al (2007) Effect of non-linear liquidus and solidus in undercooled dendrite growth: a comparative study in Ni–0.7 at.%B and Ni–1 at.%Zr systems. Scr Mater 57:413–416
Li MJ, Nagashio K, Ishikawa T et al (2008) Microstructure formation and in situ phase identification from undercooled Co–61.8 at.%Si melts solidified on an electromagnetic levitator and an electrostatic levitator. Acta Mater 56:2514–2525
Kuang WW, Wang HF, Liu F et al (2016) Modeling of eutectic dendrite growth in undercooled binary alloys. J Mater Sci 51:2141–2152
Boettinger WJ, Coriell SR, Greer AL et al (2000) Solidification microstructures: recent developments, future directions. Acta Mater 48:43–70
Wei XX, Xu W, Kang JL et al (2016) Metastable Co23B6 phase solidified from deeply undercooled Co79.3B20.7 alloy melt. J Mater Sci 51:6436–6443
Tatsuya T, Kazumasa N, Hiroshi O et al (2003) Thermodynamic assessment of the Ni–Si system by incorporating ab initio energetic calculations into the CALPHAD approach. CALPHAD Comput Coupling Phase Diagr Thermochem 27:161–168
Ahmad R, Cochrane RF, Mullis AM (2012) The formation of regular αNi-γ(Ni31Si12) eutectic structures from undercooled Ni–25 at.%Si melts. Intermetallics 22:55–61
Cao LG, Cochrane RF, Mullis AM (2014) Lamella structure formation in drop-tube processed Ni–25.3 at.%Si alloy. J Alloys Compd 615:S599–S601
Dutra AT, Milenkovic S, Kiminami CS et al (2004) Microstructure and metastable phase formation in a rapidly solidified Ni–Si eutectic alloy using a melt-spinning technique. J Alloys Compd 381:72–76
Cao LG, Cochrane RF, Mullis AM (2015) Microstructural evolution and phase formation in rapidly solidified Ni–25.3 at.%Si alloy. Metall Mater Trans A 46:4705–4715
Schwarz M, Karma A, Eckler K et al (1994) Physical mechanism of grain refinement in solidification of undercooled melts. Phys Rev Lett 73:1380–1383
Karma A (1998) Model of grain refinement in solidification of undercooled melts. Int J Non-Equilib Process 11:201–233
Wang HF, Liu F, Tan YM (2011) Modeling grain refinement for undercooled single-phase solid-solution alloys. Acta Mater 59:4787–4797
Cline HE (1971) Shape instabilities of eutectic composites at elevated temperatures. Acta Metall 19:481–490
Acknowledgements
The authors would like to thank the Natural Science Foundation of China (Nos. 51371149, 51134011 and 51431008), the China National Funds for Distinguished Young Scientists (No. 51125002), Huo Yingdong Young Teacher Fund (No. 151048), the Aeronautical Science Foundation of China (No. 2015ZF53066), the Free Research Fund of State Key Lab. of Solidification Processing (No. 92-QZ-2014) and the project of Shaanxi Young Stars of Science and Technology (No. 2015KJXX-10).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lai, C., Wang, H., Pu, Q. et al. Phase selection and re-melting-induced anomalous eutectics in undercooled Ni–38 wt% Si alloys. J Mater Sci 51, 10990–11001 (2016). https://doi.org/10.1007/s10853-016-0312-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0312-y