Abstract
Coral reefs in the Florida Reef Tract have seen protracted loss of coral over the past several decades due to a variety of disturbances from marine heat waves, cold snaps, and disease events. Corals have not recovered despite abundant herbivorous fishes and relatively low macroalgal cover, two factors thought to facilitate resilience of corals. Thus, factors affecting the replenishment of coral populations may be hindering the recovery of corals. To study the potential factors affecting coral recovery in reefs of the Florida Reef Tract, we assessed benthic abiotic variables (substrate slope, depth, structural complexity, and abundance of sediment), fish assemblages, and benthic composition in three different reef habitats (groove, spur wall, spur top) located on three low-relief reefs and three high-relief spur-and-groove reefs. Herbivorous fish biomass ranged (44.7–107 g m−2), which is above average for the Caribbean. Yet there was low coral cover (~ 1%) and low density (~ 1 coral m2) of small adult corals, which likely reflects the cumulative effects of years of disturbances. The presence and density of juvenile corals were negatively correlated with the depth of the sediment layer trapped within long, sediment-laden algal turfs (LSAT), which are particularly abundant (> 50% cover) in low complexity reef habitats (low-relief groove, low-relief spur top, and high-relief groove). Our results indicate that current unsuitable habitat conditions (high sediment load) for early life stage corals may be an important factor preventing coral recovery. Consequently, the abundance of herbivorous fishes and coral cover trajectories appear decoupled in the region, and additional management initiatives considering LSAT composition are required to aid reef resilience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both biotic factors (e.g., larval input, primary production, herbivory) and abiotic factors (e.g., light intensity, water temperature, nutrient levels, sedimentation) influence structure, function, productivity, energy cycling and other processes of coral reefs (Done et al. 1996; Harborne et al. 2016) Alterations of one or more factors (e.g., increase of nutrient availability and sediment deposition, reduction of herbivorous fishes), as well as an intensification of natural disturbances, can stress and kill corals, the foundation species of coral reefs, leading to overall ecosystem degradation (Baker et al. 2008; Bozec et al. 2015). Furthermore, anthropogenic stressors such as overfishing herbivores and eutrophication can limit the resilience of western Atlantic reefs after disturbance (Mumby 2006; Hughes et al. 2010). Therefore, protection of herbivorous fishes that control macroalgae, a major competitor of corals, and reduction of anthropogenic nutrient input, fuel for algal growth, are often suggested as solutions to facilitate coral reef resilience in the region (D’Angelo and Wieddenmann 2014; Adam et al. 2015; Steneck et al. 2019). Nevertheless, some parts of the region, particularly the coral reefs in South Florida, have failed to regain coral cover despite decades of effective protection of herbivorous parrotfishes (Toth et al. 2014). While the decline in corals is likely strongly influenced by repeated disturbances, such as marine heatwaves and disease outbreaks (Toth et al. 2014), resilience still appears lower than expected in such a grazer-rich system, even one without grazing urchins following the disease-induced mortality of Diadema urchins (Lessios 2016).
Florida’s coral reefs are somewhat atypical because they exist outside the tropics, bounded to the north by a transitional zone from tropical to temperate characteristics that limit their northward expansion (Engle and Summers 1999; Walker 2012; Walker and Gilliam 2013; Toth et al. 2021). Yet, Florida’s coral reefs were historically characterized by high, though variable, coral cover and the presence of massive and branching reef-building corals (Dustan 1977; Jaap 1984; Burns 1985; Murdoch and Aroson 1999). Repetitive disease outbreaks in the last 50 years (e.g., black band disease in Orbicella and Dendrogyra, white band disease in Acropora, Porter and Meier 1992; Aronson and Precht 2001; Precht et al. 2016; Lewis et al. 2017), marine heatwaves and cold snaps (Lirman et al. 2011; Manzello 2015) and more recently (after 2014) the spread of stony-coral-tissue-loss-disease (SCTLD) (Precht et al. 2016) have led to low (< 5%) coral cover across much of the Florida Keys (Ruzicka et al. 2013; Muller et al. 2020). Coral cover has remained low on these reefs after these mortality events, despite the possible significant if variable input of coral settlers and the implied potential for coral recovery (Toth et al. 2014; van Woesik et al. 2014).
Furthermore, upright macroalgal abundance remains relatively modest (~ 20% Keys-wide, Ruzicka et al. 2013; Jones et al. 2022), particularly on shallow forereefs, likely because Florida’s coral reefs are one of the few places in the Caribbean, where there are still large populations of herbivorous parrotfishes (Shantz et al. 2020). Large scarids such as Scarus guacamaia and S. coelestinus, absent from much of the wider Caribbean as a result of overharvesting, are abundant in the Florida Keys (Jackson et al. 2014; Shantz et al. 2020; Zuercher et al. 2023). Coral-depauperate reefs in the Caribbean have often been associated with overfishing of herbivorous fishes and/ or nutrient pollution, leading to uncontrolled algae growth (e.g., Lobophora variegata and Dictyota spp., Mumby 2009; Lapointe et al. 2011; Jackson et al. 2014). The fact that reefs in the Florida Keys currently have abundant herbivorous fishes, relatively modest algal abundance, and low coral cover despite significant coral larvae input is inconsistent with our general understanding of resilience in the wider Caribbean (Mumby et al. 2007; Steneck et al. 2019). The limited signs of recovery suggest that alternative factors (e.g., natural or human-produced sedimentation) might be compromising processes of coral recovery (Burkepile et al. 2013; Begin et al. 2015; Suchley et al. 2016; Bruno et al. 2019).
The trajectory of recovery for coral reefs following coral-killing disturbances is multifaceted and complex (Doropoulos et al. 2016; Speare et al. 2023). The abundance of algae is often emphasized as a bottleneck to coral recovery as it can limit space for coral larval settlement, and reduce the survival and growth of coral recruits (Kuffner et al. 2006; Box and Mumby 2007; Hoey et al. 2011). Other reef characteristics, such as the presence of facilitating species or the orientation and texture of the benthos, also can strongly influence coral recruitment (Birrell et al. 2005; Davies et al. 2013). For example, the abundance of certain crustose coralline algae that facilitate recruitment (e.g., Titanoderma prototypum) or taxa that inhibit recruitment, such as invertebrates and other species of crustose coralline algae, could significantly influence coral recruitment (Nozawa 2008; Arnold and Steneck 2011; Brandl et al. 2013). Coral larvae also preferentially settle and have higher survival on vertical and rough substrates that often have taxa facilitating recruitment (Arnold and Steneck 2010). In contrast, sediment trapped within algal communities on the benthos appears to be a strong inhibitor of coral recruitment, possibly negatively impacting coral larvae more than abundant macroalgae (Ricardo et al. 2017; Speare et al. 2019). In the Caribbean, however, the impact of sediment on coral settlement, recruitment, and survival is still poorly understood (Rogers and Ramos-Scharron 2022).
Sediment dynamics (e.g., production, suspension, accumulation rate, and spatial distribution) on coral reefs is a complex process driven by several hydrodynamic factors (e.g., current, waves) and habitat characteristics (Schlaefer et al. 2021; 2022; Tebbett et al. 2020a, b; 2023). Of particular importance for coral recovery is the sediment load that settles on the benthos, often trapped within turf algae, creating long sediment-laden algal turf (LSAT hereafter, Goatley et al. 2016) because it could mechanically impede coral settlement (Speare et al. 2019). In Florida, no long-term monitoring programs of coral reefs include LSAT as a category; thus, no information on LSAT abundance or composition is available. Yet the cover by major benthic groups such as stony coral, octocoral, sponge, and macroalgae is usually less than 50% (except for Dry Tortugas National Park; see Toth et al. 2019). Although the composition of the remaining substrate is quite variable (e.g., covered by Peyssonelia spp. and CCA), LSAT is ubiquitous across most habitats, suggesting that sediment accumulation might create unsuitable conditions for the settlement and growth of coral recruits.
Our study investigated the role of abiotic (sediment, substrate slope, and rugosity) and biotic factors (benthic assemblage composition) impacting juvenile corals on reefs in the Upper Florida Keys. We surveyed each reef using a taxonomic resolution suitable for evaluating the relative abundance of potential promoters and detractors of coral recruitment including LSAT, short productive algal turf (hereafter SPAT), juvenile corals, and coral abundance and physical characteristics. Coral juveniles were identified and quantified in situ, across five different reef habitats found within the two most common forereef types in the upper Florida Keys reefs: high-relief and low-relief spur-and-groove reefs. We use these data to 1) evaluate benthic composition among habitats, particularly LSAT and 2) determine potential drivers of juvenile coral densities. We predict that less rugose and flatter habitats on low-relief reefs have deeper sediments than high-relief reefs and are associated with fewer juvenile corals.
Materials and methods
Study sites
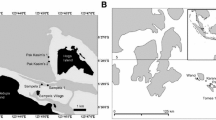
Our research was conducted during June–August 2015 on six shallow (5–8 m) forereefs [Conch Reef (24°57.695′W, 80°27.230′N), Davis Reef (80°30.361′W, 24°55.305′N), Pickles Reef (80°24.964′W, 24°59.087′N), Molasses Reef (80°22.374′W, 25°13.632′N), French Reef (80°21.009′W, 25°02.026′N), Elbow Reef (80°26.076′W, 25°00.646′N)] located approximately 10 km offshore from the upper section of the Florida Keys, USA (Fig. 1). Our sites encompassed low-relief and high-relief spur-and-groove reef formations spanning a broad range of benthic assemblages and structural complexity. Considering large-scale complexity (low relief (LR) reef vs. high relief (HR) reef) and within-reef habitats (spur top, spur wall, or groove), we classified the benthos into five habitats: low-relief groove (LR groove), low-relief spur top (LR spur top), high-relief spur groove (HR spur groove), high-relief spur wall (HR spur wall), and high-relief spur top (HR spur top). Spur walls are virtually non-existent in the low-relief reefs and thus were characterized only on high-relief reefs (Fig. 1). Across all sites, sandy substrates (sediment depth over 1 cm equivalent to > 5000 g m−2) were excluded from the analysis given that sediment loads of that magnitude are unsuitable for sessile organisms like corals (Tebbett et al. 2023). We corroborated the distinction between reef types (low-relief reefs vs. high-relief reefs) using the Rugosity Index (R.I) as a proxy of reef-scale structural complexity (Risk 1972, ESM Fig. 1).
Herbivorous fish biomass and benthic characteristics
We assessed fish community structure at each site via visual surveys along 20 belt transects (2 × 30 m) located haphazardly perpendicular to the spur and groove formation. All fishes were identified and their estimated size (total length, to the nearest cm) was recorded. Size estimates were converted to biomass for each fish using published length to weight relationships (Bohnsack and Harper 1988). Biomass of individuals from families Acanthuridae (three surgeonfish species) and scarine labrids were combined (ten parrotfish species) to estimate herbivorous fish biomass at each site. We used one-way ANOVA to compare herbivorous fish biomass across sites.
We surveyed the benthos in 25 (50 × 50 cm) quadrats placed every two meters along eight (50 m) transects per site (n = 200 plots/site). We used an Olympus camera (Tough TG-5) to photograph each 50 × 50 cm quadrat and then analyzed the benthic composition in the lab using Coral Point Count with Excel extensions version 4.1 (CPCe, Kohler and Gill 2006) with the following code modifications: long sediment-laden algal turf (LSAT), short productive algal turf algae (see below for description), hereafter “SPAT,” were classified as a relatively sediment-free, dense multi-species (e.g., Polysiphonia spp., Ceramium spp. Hypnea spp. Laurencia spp.) algal assemblage forming a layer < 1 cm tall (Connell et al. 2014). We combined all species of crustose coralline algae into a single group (CCA). We recognize that different species of CCA can have positive, neutral, or negative impacts on coral settlement and recruitment (Ritson-Williams et al. 2016), but these taxa are difficult to discriminate in the field making characterization of their distribution at this scale challenging. Species from the genus Peyssonnelia, a non-coralline crustose alga, were classified as a single group (Peyssonnelia). Percent cover from each plot was calculated from 25 points generated following a stratified-random distribution within each photo-quadrat. For each coral colony within a plot, we measured the maximum colony diameter of corals larger than 4 cm.
Since the physical characteristics of the substrate, such as slope, can determine the benthic composition (Fabricius and De’ath 2001), we collected information on rugosity, substrate slope, and depth of the sediment layer within each 0.25 m2 plot. We estimated R.I. using a 50 cm chain (link ~ 1 cm length) laid within each 50 × 50 cm quadrat parallel to the transect line and measured the linear length. We calculated habitat-scale R.I by dividing the chain’s total length (50 cm) by the linear length covered by the chain within the plot where R.I = 1 indicates the lowest complexity, and it increases with complexity. The sediment depth (mm) within each plot (n = 200 per site) was measured using a pencil calibrated with 1 mm increments. The pencil was inserted vertically into the sediment layer until it reached the hard substrate to measure the sediment depth. We recorded the slope of the substrate (i.e., the angle from horizontal) using a protractor with a small foam float placed on the 25 cm quadrat side parallel, closest to the transect tape. Small angles correspond to relatively flat (horizontal) substrates, whereas higher degree angles (up to 90°) are associated with more vertical substrates.
Sediment was usually trapped within turf algae, forming a sediment-turf matrix hereafter “long sediment-laden algal turf” (LSAT, Goatley et al. 2016). To quantify the LSAT turf-algae composition, we first measured the sediment layer using the calibrated pencil method from five plots at 1 m, 11 m, 21 m, 31 m, and 41 m along each transect (n = 40/site) of the matrix. Then, we removed sediment by perturbing the water with a manual bilge pump until we fully exposed the turf algae entangled within the sediment matrix. Finally, we used the calibrated pencil to record the length of the exposed turf algae. Here we only report turf length and sediment thickness.
Distribution of residuals and normality was checked using residual Q-Q plots. We used linear mixed models (LMM) with “study site” as a random factor to compare biotic and abiotic variables across reef habitats using R package lme4 (Bates et al. 2015). We performed pairwise post hoc analyses tailored for LMM using the R package (multcomp). To contrast benthic assemblage composition by habitat, we used a nonmetric-multidimensional scaling analysis (NMDS) followed by a permutational analysis of variance (PERMANOVA) using the R package vegan (Oksanen et al. 2017).
Drivers of juvenile corals
We surveyed coral juveniles (colonies ≤ 4 cm in diameter) in situ within the southwest quarter (25 × 25 cm) of each plot. The diameter of each juvenile was measured and identified to the genus level. We divided the number of coral juveniles by 0.0625 (area surveyed in m2) to estimate the density of juveniles m−2. We used GLMM (including study site as a random effect) to compare density of juveniles and adults among reef habitats. Because of the low abundance of corals across all habitats and sites, we tested for the influence of biotic and abiotic variables using simply the presence and absence data of juvenile corals using a multiple logistic regression model (LRM) with a binomial error structure. Collinearity was evaluated using the variation inflation factors (VIF). We included biotic and abiotic variables (e.g., rugosity index, LSAT sediment depth, substrate slope, and abundance of most dominant benthic groups (LSAT, SPAT, Dictyota spp. Gorgonian, CCA, Sponge, Coral) in our first logistic regression model. We started from a null model (only the intercept) and built eight models combining biotic and abiotic variables. We selected the model with the lowest AIC. In all cases, we reported the model fit using D2, a ratio between the logistic models’ residual deviance and null deviance. The estimates reported from logistic regression have not been transformed. We ran all descriptive analyses, graphs, and models with R version 3.2.2 (R Development Core Team 2016).
Results
Herbivorous fish biomass and benthic characteristics
Our study sites displayed a gradient of herbivorous fish biomass and reef habitat characteristics across Florida’s offshore coral reefs. Herbivorous fish biomass across all sites ranged from 44.70 (± 5.84) g m−2 at Conch Reef to 107.13 (± 13.75) g m−2 at Molasses Reef (Fig. 2). All three high-relief reefs showed higher herbivorous fish biomass than the low-relief reefs (One-way ANOVA, F5,114 = 3.965, p = 0.003).

Taken from Jackson et al 2014
Herbivorous fish biomass by study site (mean ± SE). Dash lines indicate reported parrotfish biomass in some other Caribbean areas (red, Los Roques Venezuela; purple, Saint Vincent; brown, Costa Rica; blue Flower Gardens Bank; green Bonaire).
Habitat characteristics ranged from almost complete horizontal substrates (average slope ~ 10 degrees) dominated by long sedimented-laden algal turf (LSAT) to more diverse algal communities in habitats with steeper slopes and higher physical complexity (Fig. 3, ESM Table 1). LSAT dominated the LR groove habitat, covering 70% of the benthos. In LR spur and HR groove habitats, LSAT covered approximately 50% of the benthos, whereas HR spur wall and HR spur top habitats showed a more heterogenous distribution by taxa and higher diversity compared to LR groove, LR spur, and HR groove habitats (Fig. 3C and D). We observed LSAT abundance decreased with increasing slope (Estimate = − 0.26, R2 = 0.61, p < 0.001) and physical complexity (Estimate = − 32.01, R2 = 0.64, p < 0.001) of the reef habitats. The abundance of LSAT increased over three times (from 20 to 60% cover) from high-relief reefs (spur top and wall) to low-relief groove habitats (F4,1030 = 63.85, p < 0.001). In contrast, Dictyota spp., SPAT, and crustose coralline algae became more abundant in high-relief reefs (PERMANOVA, habitat, R2 = 0.56, F4,101=30.8, p = 0.010 (Fig. 3C and D). The NMDS analysis (Fig. 3D) revealed distinct assemblages across habitats where low-relief grooves were dominated by LSAT while high-relief walls had more abundant Dictyota, SPAT, and to a lesser extent CCA. Indeed, differences in assemblage composition among habitats were almost double the variation explained by reef type (PERMANOVA, habitat, R2=0.32, F1,101=47.7, p = 0.010).
Benthic characteristics by reef habitat. A Substrate slope, B Sediment depth of LSAT across habitats, C community composition of major groups by habitat and estimate slope and structural complexity (mean ± SE). D Non-metric multidimensional analysis contrasting reef habitat composition. Notice that colored polygons indicate habitat type
Coral cover was very low (1.0 ± 0.1%) across all reef habitats (LMM, F = 1.7, p = 0.158). The density of juvenile (≤ 4 cm) corals averaged 13.1 ± 0.6 corals m−2 with assemblages dominated by Agaricia (35% of juvenile corals), Siderastrea (29% of juvenile corals), and Porites (28% of juvenile corals) (Fig. 4A&B). The density of adult corals (> 4 cm) was low across all habitats (0.8 ± 0.1 coral m−2), which represented approximately 90% fewer adult corals compared to the density of juveniles and was dominated by species of Siderastrea (Fig. 4C&D). Noticeably, juvenile and adult colonies of large reef-building corals such as Orbicella and Acropora represented approximately 3% of both juvenile (n = 25) and adult (n = 8) coral communities (Fig. 4B&D).
Drivers of juvenile coral abundance
More than 50% of all plots (604 out of 1086 total plots) did not have any juvenile corals. Thus, we analyzed presence/absence of juvenile corals as a function of benthic abiotic characteristics (rugosity, slope, and LSAT sediment depth) and biotic composition (percent cover of common groups) across all sites and habitats. Sediment depth trapped within LSAT was the best predictor (negative) of juvenile coral presence (ESM Table 2). The probability of finding juvenile coral significantly declined as mean LSAT sediment depth within the plot increased (Fig. 5A, LRM, Estimate = − 0.11, p < 0.001). The second significant predictor (positive) of juvenile coral presence was CCA cover (Fig. 5B, LRM, Estimate = 0.03, p < 0.009) with the likelihood of juvenile corals increasing as CCA cover increased.
Discussion
Across all reef habitats, we found a very low density of juvenile corals (~ 13 juvenile m−2) with communities dominated by so-called weedy species (from the genera Porites, Agaricia, and Siderastrea; Knowlton 2001), which might indicate impoverishment of coral settlement substrate. Indeed, reef habitats with lower rugosity and slope, usually dominated by LSAT, showed the lowest juvenile density, likely as a consequence of a deep (> 3.5 mm) LSAT sediment layer. Only the cover of crustose coralline algae, which tended to be more abundant on high-relief reefs, was a significant positive predictor of juvenile coral presence.
Our results show that habitat characteristics, particularly the abundance and depth of sediment, are negatively correlated with juvenile coral densities. There is substantial evidence that sediment has deleterious effects on juvenile and adult corals (Rogers 1990; Jones et al. 2015). Adverse effects of sedimentation include physiological stress and decreased photosynthesis and respiration (Riegl and Branch 1995; Weber et al. 2012; Tuttle and Donahue 2022), reduction of fertilization rates (Jones et al. 2015), recruitment (Birrell et al. 2005), coral growth (Rogers 1990), and total or partial coral colony mortality (Flores et al. 2012). Reef habitats with a high percent cover of LSAT were ubiquitous across our study sites, suggesting that sediment trapped within turf algae might create unsuitable conditions for the settlement and growth of coral recruits (Evans et al. 2020). Previous experiments conducted in the Florida Keys have shown that LSAT, and especially the sediment within LSAT, significantly reduces successful settlement of coral larvae (Speare et al. 2019). Furthermore, given that overall coral cover was very low, it was not surprising that we found few adult corals (approximately 10% of the number of juveniles) on these reefs. These adult coral communities were dominated by the genus Siderastrea. Some species of Siderastrea (e.g., Siderastrea radians) are temperature and sediment-tolerant (Lirman and Manzello 2009); thus these species may be the most likely species to thrive under high-sediment conditions.
Common benthic groups (e.g., SPAT, Dictyota spp.) did not significantly predict juvenile coral presence on high-relief reefs. Despite their low abundance, CCA was the only benthic group that showed a positive relationship with the occurrence of juvenile corals. A few CCA species (e.g., Titanoderma prototypum) have been proposed to facilitate coral recruitment (Arnold et al. 2010; Ritson-Williams et al. 2010). For the GBR, Abdul Wahab et al. (2023) tested 15 CCA species and reported Titanoderma cf. tessellatum as the most effective inducing coral larval settlement. Other taxa such as Porolithon and Sporolithon have the capacity to promote coral settlement, at least of some coral species (Abdul Wahab et al. 2023). Unfortunately, CCA species from Florida’s coral reefs are not well studied, and we are unaware of any work referring to individual species abundances or the composition of CCA assemblage, making it challenging to understand how coral recruitment patterns related to the distribution of CCA taxa. Furthermore, species-level identifications in our quadrats were not possible while underwater or from photographs as they require detailed analyses of the CCA thalli in the lab (Steneck 1986). Such eco-taxonomic analyses are needed in the future, given the importance of this group for coral reef resilience.
On high-relief reefs, Dictyota spp. was typically the dominant algal group followed by short productive algal turf (SPAT). Yet, there was no relationship between the abundance of Dictyota spp. and patterns of juvenile coral presence. Species within the genus (e.g., Dictyota pulchella) have been shown to reduce the growth of newly settled corals, specifically via physical abrasion, shading, or other physical mechanisms (Box and Mumby 2007; Kuffner et al. 2006; Rasher et al. 2011). This result suggests that species of the genus Dictyota found in our surveys might not impact coral juveniles as significantly as elsewhere in the region, perhaps because of the cooler winters or the dominant impact of LSAT. In addition, Dictyota is quite seasonal on Florida’s coral reefs (Lirman and Biber 2000; Duran et al. 2016) and maybe less abundant when coral settlement is happening. Other commonly seen algae in Caribbean coral reefs, such as Lobophora variegata, were practically absent. Macroalgae such as Lobophora variegata with prostrate morphology can decrease survival and growth of corals, likely via smothering and creating hypoxic areas at the interface with corals (Box and Mumby 2007; Ferrari et al. 2012). Similar algal species (e.g., Liagora sp) have also been shown to physically inhibit settlement and growth of corals (Box and Mumby 2007; Doropoulos et al. 2014) but were rare on our study reefs.
Considering the high abundance of LSAT and scarcity of CCA, coral larvae are unlikely to find favorable conditions to settle on the studied reefs. Thus, our results support the hypothesis proposed by van Woesik et al. (2014) that larval supply may not be the leading cause of low coral recruitment on Florida’s coral reefs, but rather, the lack of substrate with suitable habitat quality for settlement and survival of recruits appears to be a more important bottleneck for recruitment. In addition to our study, results from other work create a concerning picture of coral recruitment on Florida’s reefs. Previously, van Woesik et al. (2014) and Tougas and Porter (2002) evaluated coral recruitment across the Florida Keys and found a disproportional amount of hydrocoral recruits compared to scleractinians and the absence of recruits of massive coral species (i.e., Orbicella). Moulding (2005) studied patterns of coral recruitment on patch reefs along the Florida Reef Track and found lower density and diversity in the Upper Keys than Middle Keys and Lower Keys. Studies from Tougas and Porter (2002) and Moulding (2005) show large recruitment variability as a function of depth (deeper sites tend to have more scleractinian recruits), location, and other variables (e.g., tile position and separation from the seafloor). More recently, Harper et al. (2023) reported a dramatic 70-fold difference in coral recruitment between two years (2017–2018), with much of this variability being driven by broadcast spawning Siderastrea spp. These results indicate the urgent need to more fully study coral larval supply and recruitment patterns on these reefs (see King et al. 2023).
Unlike many other Caribbean reefs, the protection of herbivorous fishes in the Florida Keys has maintained a high abundance of large parrotfishes (Shantz et al. 2020) that can substantially impact corals via intentional or incidental predation (Burkepile 2012). For instance, Rotjan et al. (2006) showed that chronic coral predation could delay the process of coral recovery after disturbances. In the Florida Keys, the low coral cover and high abundance of parrotfishes, which often prey on corals (Burkepile et al. 2019), can lead to increases in coral predation with overall detrimental effects on coral communities (Burkepile 2012). Indeed, corallivory by parrotfishes, particularly the stoplight parrotfish (Sparisoma viride), is intense enough to hamper current coral restoration efforts in some areas of South Florida (Koval et al. 2020). Although the net effect of parrotfish grazing is generally considered positive for reefs (Mumby 2009), corallivory might lead to low coral recruitment in the Florida Keys. It is worth considering that as abundant LSAT constrains habitat availability to corals for recruitment, and that herbivorous fish tend to feed less (Bonaldo et al. 2012; Goatley and Bellwood 2013), then parrotfishes may be even more likely to negatively impact recruitment patterns.
Florida’s coral reefs have experienced severe (> 75%) loss of corals in the last 50 years caused by several disturbances, including cold-water stress events (Burns 1985; Lirman et al. 2011), coral diseases (Porter and Meier 1992; Kuta and Richardson 1996), and bleaching events (Manzello 2015), resulting in a dramatic decline in coral cover (Lewis et al. 2017). As a result, the current coral cover is less than 5% across the outer reefs on average across the Keys (Rutten et al. 2008; McClenachan et al. 2017), and is considered in an erosional state with few signs of natural recovery. We proposed that the lack of coral recovery in the last few decades highlights an additional negative feedback loop to incorporate into considerations of reef resilience. This additional feedback loop is that coral cover and complexity were high before the 1980’s (Dustan 1977; Jaap 1984; Burns 1985). After the catastrophic impacts of several coral-stress events (e.g., diseases, bleaching, cold snap), the loss of coral cover has reduced physical complexity (Perry and Harborne 2016). Based on our results, reducing physical complexity and flattening reefs facilitate sediment buildup. As sediment accumulates, it protects turf algal communities against herbivory (Gordon et al. 2016; Tebbett et al. 2020a, b, 2021). The sedimented-turf algae grow longer (ESM Fig. 2) and stabilize the turf-sediment matrix (LSAT), creating impoverished habitat conditions for coral settlement and recruitment (Speare et al. 2019, Tebbet et al. 2021).
Complexity has decreased across the region (Alvarez-Filip et al. 2009), but little is known regarding how sedimentation could influence less complex reefs (Tebbett et al. 2023), particularly in Florida (Duran et al. 2018). Further analyses (e.g., seasonal changes of sediment load, sediment sources, sediment composition, and hydrodynamic drivers of reef sediment) are needed to better to understand sediment dynamics on Florida’s coral reefs. For instance, sediment composition [grain size, grain type (carbonate vs. silica based), organic composition, and contaminats] might determine the effect of sediment on reef organisms and ultimately on coral reef resilence. Rogers and Ramos-Scharron (2022) argue that sedimentation (sediment delivery) and its impact on Caribbean coral reefs is poorly studied. However, from studies across the globe, we know that sedimentation is a complex process. For instance, Tebbett et al. (2023) showed that sedimentation of coral reefs is related to currents, waves, and parrotfish sediment production (reworked sediment). These investigators also found that the highest sediment accumulation rates in sediment traps and sediment standing stocks in turfs were on the low-complexity reef flat. Such studies are urgently needed to understand sedimentation patterns along Florida’s coral reef.
There are still fundamental knowledge gaps regarding the coral larval supply, reef sediment sources, and the overall ecological role of herbivores (corallivory vs. herbivory) on Florida’s reefs. It is feasible to think that low coral cover translates into low coral larval production but we still need to understand more about the dynamics of the coral larval pool. The abundance of coral larvae seems to vary significantly from year to year (Harper et al. 2023), some sites in Florida may export (act as sources) and receive (act as sinks) more than others resulting from local connectivity (King et al 2023). However, our study suggests that sediment load within algal turf assemblages could be a key influence on the apparent lack of resilience of corals on these reefs. While such knowledge is critical, it also has important implications for conserving Florida’s coral reefs. For example, the role of sediment and its spatial variabiliy suggest that mapping habitat-specific characteristics that make some reefs more resilient than others, improving water quality, and dealing with sediment load as part of restoration activities would benefit management efforts. More generally, our work underscores the need to urgently reduce stressors that drive coral mortality and the loss of structural complexity on reefs.
References
Abdul Wahab MA, Ferguson S, Snekkevik VK, McCutchan G, Jeong S, Severati A, Randall CJ, Negri AP, Diaz-Pullido G (2023) Hierarchical settlement behaviours of coral larvae to common coral coralline algae. Sci Rep 13:5795. https://doi.org/10.1038/s41598-023-32676-4
Adam TC, Burkepile DE, Ruttenberg BI, Paddack MJ (2015) Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar Ecol Prog Ser 520:1–20
Alvarez-Filip L, Dulvy NK, Gill JA, Cote IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc B 276(1669):3019–3025
Arnold SN, Steneck RS (2011) Settling into an increasingly hostile world: the rapidly closing “recruitment window” for corals. PLoS ONE. https://doi.org/10.1371/journal.pone.0028681
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91–105
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: a ecological assessment of long-term impacts, recovery trends, and future outlook. Estuar Coast Shelf Sci 80:435–471
Bates D, Maechler M, Bolher B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Begin C, Schelten CK, Nugues MM, Hawkins J, Callum R, Cote IM (2015) Effects of protection and sediment stress on coral reefs in Saint Lucia. PLoS ONE. https://doi.org/10.1371/journal.pone.0146855
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Poll Bull 51:408–414
Bohnsack JA, Harper DE (1988) Length-weight relationships of selected marine reef fishes from southeastern United States and the Caribbean. NOAA Tech. Mem, NMFS-SEFC-215
Bonaldo RM, Welsh JQ, Bellwood DR (2012) Spatial and temporal variation in coral predation by parrotfishes on the GBR: evidence from an inshore reef. Coral Reefs 31:263–272
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Bozec Y-M, Alvarez-Filip L, Mumby PJ (2015) The dynamics of architectural complexity on coral reefs under climate change. Glob Change Biol 21:223–235
Brandl SJ, Hoey AS, Bellwood DR (2013) Micro-topography mediates interactions between corals, algae, and herbivorous fishes on coral reefs. Coral Reefs 33:421–430
Bruno JF, Cote IM, Toth LT (2019) Climate change, coral loss, and the curios case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann Rev Mar Sci 11:307–334
Burkepile DE (2012) Context-dependent corallivory by parrotfishes in a Caribbean reef ecosystems. Coral Reefs 31:111–120
Burkepile DE, Allgeier JE, Shantz AA, Pritchard CE, Lemoine NP, Bhatti LH, Layman CA (2013) Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem. Sci Rep. https://doi.org/10.1038/srep1493
Burkepile DE, Adam TC, Ladd MC, Munsterman K, Roycroft M, Ruttenberg BI (2019) Species-specific patterns in corallivory and spongivory among Caribbean parrotfishes. Coral Reefs 38:417–423. https://doi.org/10.1007/s00338-019-01808-6
Burns TP (1985) Hard-coral distribution and cold-water disturbances in South Florida: variation with depth and location. Coral Reefs 4:117–124
Connell SD, Foster MS, Airoldi L (2014) What are algal turf? Towards a better description of turfs. Mar Ecol Prog Ser 495:299–307
D’Angelo C, Wieddenmann J (2014) Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr Opin Environ Sustain 7:82–93
Davies SW, Matz MV, Vize PD (2013) Ecological complexity of coral recruitment processes: effects of invertebrate herbivores on coral recruitment and growth depends upon substratum properties and coral species. PLoS ONE. https://doi.org/10.1371/journal.pone.0072830
Done TJ, Ogden JC, Wiebe WJ, Rosen BR (1996) Biodiversity and ecosystem function of coral reefs. In: Moonet HA, Cushman JH, Medina E, Sala OE, Schulze ED (eds) Functional roles of biodiversity: A Global Perspective. SCOPE 1996, John Wiley & Sons Ltd. U.K. 493 pp.
Doropoulos C, Roff G, Zupan M, Nestor V, Isechal AL, Mumby PJ (2014) Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs 33:613–623
Doropoulos C, Roff G, Bozec Y-M, Zupan M, Werminghausen MPJ (2016) Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr 86:20–44
Duran A, Collado-Vides L, Burkepile DE (2016) Seasonal regulation of herbivory and nutrient effects on macroalgal recruitment and succession in a Florida coral reef. PeerJ. https://doi.org/10.7717/peerj.2643
Duran A, Collado-Vides L, Palma L, Burkepile DE (2018) Interactive effects of herbivory and substrate orientation on algal community dynamics on a coral reef. Mar Bio 165:156. https://doi.org/10.1007/s00227-018-3411-2
Dustan P (1977) Vitality of reef coral populations off key Largo, Florida: recruitment and mortality. Environ Geol 2:51–58
Engle VD, Summers JK (1999) Latitudinal gradients in benthic community composition in western Atlantic Estuaries. J Biogeogr 26:1007–1023
Evans RD, Wilson SK, Fisher R, Ryan NM, Babcock R, Blakeway D, Bond T, Dorji P, Dufois F, Ferans P, Lowe R, Stoddart J, Thomson DP (2020) Early recovery of turbid coral reefs after recurring bleaching events. J Environ Manage. https://doi.org/10.1016/j.jenvman.2020.110666
Fabricius K, De’ath G (2001) Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 19:303–309
Ferrari R, Gonzalez-Rivero M, Mumby PJ (2012) Size matters in competition between corals and macroalgae. Mar Eco Prog Ser 467:77–88
Flores F, Hoogenboom MO, Smith LD, Cooper TF, Abrego D, Negri AP (2012) Chronic exposure of corals to fine sediments: lethal and sub-lethal impacts. PLoS ONE. https://doi.org/10.1371/journal.pone.0037795
Goatley CHR, Bellwood DR (2013) Ecological consequences of sediment on high-energy coral reefs. PLoS ONE 8(10):e77737
Goatley CHR, Bonaldo RM, Fox RJ, Bellwood DR (2016) Sediment and herbivory as sensitive indicators of coral reef degradation. Ecology Society. https://doi.org/10.5751/ES-08334-210129
Gordon SE, Goatley CHR, Bellwood DR (2016) Low-quality sediments deter grazing by the parrotfish Scarus rivulatus on inner-shelf reefs. Coral Reefs 35:285–291
Harborne AR, Rogers A, Bozec Y-M, Mumby PJ (2016) Multiple stressors and the functioning of coral reefs. Annu Rev Mar Sci 9:445–468
Harper LM, Huebner LK, O’cain ED, Ruzicka R, Gleason DF, Fogarty ND (2023) Multi-year coral recruitment study across the Florida Reef Tract reveals boom-or-bust pattern among broadcast sapwners and consistency among brooders. Mar Ecol Prog Ser 721:39–58
Hoey AS, Pratchett MS, Cvitanovic C (2011) High macroalgal cover and low coral recruitment undermine the potential resilience of the world’s southernmost coral reef assemblages. PLoS ONE. https://doi.org/10.1371/journal.pone.0025824
Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–642
Jaap WC (1984) The ecology of the south Florida coral reefs: a community profile. U.S. Fish Wild FWS/OBS-82/08.138
Jackson JBC, Donovan MK, Cramer KL, Lam VV (2014). Status and Trends of Caribbean Coral Reefs: 1970–2012. Global Coral Reef Monitoring Network, IUCN, Gland, Switzerland
Jones R, Ricardo GF, Negri AP (2015) Effects of sediment on the reproductive cycle of corals. Mar Pollut Bull 100:13–33
Jones PN, Ruzicka RR, Colella MA, Pratchett MS, Gilliam DS (2022) Frequent disturbances and chronic pressures constrain stony coral recovery on Florida’s Coral Reef. Coral Reefs 41:1665–1679. https://doi.org/10.1007/s00338-022-02313-z
King S, Saint-Amand A, Walker BK, Hanert E, Figueiredo J (2023) Larval dispersal patterns and connectivity of Acropora on Florida’s Coral Reef and its implications for restoration. Front Mar Sci 9:91038463. https://doi.org/10.3389/frmars.2022.1038463
Knowlton N (2001) The future of coral reefs. Proc Natl Acad Sci USA 98:5419–5425
Kohler KE, Gill SM (2006) Coral point count with Excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Koval G, Rivas N, D’Alessandro M, Hesley D, Santos R, Lirman D (2020) Fish predation hinders the success of coral restoration efforts using fragmented massive corals. PeerJ 8:e9978. https://doi.org/10.7717/peerj.9978
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Kuta KG, Richardson LL (1996) Abundance and distribution of black band disease on coral reefs in the northern Florida Keys. Coral Reefs 15:219–223
Lapointe BE, Thacker K, Hanson C, Getten L (2011) Sewage pollution in Negril, Jamaica: effects on nutrition and ecology of coral reef macroalgae. Chin J Oceanology Limnol 29:775–789
Lessios HA (2016) The great Diadema antillarum die-off: 30 years later. Ann Rev Mar Sci 8:267–283
Lewis CL, Neely KL, Richardson LL, Rodriguez-Lanetty M (2017) Temporal dynamics of black band disease affecting pillar coral (Dendrogyra cilindricus) following two consecutive hyperthermal events on the Florida Reef Tract. Coral Reefs 36:427–431
Lirman D, Biber P (2000) Seasonal dynamics of macroalgal communities of the Northern Florida Reef Tract. Bot Mar 43:305–314
Lirman D, Manzello D (2009) Patterns of resistance and resilience of the stress-tolerant coral Siderastrea radians (Pallas) to sub-optimal salinity and sediment burial. J Exp Mar Biol Ecol 369:72–77
Lirman D, Schopmeyer S, Manzello DP, Gramer LJ, Precht WF et al (2011) Severe 2010 cold-water event caused unprecedented mortality to Corals of the Florida reef tract and reversed previous survivorship patterns. PLoS ONE. https://doi.org/10.1371/journal.pone.0023047
Manzello DP (2015) Rapid recent warming of coral reefs in the Florida Keys. Sci Rep. https://doi.org/10.1038/srep16762
McClenachan L, O’Connor G, Neal BP, Pandolfi JM, Jackson JBC (2017) Ghost reefs: nautical charts document large spatial scale of coral reef loss over 240 years. Sci Adv. https://doi.org/10.1126/sciadv.1603155
Moulding AL (2005) Coral recruitment patterns in the Florida Keys. Rev Biol Trop 53:75–82
Muller EM, Sartor C, Alcaraz NI, Rv W (2020) Spatial epidemiology of the Stony-Coral-TissueLoss-Disease in Florida. Frontiers Marine Sci. https://doi.org/10.3389/fmars.2020.00163
Mumby PJ (2006) The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl 16:747–769
Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28:761–773
Mumby PJ, Hasting A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450:98–101
Murdoch TJT, Aronson RB (1999) Scale-dependent spatial variability of coral assemblages along the Florida Reef Tract. Coral Reefs 18:341–351
Nozawa Y (2008) Micro-crevice structure enhances coral spat survivorship. J Exp Mar Biol Ecol 367:127–130
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, et al. (2017) Community ecology package. https://cran.r-project.org, https://github.com/vegandevs/vegan
Perry CT, Harborne AR (2016) Coral reefs at the crossroads. Coral Reefs World. https://doi.org/10.1007/978-94-017-7567-0_4
Porter JW, Meier OW (1992) Quantification of loss and change in Floridian Reef Coral Populations. Amer Zool 32:625–640
Precht WF, Gintert BE, Robbart ML, Fura R, van Woesik R (2016) Unprecedented disease-related coral mortality in Southeastern Florida. Sci Rep 6:31374. https://doi.org/10.1038/srep31374
R Development Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, URL http://R-project.org/
Rasher DB, Stout EP, Engel S, Kubanek J, Hay ME (2011) Macroalgal terpenes function as allelopathic agents against reef corals. Proc Natl Acad Sci 108:17726–17731
Ricardo GF, Jones RJ, Nordborg M, Negri AP (2017) Settlement patterns of the coral Acropora millepora on sediment-laden surfaces. Sci Total Environ 609:277–288
Riegl B, Branch GM (1995) Effect of sediment on the energy budgets of four scleractinian (Bourne 900) and five alcyonacean (Lamouroux 1816) corals. J Exp Mar Bio Eco 186:259–275
Risk MJ (1972) Fish diversity on a coral reef in the Virgin Islands. Atoll Res Bull 153:1–6
Ritson-Williams R, Paul VJ, Arnold SN, Steneck RS (2010) Larval settlement preferences and post-settlement survival of the threatened Caribbean corals A. palmata and A. cervicornis. Coral Reefs 29:71–81
Ritson-Williams R, Arnold SN, Paul VJ (2016) Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar Ecol Prog Ser 548:127–138
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202
Rogers CS, Ramos-Scharron CE (2022) Assessing effect of sedimen delivery to coral reefs: a Caribbean Watershed perspective. Front Mar Sci. https://doi.org/10.33389/fmars.2021.773968
Rotjan RD, Dimond JL, Thornill DJ, Leichter JJ, Helmuth B, Kemp DW, Lewis SM (2006) Chronic parrotfish grazing impedes coral recovery after bleaching. Coral Reefs. https://doi.org/10.1007/s00338-006-0120-y
Rutten LM, Chiappone M, Swanson DW, Miller SL (2008) Stony coral species diversity and cover in the Florida Keys using design-based sampling. Proc 11th Int Coral Reef Symp 18:800–804
Ruzicka RR, Colella MA, Porter JW, Morrison JM, Kidney JA, Brinkhuis V, Lunz KS, Macaulay KA, Bartlett LA, Meyers MK, Colee J (2013) Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El niño. Mar Ecol Prog Ser 489:125–141
Schlaefer JA, Tebbett SB, Bellwood DR (2021) The study of sediments on coral reefs: a hydrodynamic perspective. Mar Poll Bull. https://doi.org/10.1016/jmarpolbul.2021.112580
Schlaefer JA, Tebbett SB, Bowden CL, Collins WP, Duce S, Hemingson CR, Huertas V, Mihalitsis M, Morais J, Morais RA, Siqueira AC, Streit RP, Swan S, Valenzuela J, Bellwood DR (2022) A snapshot of sediment dynamics on an inshore coral reef. Mar Env Res. https://doi.org/10.1016/j.marenvres.2022.105763
Shantz AA, Ladd MC, Burkepile DE (2020) Overfishing and the ecological impacts of extirpating large parrotfish from Caribbean coral reefs. Ecol Mono 90:e01403. https://doi.org/10.1002/ecm1403
Speare KE, Duran A, Miller MW, Burkepile DE (2019) Sediment associated with algal turfs inhibits the settlement of two endangered coral species. Mar Poll Bull 144:189–195
Speare KE, Duran A, Miller MW, Moeller HV, Burkepile DE (2023) Small-scale habitat selection by larvae of a reef-building coral. Mar Eco pro Ser 724:67–79
Steneck RS (1986) The ecology of coralline algal crusts: convergent patterns and adaptive strategies. Ann Rev Ecol Syst 17:273–303
Steneck RS, Arnold SN, Boenish R, de Leon R, Mumby PJ, Rasher DB, Wilson MW (2019) Managing recovery resilience in coral reefs against climate-induced bleaching and hurricanes: a 15 year case study from Bonaire. Dutch Caribbean Front Mar Sci 6:265. https://doi.org/10.3389/fmars.2019.00265
Suchley A, McField MD, Alvarez-Filip L (2016) Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ. https://doi.org/10.7717/peerj.2084
Tebbett SB, Goatley CHR, Streit RP, Bellwood DR (2020a) Algal turf sediment limits the spatial extent of function delivery on a coral reef. Sci Tot Environ. https://doi.org/10.1016/j.scitotenv.2020.139422
Tebbett SB, Streit RP, Bellwood DR (2020b) A 3D perspective on sediment accumulation in algal turfs: implications for coral reef flattening. J Ecol 108:70–80
Tebbett SB, Morais RA, Goatley CHR, Bellwood DR (2021) Collapsing ecosystem functions on an inshore coral reef. J Environ Manage. https://doi.org/10.1016/j.jenvman.2021.112471
Tebbett SB, Schlaefer JA, Bowden CL, Collins WP, Hemingson CR, Ling SD, Morais J, Morais RA, Siqueira AC, Streit RP, Swan S, Bellwood DR (2023) Bio-physical determinants of sediment accumulation on an offshore coral reef: A snapshot study. Sci Total Environ. https://doi.org/10.1016/j.sciotenv.2023.165188
Toth LT, van Woesik R, Murdoch TJT, Smith SR, Ogden JC, Precht WF, Aronson RB (2014) Do no-take reserves benefit Florida’s corals? 14 years of changes and stasis in the Florida Keys National Marine Sanctuary. Coral Reefs 33:565–577
Toth LT, Stathakopoulos A, Kuffner RRR, Colella MA, Shinn EA (2019) The unprecedented loss of Florida’s reef building corals and the emergence of a novel coral-ref assemblages. Ecology. https://doi.org/10.1002/ecy.2781
Toth LT, Precht WF, Modys AB, Stathakopoulos A, Robbart ML, Hudson H, Oleinik AE, Riegl BM, Shinn EA, Aronson RB (2021) Climate and the latitudinal limits of subtropical reef development. Sci Rep 11:13044. https://doi.org/10.1038/s41598-02187883-8
Tougas JI, Porter JW (2002) Differential coral recruitment patterns in the Florida Keys. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay, and coral reefs f the Florida Keys. CRC Press, Boca Raton, Fl, pp 789–811
Tuttle LJ, Donahue MJ (2022) Effects of sediment exposure on corals: a systematic review of experimental studies. Environ Evidence 11:4. https://doi.org/10.1186/s13750-022-00256-0
van Woesik R, Scott WJ, Aronson RB (2014) Lost opportunities: coral recruitment does not translate to reef recovery in the Florida Keys. Mar Pollut Bull 88:110–117
Walker BK (2012) Spatial analyses of benthic habitats to define coral reef ecosystem regions and potential biogeographic boundaries along a latitudinal gradient. PLoS ONE. https://doi.org/10.1371/journal.pone.0030466
Walker BK, Gilliam DS (2013) Determining the extent and characterizing coral reef habits of the northern latitudes of the Florida Reef Tract (Martin County). PLoS ONE. https://doi.org/10.1371/journal.pone.0080439
Weber M, Db B, Lott C, Polerecky L, Kohl K, Abed RMM, Ferdelman TG, Fabricius K (2012) Mechanisms of damage to corals exposed to sedimentation. PNAS 109(24):E1558–E1567. https://doi.org/10.1073/pnas.1100715109
Zuercher R, Kochan D, Harborne AH (2023) Factors influencing the biomass of large-bodied parrotfish species in the absence of fishing on coral reefs in Florida, USA. J Fish Biol 103:1526–1537
Acknowledgements
A State Wildlife Grant from the Florida Fish and Wildlife Conservation Commission supported this work. We thank all volunteers who assisted us during our field and lab work. This work was conducted under a permit from the Florida Keys National Marine Sanctuary #2014-083-A1. This is contribution #x from the Center for Coastal Oceans Research in the Institute for Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duran, A., Speare, K., Fuchs, C. et al. Long sediment-laden algal turf likely impairs coral recovery on Florida’s coral reefs. Coral Reefs 43, 1109–1120 (2024). https://doi.org/10.1007/s00338-024-02532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02532-6