Abstract
The Philippine archipelago’s complex bathymetry, hydrology, and geologic history indicate the likely development of unique coral communities. However, there are few studies on coral community structure in the Philippines. Adult coral composition was analyzed through classification and ordination of data from 206 sampling stations, covering all Philippine marine biogeographic regions, on upper slopes of mostly well-developed fringing reefs. Seven coral community types are distinguished: I—moderately wave-exposed, mostly southwest-facing slopes with abundant massive Porites and branching Acropora; II—highly wave-exposed, mostly northeast-facing slopes, dominated by encrusting Isopora; III—low-exposure slopes with high hard coral cover (HCC), dominated by branching Porites; IV—wave-sheltered communities with abundant Galaxea; V—low-HCC communities with stress-tolerant corals; VI—Heliopora-dominated communities; VII—communities with low HCC and high macroalgal cover. Coral composition in these types is influenced by broad-scale hydrologic regimes (i.e., wave exposure and monsoon forcing) and local stressors. Applications of the typology are proposed for the spatial management of protected seascapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hard coral cover (HCC) and species richness are coarse metrics widely used in assessing reef status (e.g., Bruno and Selig 2007; Licuanan et al. 2019a). The study of coral community structure (i.e., the composition and abundance of hard corals in an assemblage) allows for a more detailed characterization of reefs. Including information on coral community structure as a metric of assessing reef status provides a basis for more specific, scale-appropriate management regimes for coral reefs. For example, the selection and zoning of marine protected areas (MPAs) could be better guided by knowledge of available coral community types and environmental conditions prevailing in a location (Riegl and Riegl 1996). Detailed analysis of coral community structure and its drivers can also be used to refine and assess conservation goals and project future reef states (Done 1999; Pandolfi et al. 2003).
Coral community structure is shaped by the complex interactions of broad-scale environmental processes, episodic disturbance events, and local anthropogenic impacts. Known drivers of heterogeneity among coral communities include geological processes such as volcanic activity and sea-level changes (Pandolfi and Jackson 2006; Houk and Starmer 2010), broad-scale hydrological regimes such as monsoons, tides, and currents (van Woesik and Done 1997; Quibilan and Aliño 2006), wind energy and direction (Done 1982; Hongo and Kayanne 2009), degree of embayment (Licuanan and Gomez 1988) and proximity to land and terrestrial influence (Done 1982; van Woesik and Done 1997). Coral community structure is also altered by chronic impacts from fishing, tourism, sedimentation, and nutrient input (Hawkins and Roberts 1993; McManus et al. 2000; DeVantier et al. 2006), and these impacts are exacerbated by disturbance events such as mass coral bleaching due to thermal stress and storms (Loya et al. 2001; Fabricius et al. 2008; Hongo et al. 2012; Darling et al. 2013).
The Philippines, where this study was conducted, has the third-largest reef area in the world (Spalding et al. 2001) and the highest species richness of hard corals globally (DeVantier and Turak 2017). The Philippines has a complex geologic history (Carpenter and Springer 2005; Licuanan et al. 2019b) and wider latitudinal distribution than neighboring Indonesia, which has a broader longitudinal gradient. Philippine reefs are mostly fringing (Faustino 1931), have different embayment and wave exposure conditions (Licuanan and Gomez 1988; Quibilan and Aliño 2006), and marine biogeographic regions are delineated by the country’s large islands and ocean current systems (Aliño and Gomez 1994). Philippine reefs are also exposed to annual reversing northeast/southwest monsoons (Chang et al. 2005; Gordon et al. 2011).
Philippine coral communities have been altered by acute disturbance events such as typhoons (Quibilan and Aliño 2006; Anticamara and Go 2017) and mass coral bleaching (Magdaong et al. 2014). Three major bleaching events have been recorded in Philippine reefs: 1998–1999, 2010, and 2016–2017 (Chou 2000; Tun et al. 2010; Licuanan et al. 2019a). Bleaching in Philippine reefs was also recorded in 1982–1983 and 1987–1988, although data from these bleaching events are limited (Arceo et al. 2001; Magdaong et al. 2014). Coral cover loss of around 8% was reported after the 1998–1999 bleaching event from a meta-analysis of 317 reef areas across the country (Magdaong et al. 2014), while severe bleaching was reported in the southern part of Luzon Island (Fig. 1) and surrounding reef areas in 2010 (Tun et al. 2010). Bleaching was also reported in 2016–2017 on the deeper parts (> 7 m depth) of reef slopes in Philippine fringing reefs (Licuanan et al. 2019a, in the same stations examined in this study), albeit less in severity and extent compared to bleaching reported in other parts of the globe, such as the Great Barrier Reef (Hughes et al. 2017).
Map of study areas. Black circles represent the 206 assessment stations covered in the study. Marine biogeographic regions (Aliño and Gomez 1994) are indicated
Fringing reefs in the Philippines are also exposed to severe anthropogenic pressure, as population centers are rapidly urbanizing, and most reefs are within an hour’s boat ride from coastal towns (Samson and Licuanan 2002). Coastal populations and more than a million small-scale fishers rely on the reefs for livelihood, subsistence, shoreline protection, and tourism revenue (White et al. 2000; Tamayo et al. 2018). Despite the importance and uniqueness of Philippine reefs, there are still very few detailed studies on coral community structure in Philippine reefs, even with coral reefs in this area receiving scientific attention as early as the 1930s (e.g., Faustino 1931). Additionally, Philippine reefs are at risk of further degradation due to climatic and anthropogenic impacts, as the most recent assessment of Philippine reefs revealed a loss of a third of its hard coral cover (HCC) over the past 40 yr (Licuanan and Gomez 2000; Licuanan et al. 2019a).
As such, Philippine reefs offer a unique opportunity to study how coral communities are differentiated in the center of biodiversity, where reefs are exposed to various environmental conditions at different spatio-temporal scales (Reyes et al. 2022). This study identifies coral community types in shallow upper slopes of fringing reefs throughout the Philippines. The community types are differentiated by hard coral composition at the genus life-form level. Chronic stressors reflecting topography, bathymetry, and long-term hydrological regimes were examined to identify their influence on the coral community structure in each type. Possible impacts of anthropogenic disturbance and acute disturbance events, such as typhoons and coral bleaching, on the differentiation of community types, were also evaluated. Lastly, applications are suggested for the spatial management of reefs in MPAs.
Materials and methods
Study area and sampling methodology
Coral community types were determined by assessing hard coral composition at the genus life-form level from 206 fringing reef assessment stations, sampled from 2014 to 2017, covering all six Philippine marine biogeographic regions (Fig. 1; see Licuanan et al. 2019a). Sampling was focused on stations in well-developed fringing reefs, which were defined using the following criteria: (1) a reef flat of least 15-m width, which can be measured and viewed in satellite images, (2) a well-defined reef crest, observed during the initial site selection, and (3) a reef slope extending to at least 5-m depth. Stations were also established facing the dominant monsoon wind (i.e., NE or SW).
The reefs surveyed were selected through stratified random sampling according to the aggregate area of fringing reefs in each marine biogeographic region. Initial randomization was done by proportionally scattering 500 points, corresponding to WGS84 coordinates of a reef, across the six marine biogeographic regions (Table 1). Afterward, satellite images were reviewed, and the coordinates were adjusted to prioritize reef areas facing NE or SW at the scale of the landmass where the fringing reef is located. For example, if the fringing reef is located along an island, the coordinates were adjusted to the NE or SW side of the island. Similarly, if the fringing reef is located on the mainland, the coordinates were adjusted to prioritize the NE-facing or SW-facing parts of the reef, where appropriate. Each reef point was visited and subsequently sampled if it met the above criteria.
Moderately exposed reefs within gulfs and bays were also surveyed; sheltered and marginal reef areas were not initially considered and were only surveyed if there were no other reefs in the location that met the criteria described above. If no reefs in a sampling location qualified under the described criteria during a field expedition, assessments were still performed on reefs representative of the location. Additional points, following the criteria above, were also sampled during each survey trip if accommodated by the fieldwork schedule. The final number of stations sampled in each bioregion (Table 1) was determined by the availability of reefs that fit the criteria described, the accessibility of the sampling locations, and the feasibility of travel to the different bioregions. Fringing reefs in the West Philippine Sea were sampled more comprehensively than the other bioregions relative to its reef area, since the accessibility of the area for field trips and lower cost of travel allowed for more exhaustive sampling. Additionally, five monitoring locations, with at least four sampling stations each, were established and/or revisited in the West Philippine Sea (see Licuanan et al. 2019a, b) within the survey period, and reef assessments from these stations were also included in the present study. In total, 415 stations were visited, and 206 of these were sampled (Table 1). All sampled stations were included in the analysis.
Each assessment station covers a rectangular area of 1875 m2 on the upper reef slope. Each station’s dimensions are 25 × 75 m or 18.75 × 100 m, depending on the range of depths over which coral was present. Five 50-m transects were deployed in each station, parallel to the shore and following the reef's contour. First, a 75- or 100-m belt transect was deployed to demarcate the deepest limit of each station, at 5–7 m depth. The four remaining transects were deployed at least 1 m apart and parallel to the preceding transect. A random number generator was used to determine x- and y-coordinates, in meters, where the remaining four transects were deployed. This random deployment of transects within the 25 × 75 m or 18.75 × 100 m station resulted in each station spanning a depth range of 2 m (shallowest transect) to 7 m (deepest transect). The angle of the reef slope was considered in selecting stations, where five transects should fit within the depth range and station dimensions; as such, steep slopes and walls were not sampled.
The composition of the reef benthos in each station was measured using photo-transects as the primary sampling unit. Photographs of the benthos beneath each transect were taken at 1-m intervals using Sony Cyber-shot DSC-RX100 II digital cameras enclosed in Ikelite underwater cases and equipped with INON UWLH100 wide-angle lenses. Each image covered at least a 1-m2 base area. The cameras were mounted on a 1 × 1 × 1.2 m tetrapod to maintain a uniform distance from the substrate. At least 250 transect photographs were taken at each station.
Each photograph was overlain with ten random points, corresponding to pixels, using Coral Point Count with Excel extensions v4.0 (CPCe; Kohler and Gill 2006). The reef bottom type and benthos were then identified to taxonomic amalgamation units (TAUs; see Licuanan et al. 2019a). Hard corals were classified into 59 TAUs, representing a combination of genus and life form, following the taxonomy in Veron (2000)’s Corals of the World, which are optimized for processing transect images (Licuanan et al. 2019a). 2500 points were identified for each station.
Environmental parameters
Environmental data were collected from existing databases and remotely processed to match the locations or geographic coordinates of each assessment station (Table 2; see Electronic supplementary material). The variables were selected based on previous smaller-scale studies on coral composition in Philippine reefs (Licuanan and Gomez 1988; Uychiaoco et al. 1992; Samson and Licuanan 2002; Quibilan and Aliño 2006). Supplementary data were collected during reef assessments through field observations and interviews.
Geographic data were visualized in Google Earth Pro and QGIS 3.4.15. The Google Earth Pro line measurement tool was used to estimate each station’s minimum distance from land, in meters. The role of wave exposure was approximated using three different measures representing different spatial scales. First, wave exposure data were extracted from the Wave Exposure Model (WEMo) database of Villanoy et al. (2013) to generate a Relative wave Exposure Index (REI) value. Each REI value is a unitless measurement of exposure at the coastal village (i.e., “barangay”) level, where higher REI values correspond to higher levels of wave exposure on the coastlines of each “barangay” (Villanoy et al. 2013). WEMo uses an equation that accounts for wind direction, wind speed, and fetch in the eight cardinal directions (Villanoy et al. 2013).
NE and SW station orientation was categorized to approximate wind exposure at the station level relative to the direction of the Philippines’ annual reversing NE and SW monsoons. Each station was given a score from 0 to 5 for NE and SW orientation, with higher scores indicating longer fetch and approximating higher levels of wind exposure in either direction. For instance, a NE-facing station with fetch > 100 km would score 5 for NE orientation and 0 for SW orientation. Stations facing neither southwest nor northeast would score 0 for both NE and SW station orientation.
Each station’s degree of embayment was calculated to estimate wind exposure relative to a station’s location within a bay. The degree of embayment was calculated using an adapted embayment morphometric parameter (\({\gamma }_{\mathrm{e}})\) equation derived from Fellowes et al. (2019), where a higher embayment morphometric parameter indicates a higher degree of embayment (see Electronic supplementary material). Non-embayed stations were scored as 0.
The predominant geomorphology of each station was identified based on Houk and Starmer (2010), where each station was given a score from 1 to 10, representing increasing levels of reef development (see Electronic supplementary material for the corresponding scores). Geomorphology was scored at the station level by visual assessment and review of videos taken at the survey station. The observed geomorphology at the station level was recorded and scored. Coastal population sizes, at the coastal village (“barangay”) level, were obtained from the 2015 Census of Population data (Philippine Statistics Authority 2020).
Data analyses
Hierarchical cluster analysis using the unweighted pair group method with arithmetic mean (UPGMA) algorithm and principal coordinates analysis (PCoA) ordination were performed to elucidate station groupings that distinguish distinct coral community types, both using Bray–Curtis as the distance measure. Percent abundance of hard coral TAUs was used as the species data input. Average HCC and average coral generic richness were used to further describe each community type and were compared with the Philippine averages (22.8% ± 1.2 SE average HCC and 14.5 TAUs ± 0.5 SE average generic richness; Licuanan et al. 2019a), which were derived from the same dataset used in this study.
Analysis of similarity (ANOSIM) was used to examine if the groupings obtained in the classification differed significantly from one another. Groupings were then rechecked and optimized according to the ANOSIM results. The dataset was also classified using Ward’s Method as the group linkage method since it creates linkages that minimize the within-cluster variance of groups. Cross-comparisons were done between the UPGMA and Ward’s Method groupings to identify distinct coral community types. For groupings that were not resolved in Ward’s Method but were distinct in the UPGMA classification, groupings that showed marked differences in HCC and non-coral benthic composition were reported as distinct coral community types. Similarity percentage analysis (SIMPER) was also performed to identify hard coral TAUs that caused the clustering patterns.
Canonical correspondence analysis (CCA) was performed to analyze the influence of the selected environmental variables on coral composition. Prior to gradient analyses, environmental data were centered and normalized, with bi-plot scaling to a mean of zero. Axis scores were also centered and standardized to unit variance. A randomization test was performed (999 randomizations) to identify the significance of species-environment correlations. Data were analyzed using Past 3.26 (Hammer et al. 2001) and PC-ORD 7.0 (McCune and Mefford 2011).
Results
Overview of coral community types
Seven coral community types were distinguished from the surveyed assessment stations (Fig. 2).
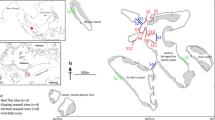
Left: Hierarchical cluster analysis (UPGMA algorithm, Bray–Curtis dissimilarity) dendrogram of coral composition in the reef assessment stations. Each branch in the dendrogram corresponds to one assessment station, labeled with a unique sample ID. Coral community types are indicated in Roman numerals, and larger groupings are indicated by capital letters (A = wave-exposed coral communities, B = sheltered coral communities, C = community types indicative of disturbance). Right: A map of the Philippines with the assessment stations plotted according to coral community type. Philippine marine biogeographic regions (Aliño and Gomez 1994) are indicated (NPS North Philippine Sea, SPS South Philippine Sea, CS Celebes Sea, SS Sulu Sea, WPS West Philippine Sea, VS Visayas Sea). Locations of the monitoring stations (Taytay Bay, Palawan [see also Abesamis et al. 2022] and Lian, Batangas) used in the successional vector analysis are also indicated
A. Wave-exposed coral communities.
Type I. Moderately wave-exposed, mostly southwest-facing slopes with abundant massive Porites and Acropora.
Type II. Highly wave-exposed, mostly northeast-facing slopes, dominated by encrusting Isopora.
B. Wave-sheltered coral communities.
Type III. Low-exposure slopes with high hard coral cover (HCC), dominated by branching Porites;
Type IV. Wave-sheltered communities with abundant Galaxea.
C. Communities indicative of disturbance.
Type V. low-HCC communities with abundant massive Porites and faviids.
Type VI. Heliopora-dominated coral communities.
Type VII. low-HCC communities with high macroalgal cover.
The groupings obtained in classification were corroborated by findings from the PCoA ordination (32.7% variance explained in axes 1–3; see Electronic Supplementary Material). All community types are significantly dissimilar from one another (ANOSIM p < 0.05; all uncorrected pairwise p < 0.05, R = 0.7602) and are characterized by high abundance of one or two unique coral TAUs (Fig. 3; Table 3). Pooled comparisons generated from SIMPER indicate that the abundant hard coral TAUs are the largest contributors to dissimilarity among community types (i.e., % contributions to dissimilarity: massive Porites = 21.7%, branching Porites = 15.5%, Heliopora = 6.1%, branching Acropora = 5.7%, Isopora = 5.0%, encrusting Porites = 4.0%, Galaxea = 3.5%, Favites = 3.1%). Massive Porites is within the five most abundant hard coral TAUs of all community types (Table 3).
Rank-abundance curves of hard coral taxonomic amalgamation units (TAUs) recorded in each coral community type. Each point indicates the rank abundance of a hard coral TAU. The ten most abundant hard coral TAUs in each coral community type are shown in Table 3
Two outlier stations from the first PCoA ordination were removed from succeeding analyses since they occluded the visualization of the remaining stations in the ordination space (see Electronic Supplementary Material). Both outlier stations are in the Sulu Sea. One station is within an embayment dominated by Anacropora, and the second outlier station is a SW-facing reef dominated by Seriatopora.
Hard coral cover, generic richness, and spatial distribution of community types
The seven coral community types differ in number of samples, average HCC, and to a lesser extent, average coral generic richness. Average HCC of the community types ranges from 0.5% ± 1.3 SE (Type VII) to 38.5% ± 3.8 SE (Type III), and average coral generic richness ranges from 2.1 TAUs ± 1.3 SE (Type VII) to 18.4 TAUs ± 0.5 SE (Type I). A more detailed examination of non-coral benthos in the three community types with the lowest average HCC (Types V, VI, and VII) reveal dominance by turf algae and macroalgae. Celebes Sea and Visayas Sea biogeographic regions have the highest number of coral community types represented, with five each. Type I is the only coral community type observed in all six biogeographic regions (Table 4).
Correlation with environmental parameters
Relative exposure and NE/SW station orientation most strongly influence differences in coral composition among community types (Fig. 4). Coastal population sizes, although appearing in the first two axes of the canonical correspondence analysis, did not appear to influence variations among the types (Fig. 4). Species-environment correlation is statistically significant, with the first three axes explaining 5.7% of the variance among stations (randomization test for species-environment correlations, 998 runs: p < 0.05). The Type I centroid is oriented toward the SW axis, while the Type II centroid lies along the NE axis. No distinct patterns were observed for Types III to VII, yet centroids in Types IV and VI are oriented more toward the SW axis, and centroids in Types III, V, and VII are oriented toward the NE axis. The REI and NE axes are also aligned in a similar direction, where centroids of Types II, III, V, and VII are oriented. Closer inspection of Type III and IV stations in maps reveals that most Type III and IV communities are in short-fetch locations (e.g., inner seas, within bays, or between islands).
Canonical correspondence analysis plot with station points labeled according to coral community type. Centroids are marked with black circles. The black arrows indicate environmental axes (NE = northeast-facing, Coastal population, SW = southwest-facing, REI = relative exposure index). Eigenvalues are indicated
Average geomorphology, horizontal distance from land, and degree of embayment did not appear on the first two axes of the CCA plot. Among the 47 embayed assessment stations, the degree of embayment ranged from 1.077 (most embayed) to 0.003 (least embayed). Average horizontal distance from land ranged from 33 m to > 4 km. Average geomorphology scores for Types V, VI, and VII are lower (6.4 ± 0.3 SE) than the average geomorphology scores for the other coral community types (8.2 ± 0.3 SE).
Description of coral community types
Type I
Type I is the most common coral community type (n = 144; Table 4). All 59 hard coral TAUs were observed in Type I, where massive (= hemispherical) Porites and branching Acropora are most abundant (Table 3; Figs. 3, 5a). The average HCC (26.6% ± 1.0 SE) and average generic richness (18.4 TAUs ± 0.5 SE) in Type I communities are both higher than the Philippine averages. 92 of the 144 Type I communities face southwest at the island or landmass scale (Fig. 2).
Coral communities in Philippine fringing reefs. a Type I: massive Porites and branching Acropora are abundant. b Type II: dominated by encrusting corals, such as Isopora spp. c Type III: dominated by stands of branching Porites. d Type IV: hard carbonate substrate interspersed with sand patches; Galaxea, branching Acropora, and soft corals are abundant. e Type V: low-relief carbonate substrates dominated by turf algae. f Type VI: dominated by Heliopora. g Type VII: dominated by macroalgae
Since Type I contained more than half of the assessment stations in the study, classification and ordination were performed to identify possible subtypes within Type I. Following the process outlined in the Methods section, the classification and ordination of only Type I stations revealed three distinct subtypes. ANOSIM validated that these three subtypes are significantly different from one another (ANOSIM p < 0.0001; R = 0.5056; all pairwise p < 0.05). These subtypes were not treated as distinct coral community types (e.g., one of the seven coral community types presented in this study) since the within-Type I groupings in the UPGMA classification were not discrete when cross-checked with Ward’s Method classification. Hence, these groupings are presented as subtypes under Type I in this study.
Subtype 1-A contains 83 of the 144 Type I stations, and it is characterized by the abundance of massive and branching Porites. Subtypes 1-B (n = 25) and 1-C (n = 36) are characterized by the dominance of massive Porites and branching Acropora, respectively. Pooled SIMPER percent (%) contributions to dissimilarity are 22.1% for massive Porites, 14.2% for branching Acropora, and 5.6% for branching Porites.
Type II
Type II consists of 16 assessment stations, most of which are dominated by encrusting and submassive Isopora, followed by Porites and Favites (Tables 2, 3; Fig. 5b). All Type II communities are well-developed reefs facing the dominant monsoon wind; 12 of 16 Type II stations have long fetch and face northeast (Fig. 2). 52 out of 59 hard coral TAUs were observed in this community type (Fig. 3; see Electronic Supplementary Material). Type II communities have lower HCC (18.7% ± 2.2 SE) but higher generic richness (15.4 TAUs ± 1.1 SE) than the Philippine averages. Foliose Montipora, Pectinia, Seriatopora, and free-living fungiids were not observed in Type II communities.
Type III
Eleven assessment stations belong to coral community Type III, which is dominated by branching Porites (> 60% average relative cover; Figs. 3, 5c), followed by massive and encrusting Porites and Galaxea (Table 3). A total of 49 out of 59 hard coral TAUs were recorded in Type III communities (Fig. 3; see Electronic Supplementary Material). Type III has the highest average HCC (38.5% ± 3.8 SE) among the seven coral community types, higher than the Philippine average by more than 15%, yet average generic richness (10.9 TAUs ± 1.1 SE) is lower than the Philippine average. Type III communities appear to be relatively more wave-sheltered (i.e., not directly facing the dominant monsoon wind or have short fetch) than the other community types (Fig. 2).
Type IV
The three assessment stations that comprise Type IV are dominated by Galaxea (> 55% average relative cover), massive and branching Porites, branching Acropora, and Goniopora. The average HCC (18.3% ± 3.0 SE) of Type IV is below the Philippine average (Table 4). Only 34 out of 59 hard coral TAUs are present in Type IV (Fig. 3; see Electronic Supplementary Material), yet it has the highest average generic richness among the community types (18.7 ± 4.9 TAUs) (Table 4). All Type IV communities are relatively remote from urban areas and are in wave-sheltered environments, such as within embayments or between islands (Fig. 2). However, the distribution and prevalence of Type IV communities are likely underrepresented since the assessments focused on outer reef slopes. Type IV substrates are carbonate rock interspersed with sand patches (Fig. 5d). Similar to Type III, Type IV communities exhibit composition patterns characteristic of inshore environments, yet Type IV has higher relative abundance of branching acroporids and lower relative abundance of Heliopora (Table 3).
Type V
Type V consists of 18 assessment stations, 15 of which are well-developed reefs facing the dominant monsoon wind. Massive Porites has the highest relative abundance, followed by Favites, other encrusting corals, branching Acropora and Pocillopora (Table 3). However, Type V communities do not exhibit strong patterns in rank-abundance (Fig. 3). Type V communities have an average HCC of 4.0% ± 0.6 SE, nearly one-fifth the Philippine average, and an average generic richness of 8.3 TAUs ± 1 SE, almost half the Philippine average. Type V communities typically have a low-relief substrate dominated by non-coral benthos, such as turf algae (51.5% ± 6.1 SE average cover) and macroalgae (24.1% ± 6.5 SE average cover) (Fig. 5e). Macroalgal cover in Type V communities is nearly three times higher than the Philippine average (8.7% ± 1.2 SE; Licuanan et al. 2019a).
Type VI
The seven stations classified under Type VI are dominated by Heliopora (61.8% ± 5.4 SE average cover), followed by massive and branching Porites, Seriatopora, and other massive corals (Table 3; Fig. 5f). The average HCC (12.5% ± 2.9 SE) and generic richness (8.6 TAUs ± 1.7 SE) of Type VI communities are nearly half the Philippine averages. A total of 45 out of 59 hard coral TAUs are present in Type VI (Fig. 3; see Electronic Supplementary Material). Acroporids such as branching and robust-branching Acropora, encrusting and foliose Montipora, and Isopora were not recorded in Type VI. Type VI communities also have relatively high abiotic cover (23.8% ± 4.1 SE combined average cover of sand, silt, and rubble) compared to the other types.
Type VII
Five stations comprise coral community Type VII, which has the lowest average HCC (0.5% ± 1.3 SE) and generic richness (2.1 ± 0.5 SE TAUs) among community types (Table 4). Only 19 of 59 hard coral TAUs were observed in Type VI communities. Massive and branching Porites, Favites, and other massive corals are most abundant (Fig. 3; Table 3; see Electronic Supplementary Material). Benthic cover in Type VII communities is dominated by macroalgae (56.0% ± 7.4 SE average cover) (Fig. 5g). Compared with the other assessment stations, most coral TAUs in Type VII are ubiquitous (i.e., present in the top 10 TAUs, when ranked by average cover, of the seven coral community types; Table 3).
Discussion
This study identifies and examines seven coral community types in Philippine fringing reefs. These coral community types are distinguished by the relative abundance of their dominant coral genera. At the scale of the Philippine archipelago, broad-scale hydrologic regimes (i.e., wave exposure and orientation relative to the dominant monsoon winds) have a stronger influence on coral composition in the fringing reef stations than station-specific chronic stressors such as degree of embayment and distance from land.
Broad-scale environmental regimes: wave exposure and monsoon forcing
Differentiation of coral community types in the examined reef areas is most strongly influenced by the degree of wave exposure and the periodic, reversing monsoon system. Most Southeast Asian landmasses experience an annual reversal of surface monsoon winds, which are then modified depending on topography and bathymetry to create mesoscale differences in rainfall, wind exposure, and oceanographic features (Chang et al. 2005; Gordon et al. 2011). The reversing monsoons also cause variations in water circulation at the archipelagic level (Gordon et al. 2011) and lead to local variations in wind energy and wave exposure throughout a year (Villanoy et al. 2012). The distribution of coral community types identified in the study showed stronger differentiation based on NE/SW orientation and wave exposure at an island/landmass scale and the Philippine archipelago scale (Fig. 2; Table 4) compared to variation according to marine biogeographic region. The latter was proposed based on basin boundaries and major current systems (Aliño and Gomez 1994).
In the Philippines, the NE monsoon blows relatively stronger and drier northeasterly winds from around November to April, and the SW monsoon blows relatively wetter southwesterly winds from around June to October annually (Chang et al. 2005; Villanoy et al. 2013). A SW-facing reef slope would be windward during months when the SW monsoon is prevailing but leeward when the NE monsoon is prevailing. Degrees of wave exposure and the reversing monsoon system have been reported to influence patterns of coral community structure in Philippine reefs (Quibilan and Aliño 2006; Licuanan et al. 2019b). The reversing monsoon system has also been reported to reduce windward-leeward differentiation (Licuanan and Gomez 1988; Quibilan and Aliño 2006), a known driver of coral community structure variations in the Great Barrier Reef in Australia (Done 1982; van Woesik & Done 1997). In this study, Types I, II, III, and IV reflect different wave exposure conditions and the effect of the reversing monsoon system on the structuring of coral communities.
High degrees of wave exposure due to long fetch (> 25 km) and orientation toward the stronger NE monsoon led to the differentiation of Type II communities. Most Type II communities are also found in the North Philippine Sea and South Philippine Sea bioregions on the Pacific coast of the Philippines (Fig. 2; Table 4), which faces an open ocean where wave action is strong and is the site of the North Equatorial Current bifurcation (Cabrera et al. 2015). Thus, Type II communities are composed of encrusting and hardy coral forms that can withstand strong water movement (Done 1982; Quibilan and Aliño 2006; Darling et al. 2012), such as the fully encrusting form of Isopora. Delicate branching and foliose forms of Montipora, Pectinia, and Seriatopora were not recorded in Type II communities. Free-living fungiids that are often associated with sheltered reef habitats (Done 1982) were rare.
Type I communities are prevalent in moderately exposed, windward environments. Type I communities were found in all Philippine marine bioregions, with nearly half of Type I stations in the West Philippine Sea, which directly faces the SW monsoon (Fig. 2; Table 4). At the island- or landmass-scale, most Type I stations also face the SW monsoon (Fig. 2). Type I stations have a high abundance of primary coral framework-builders such as massive Porites and branching Acropora, which are associated with reef accretion (van Woesik and Done 1997; Montaggioni 2005). The presence of large, long-lived colonies (e.g., massive Porites; van Woesik and Done 1997; Goodkin et al. 2011) indicate sustained reef-building capacity across geologic timescales (Shen et al. 2010).
In wave-exposed reefs, strong water circulation and flushing of sediments promote coral growth, reef framework-building, and accretion (van Woesik and Done 1997; Houk and van Woesik 2010; Houk and Starmer 2010). Between Types I and II, exposure to the stronger NE monsoon, stronger water movement (Done 1982) and higher incidence of typhoons (Quibilan and Aliño 2006) may have contributed to lower HCC and coral generic richness in Type II communities. Bathymetry and tectonic activity in the eastern Philippines (Yumul et al. 2008) may also affect reef development and the structure of Type II communities.
Type III and IV communities are in wave-sheltered, short-fetch environments such as the inner seas of the Visayas Sea marine biogeographic region, between islands, within channels, or in low-wave energy environments such as embayments (Fig. 2; Table 4). Furthermore, the slopes of Type III and IV coral communities do not directly face either of the dominant monsoon winds. Types III and IV have high abundances of coral taxa that thrive in wave-sheltered environments (Table 3; Done 1982; DeVantier et al. 2006). However, this pattern is not strongly represented in the canonical correspondence analysis, likely due to the small number of Type IV stations and the relatively high coastal village-level REI values, which may not completely reflect wave-sheltered conditions at the embayment- or station-level. Fewer typhoons make landfall along the latitude zones spanning the Visayas (central Philippines; where most Type III communities were recorded) compared to Luzon (Fig. 1) (Tagaki and Esteban 2016), and shallow lagoon environments shelter some coral forms (e.g., massive and tabular) from typhoon impacts (Hongo et al. 2012). Coupled with the weedy life-history strategy (Darling et al. 2012) of the dominant branching Porites, these prevailing conditions may have contributed to high average HCC in Type III communities (Tables 2, 3).
Reef accretion may be slower in wave-sheltered reefs since chronic wave exposure has been shown to positively impact reef development (van Woesik and Done 1997; Houk and Starmer 2010). The observed wave-sheltered reefs may also be in their incipient stage (Goodkin et al. 2011). Incipient reefs and similar marginal environments have different coral communities than those in well-developed reefs and possibly harbor rare coral taxa (DeVantier et al. 1998). In this study, Types III and IV are distinct wave-sheltered coral communities that may have been differentiated by other environmental factors not considered in this study, such as bathymetry and reef development. While predominant geomorphology observed at the station level was considered in this study, follow-up studies may benefit from the inclusion of more quantitative measures of reef and island geomorphology (e.g., bathymetry charts, detailed maps, or echo sounding) while incorporating broader-scale perspectives (e.g., the entire reef area or shelf where a station belongs).
The possible roles of local human impacts and thermal stress events in structuring coral communities
This study hypothesizes that in addition to broad-scale hydrologic regimes, anthropogenic impacts, and local disturbance also affect coral community structure in Philippine fringing reefs, especially in shallow upper reef slopes, which are more susceptible to heightened temperatures, typhoon impacts, trampling, and fishing (DeVantier et al. 2020). It is also possible that variations in the response of coral communities to thermal stress and bleaching events over time contribute to the structuring and differentiation of coral communities in Philippine reefs. Additionally, the low percent variance explained by environmental factors in the canonical correspondence analysis suggests that the sampled communities are exposed to other factors (e.g., anthropogenic stressors, episodic disturbance, and other natural sources of variation) that could not be isolated from one another in this study. While the present study did not initially seek to quantify anthropogenic disturbance in Philippine reefs, the differentiation of coral communities, especially in Types V, VI, and VII, could not be attributed to any of the broad-scale environmental patterns that were tested for in the present analysis (Table 2). Examination of the characteristics of the benthos in these coral community types revealed patterns that may be attributed to local anthropogenic stressors. The possible roles of anthropogenic disturbance and thermal stress events are outlined below.
To identify if different coral community types may respond differently to bleaching events and may therefore result in variation between or within coral community types, coral composition data from four (4) annually monitored stations were examined over time. These four monitoring stations are from two different locations, Lian, Batangas (year 2010–2019) and Taytay Bay, Palawan (year 2011–2018), which were both impacted by the 2010 mass coral bleaching event (see Abesamis et al. 2022; Reyes et al. 2022). Coral composition data from the four monitoring stations were plotted as successional vectors in a PCoA ordination first containing all coral community types, where all monitoring stations across all years belonged to Type I (see Electronic Supplementary Material).
To examine variability within Type I stations and to validate the presence of subtypes, the coral composition monitoring data were plotted as successional vectors with Type I stations only (Fig. 6). These four monitoring stations appeared to exhibit a directional change in coral community composition within Type I. Specifically, directional change was observed in Tecas, Taytay over the year 2011–2018 while remaining within Subtype 1-A. In contrast, Nabat, Taytay showed a directional shift from Subtype 1-A toward the branching Acropora-dominated Subtype 1-C from 2011 to 2018 after being heavily impacted by the 2010 mass bleaching event (see Abesamis et al. 2022). Galvez in Lian, Batangas showed an opposite trend, where the 2010 coral composition appeared to change from Subtype 1-C to Subtype 1-A in the years following the 2010 bleaching event. Lastly, Clubhouse in Lian, Batangas, appeared to have a directional change in composition while remaining in Subtype 1-B. Except for Galvez, hard coral cover increased from 2010 in all these monitored stations (not shown).
Principal coordinates analysis ordination plot of Type I stations with Lian, Batangas and Taytay Bay, Palawan monitoring data. Stations are labeled according to coral community subtypes. Successional vectors (black arrows) are indicated, along with each station’s first and last monitoring years. Meanings of the hard coral TAU abbreviations are indicated in the Electronic Supplementary Material
Since the monitoring stations all remained throughout Type I within the monitoring period, this may indicate that thermal stress impacts may affect HCC and non-coral benthic cover (e.g., turf and macroalgae; see Abesamis et al. 2022) more strongly than coral community composition. For example, while Tecas was severely impacted by the 2010 bleaching event and was dominated by macroalgae in the years immediately following the bleaching event (43% macroalgal cover in 2011; see Abesamis et al. 2022), changes in coral composition, species density, and even HCC may not have been large enough for Tecas to be classified under another coral community type. In 2011, Tecas had an HCC of 11.5%, where 24 hard coral TAUs were recorded; this is still higher than the average values in Types V and VII. Additionally, the abundant hard corals in Tecas remained characteristic of Type I stations, as massive Porites remained most abundant throughout the monitoring period, with increases in relative abundance of Acropora over time (Abesamis et al. 2022).
The observed directional change in the coral composition of the monitoring stations and the occurrence of subtypes within Type I suggest that coral communities may still respond differently to mass bleaching events on a local scale, but these changes may not be enough to create distinct coral community types. Succession is dynamic in space and time (Karlson and Hurd 1993), and the occurrence of successional states is determined by prevailing environmental conditions, coral life-history strategy, and the type and extent of disturbance (Jackson 1991). On one hand, dominance patterns and abundant coral forms may change (Karlson and Hurd 1993; DeVantier et al. 1998) after an episodic disturbance such as mass coral bleaching or due to the impact of chronic anthropogenic stressors. On the other hand, coral composition may not change markedly in a reef exposed to disturbance, despite large changes in HCC and non-coral benthic cover, as seen in the present study. Hence, these findings suggest the importance of examining coral composition along with tracking changes in HCC and non-benthic cover, where appropriate, to adequately characterize coral communities affected by disturbance and diagnose the response of coral communities to episodic and chronic stressors over time.
Low HCC, low coral generic richness, and high abundance of hardy, stress-tolerant coral genera (e.g., massive Porites, Heliopora, Favites) (Done and Potts 1992; Kayanne et al. 2002; Darling et al. 2012, 2013) in coral community types V, VI, and VII may reflect the effects of chronic anthropogenic disturbance (e.g., sedimentation, overfishing) or reefs in different recovery states from episodic disturbance, especially in the absence of clear environmental and geographic differences between these types (Table 4; Fig. 4). The relative abundance of stress-tolerant massive Porites in Indo-Pacific reefs is associated with high levels of disturbance (DeVantier et al. 2020). Philippine reefs show a similar pattern, where massive Porites is abundant in all community types (Table 3) and have a higher relative abundance in low-HCC reefs (Licuanan et al. 2019a).
High non-coral benthic cover and prevalence of stress-tolerant (Darling et al. 2012, 2013) and ubiquitous coral genera suggest that Type V communities are degraded. First, Type V communities have average turf algae cover of 51.5% ± 6.1 SE and average macroalgae cover of 24.1% ± 6.5 SE compared to an average HCC of 4.0% ± 0.6 SE, which indicates the prevalence of algae over hard corals in this community type. Further, among the hard corals present in Type V communities, the 20 most abundant coral TAUs in Type V are similar to those in Type I. However, rank-abundance patterns reveal that Type V has a high cover of faviids and low Acropora cover; the opposite trend is observed in Type I (Fig. 3; Table 3; see Electronic Supplementary Material). Faviids are resistant to wide-scale, high-intensity disturbance, and acroporids are more susceptible to thermal stress and sedimentation (Loya et al. 2001; Trapon et al. 2011; but see Muir et al. 2017). Patches of hard coral along a transect line were also fewer but larger in Type I stations than in Type V stations (not shown).
Combinations of local stressors may have contributed to the low HCC and low generic richness of Types VI and VII. For instance, Heliopora dominance, low HCC, and high algal cover in Type VI stations suggest that the reefs are exposed to chronic stressors such as thermal stress (Kayanne et al. 2002), sedimentation (Rogers 1990), or low salinity (e.g., submarine groundwater discharge; Cardenas et al. 2010; Senal et al. 2011). Nutrient enrichment from aquaculture effluent and sewage is also associated with declines in HCC (Abaya et al. 2018), high algal cover (Quimpo et al. 2020), and the development of harmful algal blooms (Lapointe et al. 2005), with fringing and inshore reefs more severely affected than offshore reefs. Type VII communities have a hard calcareous substrate, which suggests past reef accretion, yet these stations are almost devoid of hard corals and are instead dominated by large, fleshy macroalgae. This suggests that Type VII communities may be undergoing a coral-algal phase shift, where widespread coral mortality and inability to recover from disturbance leads to macroalgal dominance and the long-term disruption of reef ecosystems (Done 1992; Hughes 1994).
Anthropogenic stressors may also reduce the levels of reef development, especially when a given reef area is algae-dominated or colonized by ephemeral, short-lived species yet have a hard, carbonate substrate indicating past reef formation (van Woesik and Done 1997). The low HCC in Types V and VII (i.e., < 10% average live coral cover) makes these types vulnerable to reef erosion, as a minimum of 10% live coral cover may be required to maintain positive reef accretion rates (Perry et al. 2013). Further, turf algae-dominated reefs may have undergone mass mortality of corals and are still recovering from an episodic disturbance such as a mass coral bleaching event (e.g., Abesamis et al. 2022) or typhoon (e.g., Anticamara and Go 2017). Hence, long-term monitoring is needed to capture and diagnose drivers of change in a reef and to identify the prognosis of degraded reefs (Flower et al. 2017).
Areas for future study and refining the typology of Philippine fringing reefs
This study reports the first typology of Philippine fringing reefs according to coral community structure, identifies the significant roles of monsoon forcing and wave exposure on the structuring of coral communities, and suggests the possible roles of disturbance in the differentiation of coral communities. The percent variance explained in the ordinations (i.e., 32.7% in PCoA, 5.7% in CCA) indicates other areas of study to refine the typology. First, since the sampling scheme prioritized well-developed fringing reefs facing the dominant monsoon wind, sampling of sheltered and marginal reef areas may be performed to glean a more representative spatial distribution and prevalence of less-exposed coral communities (e.g., Type IV) and degraded coral community types (e.g., Types V, VI, VII). Second, since the present study focused on shallow reef slopes, the typology could be expanded to include coral community types in other reef zones, such as reef flats, or across a range of depths within a reef. This expansion may improve estimates of the distribution and prevalence of coral community types and possibly lead to the identification of additional coral community types.
The study focused on hydrologic regimes; as such, natural sources of variation such as island and reef geomorphology, bathymetry, slope and aspect, and geologic history on an island scale may be examined in greater detail to identify their roles in the structuring of coral communities (Houk and Starmer 2010; Licuanan et al. 2019a, b). Identification of specific local stressors, both anthropogenic and non-anthropogenic (e.g., freshwater input), that affect the structuring of coral communities may also be performed to validate the possible roles of anthropogenic impacts hypothesized for Types V, VI, and VII. Lastly, findings from the successional vector analysis (Fig. 6) show that analysis of long-term monitoring data can be used to refine the typology by identifying coral community types that may be successional stages after chronic or episodic disturbance.
Implications for management
Corals are not homogeneously distributed across the Philippines. Figure 7 aims to summarize the findings of this study and illustrates how coral community types may be distributed at different spatial scales. This study reports that in that monsoon forcing and wave exposure account for some of the differentiation of coral community types at the scale of the Philippine archipelago (Table 4; Fig. 7a; see Fig. 2 for the actual distribution of community types). Local oceanographic features, orientation toward dominant monsoon wind, and station-level wave exposure may lead to the formation of different coral community types within the scale of an embayment (Fig. 7b) or an island (Fig. 7c). Further, the size and shape of an island or embayment may result in the presence of more than one coral community type along the same island or bay (Figs. 7b, c). The diagrams presented in Fig. 7 may be used as an initial guide for identifying coral community types within a location or a proposed protected seascape. However, the presence of these coral community types must be validated with reconnaissance visits and surveys.
Generalized spatial distribution of coral community types in Philippine fringing reefs at different orientations relative to the stronger monsoon wind and different degrees of wave exposure at the A scale of the Philippine archipelago, B embayment scale, and C island scale. The land is shaded in gray
The presence of degraded coral community types (e.g., Types V, VI, and VII) with high turf algae and macroalgal cover may indicate local disturbance and human-induced stressors in addition to broad-scale hydrologic regimes such as monsoons and wave exposure, which were primary determinants of coral community Types I, II, III, and IV identified in this study. Further, episodic disturbances such as mass coral bleaching and typhoon impacts may also lead to variations in coral composition, as exhibited by the subtypes of Type I (Fig. 6). The extent and spatial distribution of coral community types should be considered in designing MPAs and establishing reef monitoring stations to represent the various types adequately, especially in areas susceptible to episodic disturbance and where local stressors are prevalent. Monitoring temporal changes in coral community structure is also suggested to identify the effects of local stressors, human impacts, and episodic disturbance that may not be reflected in measurements of HCC and generic richness alone.
Refinements in data interpretation and analysis should accompany the inclusion of coral community structure in reef assessment and monitoring metrics. For instance, type-specific baselines for HCC, generic richness, and rank-abundance patterns allow for the development of more realistic management goals (e.g., Type II coral communities are not likely to have the HCC of Type III communities) and more appropriate restoration goals (e.g., empirical baselines for determining the appropriateness of passive or active management, identifying when active restoration is necessary). Using coral community structure as a metric for setting restoration goals would be helpful in the Philippines, where many coral gardening projects are attempted (Feliciano et al. 2018).
Lastly, the typology in this study may assist in the interpretation of reef assessment and monitoring data by local MPA managers and field technicians. These personnel may be trained to determine coral community types by identifying each type’s dominant taxa and relative abundances of key benthos (e.g., massive Porites, Acropora, Heliopora, macroalgae), as presented in this study. Type-specific scales for HCC, generic richness, and coral community structure may also be developed for evaluating the success of management efforts in a monitored reef. These evaluations may be standardized and simplified through tools such as comparable scorecards for reef status and decision trees for diagnosing drivers of change, as well as corresponding citizen science field methods (e.g., Licuanan et al. 2021).
References
Abaya LM, Wiegner TN, Beets JP, Colbert SL, Carlson KM, Kramer KL (2018) Spatial distribution of sewage pollution on a Hawaiian coral reef. Mar Pollut Bull 130:335-347. https://doi.org/10.1016/j.marpolbul.2018.03.028
Abesamis MRR, Ang JL, Robles RC, Licuanan WY (2022) Recovery of coral cover on inshore fringing reefs following mass coral bleaching in the Philippines. Coral Reefs. https://doi.org/10.1007/s00338-022-02322-y
Aliño PM, Gomez ED (1994) Philippine coral reef conservation: its significance to the South China Sea. In: Yamazato K, Ishijima S, Sakihara S, Taira H, Shimabukuro Z, Teruya F, Nishihira F (eds) Development and conservation in the Asia-Pacific Region: Proceedings of the Regional Conference of East-West Center Association: Regional Development in the 21st Century, Think Globally, Act Locally. The East-West Center Association Okinawa Chapter, Okinawa, pp 222–229
Anticamara JA, Go KTB (2017) Impacts of super-typhoon Yolanda on Philippine reefs and communities. Reg Environ Change 17:703-713. https://doi.org/10.1007/s10113-016-1062-8
Arceo HO, Quibilan MC, Aliño PM, Lim G, Licuanan WY (2001) Coral bleaching in Philippine reefs: coincident evidences with mesoscale thermal anomalies. B Mar Sci 69:579-93.
Bruno JF, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: Timing, extent, and subregional comparisons. PLoS One 2:e711. https://doi.org/10.1371/journal.pone.0000711
Cabrera OCC, Villanoy CL, Alabia ID, Gordon AL (2015) Shifts in chlorophyll-a off eastern Luzon, Philippines, associated with the North Equatorial Current bifurcation latitude. Oceanography 28:46-53. https://doi.org/10.5670/oceanog.2015.80
Cardenas MB, Zamore PB, Siringan FP, Lapus MR, Rodolfo RS, Jacinto GS, San Diego-McGlone ML, Villanoy CL, Cabrera O, Senal MI (2010) Linking regional sources and pathways for submarine groundwater discharge at a reef by electrical resistivity tomography, 222Rn, and salinity measurements. Geophys Res Lett 37:L16401. https://doi.org/10.1029/2010GL044066
Carpenter KE, Springer VG (2005) The center of the center of marine shore fish biodiversity: the Philippine Islands. Environ. Biol. Fishes 72:467-480. https://doi.org/10.1007/s10641-004-3154-4
Chang CP, Wang Z, McBride J, Liu CH (2005) Annual cycle of Southeast Asia-Maritime Continent rainfall and the asymmetric monsoon transition. J Clim 18:287-301. https://doi.org/10.1175/JCLI-3257.1
Chou LM (2000) Southeast Asian reefs – status update: Cambodia, Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. In: C Wilkinson (ed) Status of Coral Reefs of the World: 2000. Global Coral Reef Monitoring Network and Australian Institute of Marine Science, Townsville, pp 117–129
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378-1386. https://doi.org/10.1111/j.1461-0248.2012.01861.x
Darling ES, McClanahan TR, Côté IM (2013) Life histories predict coral community disassembly under multiple stressors. Glob Chang Biol 19:1930-1940. https://doi.org/10.1111/gcb.12191
DeVantier L, Turak E (2017) Species richness and relative abundance of reef-building corals in the Indo-West Pacific. Diversity 9:254. https://doi.org/10.3390/d9030025
DeVantier LM, De’ath G, Done TJ, Turak E (1998) Ecological assessment of a complex natural system: a case study from the Great Barrier Reef. Ecol Appl 8:480-496. https://doi.org/10.2307/2641088
DeVantier LM, De’ath G, Turak E, Done TJ, Fabricius KE (2006) Species richness and community structure of reef-building corals on the nearshore Great Barrier Reef. Coral Reefs 25:329-340. https://doi.org/10.1007/s00338-006-0115-8
DeVantier LM, Turak E, Szava-Kovats R (2020) Species richness and abundance of reef-building corals in the Indo-West Pacific: the local-regional relation revisited. Front Mar Sci 7:487. https://doi.org/10.3389/fmars.2020.00487
Done TJ (1982) Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1:95-107. https://doi.org/10.1007/BF00301691
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121-132. https://doi.org/10.1007/BF00008211
Done TJ (1999) Coral community adaptability to environmental change at the scales of regions, reefs, and reef zones. Amer Zool 39:66–79. https://www.jstor.org/stable/3884227
Done TJ, Potts DC (1992) Influences of habitat and natural disturbances on contributions of massive Porites corals to reef communities. Mar Biol 114:479-493. https://doi.org/10.1007/BF00350040
Fabricius KE, De’ath G, Puotinen ML, Done T, Cooper TF, Burgess SC (2008) Disturbance gradients on inshore and offshore coral reefs caused by a severe tropical cyclone. Limnol Oceanogr 53:690-704. https://doi.org/10.4319/lo.2008.53.2.0690
Faustino LA (1931) Coral reefs of the Philippine islands. Philipp J. Sci. 44:291-308.
Feliciano GNR, Mostrales TPI, Acosta AKM, Luzon KS, Bangsal JCI, Licuanan WY (2018) Is gardening corals of opportunity the appropriate response to reverse Philippine reef decline? Restor. Ecol. 26:1091-1097. https://doi.org/10.1111/rec.12683
Fellowes TE, Vila-Concejo A, Gallop SL (2019) Morphometric classification of swell-dominated embayed beaches. Mar Geol 411:78-87. https://doi.org/10.1016/j.margeo.2019.02.004
Flower J, Ortiz JC, Chollett I, Abdullah S, Castro-Sanguino C, Hock K, Lam V, Mumby PJ (2017) Interpreting coral reef monitoring data: A guide for improved management decisions. Ecol. Indic. 72:848-869. https://doi.org/10.1016/j.ecolind.2016.09.003
Goodkin NF, Switzer AD, McCorry D, DeVantier L, True JD, Hughen KA, Angeline N, Yang TT (2011) Coral communities of Hong Kong: long-lived corals in a marginal reef environment. Mar Ecol Prog Ser 426:185-196. https://doi.org/10.3354/meps09019
Gordon AL, Sprintall J, Field A (2011) Regional oceanography of the Philippine archipelago. Oceanography 24:14-27. https://doi.org/10.5670/oceanog.2011.01
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, Volume 1. 9pp. https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hawkins JP, Roberts CM (1993) Effects of recreational scuba diving on coral reefs: Trampling on reef-flat communities. J Appl Ecol 30:25-30. https://doi.org/10.2307/2404267
Hongo C, Kayanne H (2009) Holocene coral reef development under windward and leeward locations at Ishigaki Island, Ryukyu Islands, Japan. Sediment Geol 214:62-73. https://doi.org/10.1016/j.sedgeo.2008.01.011
Hongo C, Kawamata H, Goto K (2012) Catastrophic impact of typhoon waves on coral communities in the Ryukyu Islands under global warming. J. Geophys. Res. Biogeosci. 117:G02029. https://doi.org/10.1029/2011JG001902
Houk P, Starmer J (2010) Constraints on the diversity and distribution of coral-reef assemblages in the volcanic Northern Mariana Islands. Coral Reefs 29:59-70. https://doi.org/10.1007/s00338-009-0545-1
Houk P, van Woesik R (2010) Coral assemblages and reef growth in the Commonwealth of the Northern Mariana Islands (Western Pacific Ocean). Mar Ecol 31:318-329. https://doi.org/10.1111/j.1439-0485.2009.00301.x
Hughes TP (1994) Catastrophes, phase shifts and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551. https://doi.org/10.1126/science.265.5178.1547
Hughes TP, Kerry JT, Alvarez-Noriega M, et al. (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373-377. https://doi.org/10.1038/nature21707
Jackson JBC (1991) Adaptation and diversity of reef corals. BioScience 41:476-482. https://doi.org/10.2307/1311805
Karlson RH & Hurd LE (1993) Disturbance, coral reef communities, and changing ecological paradigms. Coral Reefs 12:117-125. https://doi.org/10.1007/BF00334469
Kayanne H, Harii S, Ide Y, Akimoto F (2002) Recovery of coral populations after the 1998 bleaching on Shiraho Reef, in the southern Ryukyus, NW Pacific. Mar Ecol Prog Ser 239:93-103. https://doi.org/10.3354/meps295091
Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269. https://doi.org/10.1016/j.cageo.2005.11.009
Lapointe BE, Barile PJ, Littler MM, Littler DS (2005) Macroalgal blooms on southeast Florida coral reefs: II. Cross-shelf discrimination of nitrogen sources indicates widespread assimilation of sewage nitrogen. Harmful Algae 4:1106-1122. https://doi.org/10.1016/j.hal.2005.06.002
Licuanan WY, Gomez ED (1988) Coral reefs of the northwestern Philippines: a physiognomic-structural approach. Proc 6th Int Coral Reef Symp 1:275–280
Licuanan WY, Gomez ED (2000) Philippine coral reefs: Status and the role of the academe to improve their management. Proc 9th Int Coral Reef Symp 1:835–840
Licuanan WY, Robles R, Reyes M (2019a) Status and recent trends in coral reefs of the Philippines. Mar Pollut Bull 142:544-550. https://doi.org/10.1016/j.marpolbul.2019.04.013
Licuanan WY, Cabreira RW, Aliño PM (2019b) The Philippines. In: Sheppard C (ed) World seas: an environmental evaluation, Indian Ocean to the Pacific, volume II. Academic Press, London, pp 515-537
Licuanan WY, Mordeno PZB, Go MV (2021) C30 – a simple, rapid, scientifically valid, and low-cost method for citizen-scientists to monitor coral reefs. Reg. Stud. Mar. 101961. https://doi.org/10.1016/j.rsma.2021.101961
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and losers. Ecol Lett 4:122-131. https://doi.org/10.1046/j.1461-0248.2001.00203.x
Magdaong ET, Fujii M, Yamano H, Licuanan WY, Maypa A, Campos WL, Alcala AC, White AT, Apistar D, Martinez R (2014) Long-term change in coral cover and effectiveness of marine protected areas in the Philippines: a meta-analysis. Hydrobiologia 733:5-17. https://doi.org/10.1007/s10750-013-1720-5
McCune B, Mefford MJ (2011) PC-ORD. Multivariate analysis of ecological data. MjM Software, Oregon.
McManus JW, Meñez LAB, Kesner-Reyes KN, Vergara SG, Ablan MC (2000) Coral reef fishing and coral-algal phase shifts: implications for global reef status. ICES J. Mar. Sci. 57:572-578. https://doi.org/10.1006/jmsc.2000.0720
Montaggioni LF (2005) History of Indo-Pacific coral reef systems since the last glaciation: development patterns and controlling factors. Earth Sci Rev 71:1-75. https://doi.org/10.1016/j.earscirev.2005.01.002
Muir PR, Marshall PA, Abdulla A, Aguirre JD (2017) Species identity and depth predict bleaching severity in reef-building corals: shall the deep inherit the reef? Proc Biol Sci 284:20171551. https://doi.org/10.1098/rspb.2017.1551
Pandolfi JM, Jackson JBC (2006) Ecological persistence interrupted in Caribbean coral reefs. Ecol Lett 9:818-826. https://doi.org/10.1111/j.1461-0248.2006.00933.x
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958. https://doi.org/10.1126/science.1085706
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun 4:1402. https://doi.org/10.1038/ncomms2409
Philippine Statistics Authority (2020) Census of Population and Housing: Statistical Tables. https://psa.gov.ph/population-and-housing/statistical-tables
Quibilan MCC, Aliño PM (2006) Coral community structure of western Philippine reefs I: Spatial patterns. Proc 9th Int Coral Reef Symp 1:341–350
Quimpo TJR, Requilme JNC, Gomez EJ, Sayco SLG, Tolentino MPS, Cabaitan PCC (2020) Low coral bleaching prevalence at the Bolinao-Anda Reef Complex, northwestern Philippines during the 2016 thermal stress event. Mar Pollut Bull 160:111567. https://doi.org/10.1016/j.marpolbul.2020.111567
Reyes M, Robles R, Licuanan W (2022) Multi-scale variation in coral reef metrics on four Philippine reef systems. Reg. Stud. Mar. 102310. https://doi.org/10.1016/j.rsma.2022.102310
Riegl B, Riegl A (1996) Studies on coral community structure and damage as a basis for zoning marine reserves. Biol Conserv 77:269-277. https://doi.org/10.1016/0006-3207(95)00138-7
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202. https://doi.org/10.3354/meps062185
Samson MR, Licuanan WY (2002) The socio-economic conditions of the coastal communities and coastal resource management along the Pacific coast of the Philippines. Philippine Journal of Natural Sciences 1&2:169-178
Senal MIS, Jacinto GS, San Diego-McGlone ML, Siringan F, Zamora P, Soria L, Cardenas MB, Villanoy C, Cabrera O (2011) Nutrient inputs from submarine groundwater discharge on Santiago reef flat, Bolinao, Northwestern Philippines. Mar Pollut Bull 62:195-200. https://doi.org/10.1016/j.marpolbul.2011.05.037
Shen C, Siringan FP, Lin K, Dai C, Gong S (2010) Sea-level rise and coral-reef development of Northwestern Luzon since 9.9ka. Palaeogeogr Palaeoclimatol Palaeoecol 292:465-473. https://doi.org/10.1016/j.palaeo.2010.04.007
Spalding MD, Ravilious C, Green EP (2001) World Atlas of Coral Reefs. University of California Press, Berkeley.
Tagaki H, Esteban M (2016) Statistics of tropical cyclone landfalls in the Philippines: unusual characteristics of 2013 Typhoon Haiyan. Nat Hazards 80:211-222. https://doi.org/10.1007/s11069-015-1965-6
Tamayo NCA, Anticamara JA, Acosta-Michlik L (2018) National estimates of values of Philippine Reefs’ Ecosystem Services. Ecol Econ 146:633-644. https://doi.org/10.1016/j.ecolecon.2017.12.005
Trapon ML, Pratchett MS, Penin L (2011) Comparative effects of different disturbances in coral reef habitats in Moorea, French Polynesia. Hindawi Journal of Marine Sciences 2011:807625. https://doi.org/10.1155/2011/807625
Tun K, Chou LM, Low J, Yeemin T, Phongsuwan N, Setiasih N, Wilson J, Amri AY, Adzis KAA, Lane D, van Bochove JW, Kluskens B, van Long N, Si Tuan V, Gomez E (2010) A regional overview on the 2010 coral bleaching event in southeast Asia. In: Status of Coral Reefs in East Asian Seas Region: 2010, Ministry of the Environment, Tokyo, pp 9–27
Uychiaoco AJ, Aliño PM, Bermas NA (1992) Some structuring forces and distribution patterns of coral and benthic lifeform communities in the Philippines. Proc 7th Int Coral Reef Symp 1:575–579
van Woesik R, Done TJ (1997) Coral communities and reef growth in the southern Great Barrier Reef. Coral Reefs 16:103-115. https://doi.org/10.1007/s003380050064
Veron JEN (2000). Corals of the World (Volumes 1–3). Australian Institute of Marine Science, Townsville, 1410pp
Villanoy C, David L, Cabrera O, Atrigenio M, Siringan F, Aliño P, Villaluz M (2012) Coral reef ecosystems protect shore from high-energy waves under climate change scenarios. Clim Change 112:493-505. https://doi.org/10.1007/s10584-012-0399-3
Villanoy CL, Salamante E, Cabrera O (2013) Chapter 3 – Exposure: waves and storm surges. In: Vulnerability assessment tools for coastal ecosystems: a guidebook. Marine Environment and Resources Foundation Inc., Quezon City, pp 44-54
White AT, Vogt HP, Arin T (2000) Philippine coral reefs under threat: the economic losses caused by reef destruction. Mar. Pollut. Bull. 40:598-605. https://doi.org/10.1016/S0025-326X(00)00022-9
Yumul GP, Dimalanta CB, Maglambayan VB, Marquez EJ (2008) Tectonic setting of a composite terrane: A review of the Philippine island arc system. J Geosci 12:7. https://doi.org/10.1007/s12303-008-0002-0
Acknowledgements
We thank the Department of Science and Technology—Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (DOST-PCAARRD) for funding the National Assessment of Coral Reef Environments Project 1 (QSR-MR-COR03.001) and the Acidification Impacts on the Demography of Corals Project 4 (QMSR-MRRD-MEC-295-1450). We also thank the Department of Environment and Natural Resources’ Philippine Coral Reef and Mangrove Remote Sensing Project and Coral Reef Visualization and Assessment Project for financing part of the assessments. We thank the current and past members of the DLSU-SHORE Center for their contributions to the projects. Dr. OC Cabrera’s insights were instrumental in improving the study. This study is part of the masters’ thesis of GNR Feliciano, who was a graduate scholar of the DOST-PCAARRD Graduate Research and Education Assistantship for Technology Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
338_2023_2391_MOESM1_ESM.xlsx
Sheet 1: Coral composition data (% cover of hard coral taxonomic amalgamation units (TAUs) for the 206 sampling stations); Sheet 2: Data sources and explanation of values for the environmental parameters used in the canonical correspondence analysis (CCA); Sheet 3: Environmental data of the 206 assessment stations used in the CCA; Sheet 4: List of 59 hard coral TAU codes and their descriptions; Sheet 5: List of hard coral TAUs for each coral community type arranged according to rank abundance; Sheet 6: PCoA ordination plots with Eigenvalues and percent variance explained of (1) 204 sampling stations grouped into seven hard coral community types and (2) 204 sampling stations grouped into seven hard coral community types including succession vectors of the four monitoring stations (XLSX 389 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feliciano, G.N.R., Rollon, R.N. & Licuanan, W.Y. Coral community structure of Philippine fringing reefs is shaped by broad-scale hydrologic regimes and local environmental conditions. Coral Reefs 42, 873–890 (2023). https://doi.org/10.1007/s00338-023-02391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02391-7