Abstract
Push-coring is used to collect Holocene-aged coral sub-fossils, but its applications can be limited by underlying reef geology. Here, we report on a new approach of coring inundated coastal karst formations—i.e., coral holes—to determine the historical context of coral reefs in Guam. Three cores were extracted and processed to recover coral fragments as well as abundances of the photosymbiotic foraminifera Baculogypsina sphaerulata, a proxy for water clarity. Coral fragments, with the oldest dating to a median calibrated age of 221 cal. BP (1729 CE), revealed greater than 100 years of consistent accumulation and composition, suggesting a period of relative stability for macrobenthos. However, we documented a drop in B. sphaerulata abundance from approximately 100 years cal. BP, suggesting a period of environmental decline in Guam. Our results provide the first multi-centennial record of coral assemblages from Guam and provide a proof of concept for future historical investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historical analysis of coral reef communities can provide insights into ecological dynamics that span human timescales (Pandolfi 2011) and can help alleviate the shifting baseline syndrome (Pauly 1995). Paleontological data, from coring efforts and studies of exposed facies, have revealed community structure and stability for millennia prior to rapid change over the last half century (McCulloch et al. 2003; Aronson et al. 2004). In many cases, this recent and rapid shift in the ecological state of corals reefs has resulted from the increased human influence on the environment (Jackson 1997).
Push-coring through unconsolidated coral reef framework is a popular method to obtain Holocene reef fossils given its wide applicability, efficiency, and low environmental impact (Dardeau et al. 2000; Aronson et al. 2002). Yet, unconsolidated reef matrices are scare on many carbonate islands. For instance, although corals are abundant on the nearshore reefs of Guam, the largest island in Micronesia, the local geology consists mainly of basement rock covered with a thin layer (< 0.5 m) of marine sediment. Moreover, there are no known raised or inundated fossil reefs from previous sea-level high stands, making the Holocene history of Guam’s coral reefs challenging to study.
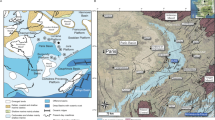
However, in several locations the Pliocene–Pleistocene basement limestone features karst dissolution caves, or “coral holes,” which formed during previous glacial maximums. These coral holes, which occur behind the reef crest, can be discerned in satellite images by their discrete shapes and lighter colorations among patches of coral and hard bottom (Fig. 1; Burdick 2005). Since these holes trap sediment, live coral, and rubble from the surrounding reef, they might offer a way to reconstruct reef community histories in this region.
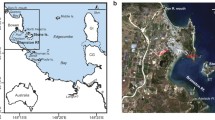
Study area, Guam. A Location of West Hagåtña (Agaña) Bay and the coral hole cored (red oval). B Location of Shark's Hole, another coral hole cored and referenced in the manuscript, although data not presented. Panel B was included to show the distinction in the coral holes from the surrounding reef framework via satellite imagery. Imagery obtained from Google Earth Pro on August 30, 2023
Included within these reef sediments are assemblages of symbiont-bearing foraminifera. Many species are sensitive to nutrient loading (Hallock 1981, 2000; Zamora-Duran et al. 2020), making foraminifera useful proxies of environmental change associated with nutrient pollution (Barmawidjaja et al. 1995; Carnahan et al. 2009). This idea has been applied to coral reef studies with the establishment of the FORAM Index (Hallock et al. 2003), which quantifies reef viability based on the relative abundances of different foraminifera groups. According to this index, areas that support the healthiest reefs are characterized by having a large abundance of photosymbiotic foraminifera, which thrive in oligotrophic waters.
Like other reefs in the North Pacific, the vitality of Guam’s coral reefs has been declining over the last 40 years (Burdick et al. 2008; Andrew et al. 2011), though the full extent of loss is difficult to establish without a pre-anthropogenic baseline. Recent threats to reefs include declining water quality from development, jungle burning-induced sedimentation (Wolanski et al. 2003; Williams et al. 2015), sewage outflow from inadequate treatment plants (Redding et al. 2013; Pinkerton et al. 2015; Duprey et al. 2017), increased outbreaks of coral predators such crown-of-thorns sea star Acanthaster planci (COTS; Colgan 1987), overfishing (Houk et al. 2012; Bejarano et al. 2013), and coral diseases (Redding et al. 2013). Additionally, Guam’s reefs have not escaped global threats such as climate change and thermal bleaching. Between 2013 and 2017, bleaching events led to the loss of approximately 30–60% of coral cover on reefs around the island (Raymundo et al. 2019). Acropora spp. were particularly impacted, with a documented loss of over 36% due to their highly sensitive biology (Raymundo et al. 2019), a collapse mirrored on a global scale (Renema et al. 2016). Though these threats reduced coral cover, less is known about changes in community structure, particularly over the Guam’s longer-term history.
Here, we present the first known historical record of coral biodiversity in Guam over the last two hundred and eighty years. We employ a sediment push-coring method to collect coral sub-fossil data from karst-formed coral holes. We also present a record of changes in relative abundance of Baculogypsina sphaerulata, a photosymbiotic foraminifer that thrives in oligotrophic waters, as a proxy for water clarity. This study offers a proof-of-concept for documenting historical changes in coral diversity on reefs that lack significant reef matrix substrate.
Materials and methods
Guam is the southernmost island of the Mariana Islands chain, located in the Micronesia sub-region of Oceania in the western Pacific. Manual push-cores (n = 3) were taken from a coral hole (approx. 20 × 40 m wide and 0.5 m below the height of the surrounding reef) located in West Hagåtña (Agaña) Bay (13.48244 N, 144.7463 E) on the western coast of Guam (Fig. 1; Burdick 2005). Cores (AG-1, AG-2 and AG-3) were taken at 5 m intervals across the hole using an open-barrel coring technique (Dardeau et al. 2000; Aronson et al. 2002). A galvanized steel tube (2.5 m length × 6.35 cm diameter) was driven into the coral hole, capped at the top, extracted from the sediment and rubble matrix, and immediately capped at the bottom to retain the internal sediment structure. Cores were then extruded into 5 cm segments at the University of Guam’s marine laboratory. Sediment samples were dried at 80 °C and weighed to determine dry weight per core segment. Each segment was sifted using an 8000, 1000, and 500 µm mesh soil sifters. Coral fragments from the > 8000 µm fraction were identified to family and all coral materials from the > 1000 µm fraction were weighed to determine total coral abundance per 5 cm segment. Corals were identified using a published coral atlas (Veron 2000), compared to field photographs of coral from Guam, or referred to coral taxonomists expert in the regional fauna. Foraminifera counts were made from 4 g subsamples of the same core segments that corals were analyzed, using a sediment splitter from the > 500 µm fraction of AG-1 and AG-2 cores. Each sample was washed with deionized water over a 63-μm sieve, air-dried on filter paper, and then counted in triplicate.
For a given segment, fragments from each coral family were weighed, converted to proportional abundance to assess community composition of the surrounding reef, and total coral weight was calculated. Coral fragments from the deepest segment of all cores and one sample from the middle segment of AG-2 and AG-3 were radiocarbon-dated at the National Ocean Sciences Accelerator Mass Spectrometry Facility at Woods Hole Oceanographic Institution (Massachusetts, USA). Coral fragments chosen for radiocarbon dating were well-preserved and showed no signs of diagenesis externally (i.e., severe encrustation, pitting, biotic boring, abiotic weather) or when cut-open. The top segment of each core was assumed to correspond to the collection year (2015) since cores were taken through recently fragmented, yet living coral rubble found at the top of the coral hole.
Radiocarbon ages were calibrated using the Marine20 curve (Heaton et al. 2020), and age–depth models were calculated using Bayesian modeling in OxCal version 4.4 (Ramsey 2009). The ΔR value used to calibrate coral samples was − 141 ± 50 (Southon et al. 2002), retrieved from CALIB (calib.org). The additional ΔR (− 14 ± 50) reported for Guam was not included for analysis because it was sampled from a mobile marine gastropod (Gibberulus gibberulus: Linnaeus, 1758) with a different life history than a sessile coral, meaning its carbon assimilation sources would be different. Additionally, the gastropod’s ΔR was an order of magnitude different than the next eight closest available ΔR dates for the region. A P_sequence model was used in OxCal with a variable k-value (Ramsey and Lee 2013). This model allowed for the incorporation of depth and sequence data (P_sequence) from our cores and accounted for variable sedimentation rates (variable k-values). Ages are reported in both calibrated years before present (cal. BP) and calendar years CE.
Total percent coral abundances (g), coral community composition (relative abundance by family), and foraminifera abundances (per 4 g sub-sample, corrected by the time each 5 cm segment encompasses) were analyzed against core segment age to determine temporal variation. Then, z-scores were calculated to compare total coral abundance and foraminifera abundance to allow for comparative analysis. Z-score data were combined by time, and a nonparametric Loess smooth curve was fit to identify composition trends using the ggplot2 package and geom_smooth function (Wickham 2016). The span chosen for corals and foraminifera (0.55, 0.60, respectively) for the LOESS smooth function that generated the least error was determined by generalized cross validation (Takezawa 2005). Analyses were performed in RStudio, version 2022.02.3 (R Core Team 2022).
Results and discussion
All cores contained coral fragments in most segments, embedded within a gravelly, muddy matrix. On average, each 5 cm layer contained 40.5 ± 37.8 g of coral and accounted for 20.7 ± 17.9% of total fragment weight. Baculogypsina sphaerulata was the dominant foraminifera species found in all cores. Acropora spp. fragments from bottom segments of the three cores dated to approximately 104, 143, 221 cal. BP, or 1848, 1807, and 1729 CE (Table 1).
Taxonomically, 99% of coral fragments from the cores belonged to four families: Acroporidae, Pocilloporidae, Faviidae, and Poritidae. Acroporidae was the most abundant coral family in cores AG-1 and AG-3 for ~ 200-years; whereas, Pocilloporidae was most abundant in core AG-2. In all cores, Pocilloporidae abundance was variable and was often inversely related to Acroporidae. Over the last 50 years in AG-1 and AG-2 Pocilloporidae and/or Poritidae increased in relative dominance (Fig. 2). This shift is consistent with community patterns documented elsewhere (Greenstein et al. 1998), including in Guam (Raymundo et al. 2019; Greene et al. 2020). One possible explanation for this pattern of decreasing Acroporid abundance is their life-history strategy. Acroporids tend to be highly competitive and fast growing, making them prominent reef developers; however, they are also highly sensitive to environmental change (Darling et al. 2012) which has led to their recent global decline (Renema et al. 2016; Cramer et al. 2020; Cybulski et al. 2020). The compiled core record (Figs. 1 and 2) could be documenting the decline of sensitive coral groups such as Acroporids, previously seen in nearby Guamanian reefs from COTS outbreaks (Colgan 1987) and bleaching (Raymundo et al. 2019). Alternatively, some of the variability in coral relative abundance (Fig. 1) could be a result of how the coral rubble is deposited in the coral hole. Deposition of coral sub-fossils into coral holes is likely less uniform than the accumulation of reef matrix layers, the typical substrate sampled using push-cores. However, even if this pattern is taphonomic and driven by variable sedimentation, it is still indicative of changes in environmental conditions that led to decreased instances of Acroporid deposition.
Starting at approximately 1865 CE, we documented a sharp decline in B. sphaerulata z-scores (Fig. 3). Such a severe decline, with a lag in coral abundance and taxonomic changes (Figs. 2 and 3) suggests one or multiple stressors that impacted each taxon differently. The drop in B. sphaerulata could be linked to a loss of habitat, or water clarity declines driven by increased nutrients and/or sedimentation (Hallock 1981, 2000). Storm-related deteriorations to suitable habitat for B. sphaerulata, followed by the additional anthropogenic stressors from human development could be one explanation to the decline and subsequent stagnation of our foraminifera record. For example, although the timing based on our current age model is not exact a possible cause in abundance declines could have been two severe storms that were documented on the island in 1848 and 1849 CE (Carano 1964). The fact that the foraminifera abundance remains low after the storm events with no recovery suggests that this decline was not due to the storms directly, or at least not the storms alone. The arrival of the US Navy in 1899 CE increased the population fivefold from ~ 10,000 to ~ 50,000 in 50 years. This sparked coastal development around nearby Hagåtña—the original capital (Bayman and Peterson 2016), likely deteriorating water quality and clarity. This would have been detrimental to B. sphaerulata recovery.
Core summaries. A Total percent weight of coral fragments in the > 1000 µm sieve fraction, by core; and B average Baculogypsina sphaerulata counts by 4 g sediment sample, corrected by time by dividing the total number by the years represented in the 5 cm core segment. All data were fitted with a LOESS smooth with a span of 0.95. Panel C shows both data sets converted to z-scores, and then LOESS smoothed separately with a span of 0.55 and 0.60 chosen for corals and B. sphaerulata,, respectively. Significant events in Guam’s history are highlighted by arrows. “Severe Storms” are those labeled as such and documented to have caused extreme damage and loss of life in Carano and Sanchez (1964)
The gradual decrease in coral abundance might indicate initial resilience to factors that caused the severe B. sphaerulata decline. However, sediment loading and added nutrients often associated with terrestrial runoff from coastal development (Fabricius 2005) as well as repeated intense storms between 1900 and 1965 CE (Scoffin 1993) could have been what caused the more drastic coral declines starting around 1915 CE (Fig. 3). Similar impacts from acute storm events and increased population and development resulted in widespread Acropora mortality on the inshore Great Barrier Reef (Roff et al. 2013).
Alternatively, it is possible that our abundance declines are not environmental or anthropogenic, but either taphonomic or an artifact of age model uncertainty. These holes could have rapidly filled after Holocene sea-level rise, resulting in repeated replacement and time-averaging of its top portion. However, because of the linear age progression of the dates, this is unlikely. A more parsimonious interpretation is that the coral reef accreted enough around the hole to create more headspace for debris accumulation. Additionally, the wide variation in our dates (between ± 23 and 80 years) makes associating specific events to declines in corals and foraminifera difficult. Additional coring and dating are needed to strengthen links between environmental and biotic changes.
Regardless, our top and bottom dates offer a snapshot of past coral communities and foraminifera assemblages in Guam for over 280 years. We show clear declines in both foraminifera and coral abundances, although timings of these declines vary, and the direct causes are unclear. This method could be expanded to other known coral holes around Guam such as Shark’s Hole (Fig. 1; 13.558630 N, 144.815933 E, approx. 145 × 45 m wide) and Agat Bay (13.372192 N, 144.647095 E approx. 80 × 40 m wide; Burdick 2005) which are known to accumulate coral fragments, or other Pleistocene karst-formed reef islands that are difficult to core such as Okinawa or Dongsha Atoll.
Data availability
All data needed to complete the analyses in this manuscript will be available on a GitHub repository after publication.
References
Andrew C, Thierry L de L, Katie R, Serge P, Karin G, Eric C, Lauretta B, Clive W (2011) Status of coral reefs of the Pacific and outlook: 2011. Global coral reef monitoring network
Aronson R, Precht W, Toscano M, Koltes K (2002) The 1998 bleaching event and its aftermath on a coral reef in Belize. Marine Biology 141:435–447
Aronson RB, Macintyre IG, Wapnick CM, O’Neill MW (2004) Phase Shifts, Alternative States, and the Unprecedented Convergence of Two Reef Systems. Ecology 85:1876–1891
Barmawidjaja DM, Zwaan GJ van der, Jorissen FJ, Puskaric S (1995) 150 years of eutrophication in the northern Adriatic Sea: Evidence from a benthic foraminiferal record. Mar Geol 122:367–384
Bayman JM, Peterson JA (2016) Archaeologies of Early Modern Spanish Colonialism. Contr Glob Hist Arch 229–252
Bejarano S, Golbuu Y, Sapolu T, Mumby P (2013) Ecological risk and the exploitation of herbivorous reef fish across Micronesia. Mar Ecol Prog Ser 482:197–215
Burdick, David, Valerie B, Jacob A, Mike G, Lee G, Amy H, Jean K, Trina L, Emily L, Jenny M, Miller J, Minton D, Nadon M, Pioppi N, Raymundo L, Richards B, Schroeder R, Schupp P, Smith E, Zgliczynski B (2008) The state of coral reef ecosystems of Guam. 465–509
Burdick D (2005) Guam Coastal Atlas. University of Guam Marine Laboratory. Technical Report 114. 149 pp.
Carano, Paul; Sanchez, Pedro C. (1964). A Complete History of Guam. Tokyo: Charles E. Tuttle Company. OCLC 414965.
Carnahan EA, Hoare AM, Hallock P, Lidz BH, Reich CD (2009) Foraminiferal assemblages in Biscayne Bay, Florida, USA: Responses to urban and agricultural influence in a subtropical estuary. Mar Pollut Bull 59:221–233
Colgan MW (1987) Coral Reef Recovery on Guam (Micronesia) After Catastrophic Predation by Acanthaster Planci. Ecology 68:1592–1605
Cramer KL, Jackson JBC, Donovan MK, Greenstein BJ, Korpanty CA, Cook GM, Pandolfi JM (2020) Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Science Advances 6:eaax9395
Cybulski JD, Husa SM, Duprey NN, Mamo BL, Tsang TPN, Yasuhara M, Xie JY, Qiu J-W, Yokoyama Y, Baker DM (2020) Coral reef diversity losses in China’s Greater Bay Area were driven by regional stressors. Science Advances 6:eabb1046
Dardeau MR, Aronson RB, Precht WF, Macintyre IG (2000) Use of a hand-operated, open-barrel corer to sample uncemented holocene coral reefs.
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM, Bellwood D (2012) Evaluating life-history strategies of reef corals from species traits. Ecology Letters 15:1378–1386
David B, Valerie B, Jacob A, Mike G, Lee G, Amy H, Jean K, Trina L, Emily L, Jenny M (2008) The state of coral reef ecosystems of Guam. 465–509
Duprey NN, Wang XT, Thompson PD, Pleadwell JE, Raymundo LJ, Kim K, Sigman DM, Baker DM (2017) Life and death of a sewage treatment plant recorded in a coral skeleton δ 15 N record. Marine Pollution Bulletin
Fabricius K (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Pollution Bulletin 50:125–146
Greene A, Donahue MJ, Caldwell JM, Heron SF, Geiger E, Raymundo LJ (2020) Coral Disease Time Series Highlight Size-Dependent Risk and Other Drivers of White Syndrome in a Multi-Species Model. Frontiers Mar Sci 7:601469
Greenstein BJ, Curran HA, Pandolfi JM (1998) Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: a Pleistocene perspective. Coral Reefs 17:249–261
Hallock P (1981) Algal symbiosis: A mathematical analysis. Mar Biol 62:249–255
Hallock P, Lidz BH, Cockey-Burkhard EM, Donnelly KB (2003) Foraminifera as Bioindicators in Coral Reef Assessment and Monitoring: The FORAM Index. Environmental Monitoring and Assessment 81:221–238
Hallock P (2000) Environmental Micropaleontology, The Application of Microfossils to Environmental Geology. Top Geobiol 121–150
Heaton TJ, Köhler P, Butzin M, Bard E, Reimer RW, Austin WEN, Ramsey CB, Grootes PM, Hughen KA, Kromer B, Reimer PJ, Adkins J, Burke A, Cook MS, Olsen J, Skinner LC (2020) Marine20—The Marine Radiocarbon Age Calibration Curve (0–55,000 cal BP). Radiocarbon 62:779–820
Houk P, Rhodes K, Cuetos-Bueno J, Lindfield S, Fread V, McIlwain JL (2012) Commercial coral-reef fisheries across Micronesia: A need for improving management. Coral Reefs 31:13–26
Jackson JBC (1997) Reefs since Columbus. Coral Reefs 16:S23–S32
McCulloch M, Fallon S, Wyndham T, Hendy E, Lough J, Barnes D (2003) Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421:727–730
Pandolfi JM (2011) The Paleoecology of Coral Reefs. In: Dubinsky Z., Stambler N. (eds) Springer Netherlands, pp 13–24
Paulay G, Kirkendale L, Lambert G, Meyer C (2002) Anthropogenic Biotic Interchange in a Coral Reef Ecosystem: A Case Study from Guam. Pac Sci 56:403–422
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution 10:430
Pinkerton K, Baker DM, Cuddy MR, Raymundo LJ, Meyer KA, Kim K (2015) Nitrogen dynamics on Guam as revealed by the seagrass Enhalus acoroides. Marine Ecology Progress Series 528:117–126
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/.
Ramsey CB (2009) Bayesian Analysis of Radiocarbon Dates. Radiocarbon 51:337–360
Ramsey CB, Lee S (2013) Recent and Planned Developments of the Program OxCal. Radiocarbon 55:720–730
Raymundo LJ, Burdick D, Hoot WC, Miller RM, Brown V, Reynolds T, Gault J, Idechong J, Fifer J, Williams A (2019) Successive bleaching events cause mass coral mortality in Guam, Micronesia. Coral Reefs 38:677–700
Redding JE, Myers-Miller RL, Baker DM, Fogel M (2013) Link between sewage-derived nitrogen pollution and coral disease severity in Guam. Marine pollution Bulletin
Renema W, Pandolfi JM, Kiessling W, Bosellini FR, Klaus JS, Korpanty C, Rosen BR, Santodomingo N, Wallace CC, Webster JM, Johnson KG (2016) Are coral reefs victims of their own past success? Science Advances 2:e1500850
Roff G, Clark TR, Reymond CE, Zhao J, Feng Y, McCook LJ, Done TJ, Pandolfi JM (2013) Palaeoecological evidence of a historical collapse of corals at Pelorus Island, inshore Great Barrier Reef, following European settlement. Proceedings: Biological Sciences 280:20122100–20122100
Scoffin TP (1993) The geological effects of hurricanes on coral reefs and the interpretation of storm deposits. Coral Reefs 12:203–221
Southon J, Kashgarian M, Fontugne M, Metivier B, Yim WW-S (2002) Marine Reservoir Corrections for the Indian Ocean and Southeast Asia. Radiocarbon 44:167–180
Takezawa K (2005) Introduction to nonparametric regression. John Wiley & Sons,
Veron J (2000) Corals of the world, Vol. 1–3. Australian Institute of Marine Science
Wickham. (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York.
Williams ID, Baum JK, Heenan A, Hanson KM, Nadon MO, Brainard RE (2015) Human, Oceanographic and Habitat Drivers of Central and Western Pacific Coral Reef Fish Assemblages. Plos One 10:e0120516
Wolanski E, Richmond RH, Davis G, Bonito V (2003) Water and fine sediment dynamics in transient river plumes in a small, reef-fringed bay, Guam. Estuar Coast Shelf Sci 56:1029–1040
Zamora-Duran MA, Aronson RB, Leichter JJ, Flannery JA, Richey JN, Toth LT (2020) Imprint of Regional Oceanography on Foraminifera of Eastern Pacific Coral Reefs. J Foraminifer Res 50:279–290
Acknowledgements
Support for portions of this work was provided by a National Oceanic and Atmospheric Administration Coral Reef Conservation Program Grant (NA15NOS4820084) to LJR and KK. We gratefully acknowledge Dick Randall’s and David Burdick’s invaluable taxonomic and geologic insights and Amelia Crabtree, Sergio Morales, and Liza Wilson for their help with sample processing, and Erin Dillon for her insights and manuscript reviews.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cybulski, J.D., Doherty, J.M., LaRoche, C. et al. Using coral holes to explore the historical ecology of Guam’s coral reefs. Coral Reefs 42, 1411–1417 (2023). https://doi.org/10.1007/s00338-023-02442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02442-z