Abstract
Advances in our knowledge of eastern tropical Pacific (ETP) coral reef biogeography and ecology during the past two decades are briefly reviewed. Fifteen ETP subregions are recognized, including mainland and island localities from the Gulf of California (Mexico) to Rapa Nui (Easter Island, Chile). Updated species lists reveal a mean increase of 4.2 new species records per locality or an overall increase of 19.2 % in species richness during the past decade. The largest increases occurred in tropical mainland Mexico, and in equatorial Costa Rica and Colombia, due mainly to continuing surveys of these under-studied areas. Newly discovered coral communities are also now known from the southern Nicaraguan coastline. To date 47 zooxanthellate scleractinian species have been recorded in the ETP, of which 33 also occur in the central/south Pacific, and 8 are presumed to be ETP endemics. Usually no more than 20–25 zooxanthellate coral species are present at any given locality, with the principal reef-building genera being Pocillopora, Porites, Pavona, and Gardineroseris. This compares with 62–163 species at four of the nearest central/south Pacific localities. Hydrocorals in the genus Millepora also occur in the ETP and are reviewed in the context of their global distributions. Coral community associates engaged in corallivory, bioerosion, and competition for space are noted for several localities. Reef framework construction in the ETP typically occurs at shallow depths (2–8 m) in sheltered habitats or at greater depths (10–30 m) in more exposed areas such as oceanic island settings with high water column light penetration. Generally, eastern Pacific reefs do not reach sea level with the development of drying reef flats, and instead experience brief periods of exposure during extreme low tides or drops in sea level during La Niña events. High rates of mortality during El Niño disturbances have occurred in many ETP equatorial areas, especially in Panama and the Galápagos Islands during the 1980s and 1990s. Remarkably, however, no loss of resident, zooxanthellate scleractinian species has occurred at these sites, and many ETP coral reefs have demonstrated significant recovery from these disturbances during the past two decades.
By consensus, authors are arranged alphabetically after lead author P. W. Glynn
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Geographic isolation, variable oceanographic conditions, and a peninsula-like shallow shelf spanning a relatively narrow latitudinal corridor, have all figured prominently in the formation and development of the eastern tropical Pacific coral reef region. The vast majority of eastern Pacific corals belong to species occurring in the central and south Pacific (e.g., Wells 1983; Cortés 1986; Grigg and Hey 1992; Veron 1995; Glynn and Ault 2000). Dana (1975) recognized this faunal affinity, and suggested the existence of genetic connectivity between the central Pacific Line Islands and the equatorial eastern Pacific by larval dispersal via the North Equatorial Counter Current (NECC). A few species are eastern Pacific endemics, and there is a near absence of Caribbean and western Atlantic species along western American shores. The narrowing and eventual closing of the Central American isthmus, 3.5-3.0 Ma (Coates and Obando 1996), brought about extinctions of once-shared transoceanic species, and the invasion of coral species from the west by long-distance dispersal (see Chap. 2, López-Pérez, and Chap. 16, Lessios and Baums; López-Pérez and Budd 2009).
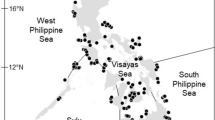
The occurrence of reef-building corals along coastal western America is highly skewed toward the northern hemisphere, with a strong presence of corals from the northern Gulf of California, Mexico (~30°N) to just a few degrees south of the equator along coastal Ecuador (2°S). Modern zooxanthellate coral communities have not been reported on continental shores south of the Gulf of Guayaquil, Ecuador, which are under the influence of the cool, north-flowing Peru Coastal Current (Fig. 5.1). Five relatively isolated oceanic island groups, from the northern-most Revillagigedo Islands off Mexico to the Galápagos Islands lying astride the equator, also belong to the eastern Pacific coral reef region. Vibrant coral communities and incipient reef development also appear far to the south and west, about 4000 km from the Galápagos Islands and coastal Ecuador. Rapa Nui (Easter) and Salas y Gómez Islands, at 27°S and over 1000 km west of Chile, represent the most isolated ETP coral outpost (Glynn et al. 2003, 2007; Hubbard and Garcia 2003; see Chap. 6, Toth et al.).
The most recent overview of the eastern tropical Pacific (ETP) coral reef region was published over 10 years ago, in Latin American Coral Reefs edited by Jorge Cortés (2003). This work comprised seven chapters dedicated to the ETP, describing coral communities and coral reefs from mainland Mexico to Ecuador, including offshore islands from the Revillagigedo Islands, Mexico to Rapa Nui, Chile. These summaries included historical sketches of coral reef research, site descriptions, species lists, natural and anthropogenic disturbances, and issues pertaining to protection and management. The present chapter updates Cortés (2003), and several earlier reviews of eastern Pacific coral reef biology (e.g., Durham and Barnard 1952; Squires 1959; Durham 1966; Glynn and Wellington 1983; Cortés 1986, 1997, 2011; Glynn 2001).
An updated biogeographic analysis of zooxanthellate Scleractinia is examined first to review species relationships within the eastern Pacific region, and secondly extra-regional faunal affinities. This analysis will serve as a framework for brief introductions to the main coral reef localities from Mexico to Ecuador, including the Galápagos Islands. An overview of the more distant eastern tropical Pacific coral outposts of Rapa Nui and Salas y Gómez Islands will conclude the chapter. Eastern Pacific Millepora records are also reviewed, and compared to hydrocoral occurrences in the central and southeastern Pacific. Each eastern Pacific coral reef locality is examined in terms of its community structure, species richness , physical environmental conditions, and chief ecological interactions. The treatments are admittedly unbalanced, with more information provided for newly discovered or less well-known areas. It is hoped that this overview will serve to stimulate further research, and provide information for comparisons with other coral reef regions. A comprehensive review of ETP Holocene reef development—including coral framework structures, time of reef growth initiation, thicknesses, and accumulation rates—can be found in Toth et al., Chap. 6.
5.2 Eastern Pacific Coral Reef Region
The eastern tropical Pacific region consists of a distinct biotic assemblage of marine species isolated from the Caribbean Sea by the Central American land bridge. The west American marine biota is also separated from the central/western Pacific region by a wide expanse (5000–8000 km) of open ocean waters, namely the eastern Pacific Barrier or Ekman’s Barrier (e.g., Darwin 1880; Ekman 1953; Briggs 1974; Grigg and Hey 1992; Veron 1995). Abundant recent evidence indicates that this barrier, which Darwin considered impassable, is best viewed as a filter bridge since numerous shallow-living species from several major taxa—scleractinian corals (Glynn and Ault 2000), bryozoans (Banta 1991), crustaceans (Garth 1991), molluscs (Scheltema 1988; Emerson 1991), echinoderms (Lessios et al. 1998), and fishes (Robertson and Allen 1996; Robertson et al. 2004; Lessios and Robertson 2006; see Chap. 16, Lessios and Baums)—have crossed this deep water stretch by larval dispersal or rafting. In particular, many marine species shared between the eastern Pacific and central/western Pacific are common members of coral communities and coral reefs. Indeed, the majority of ETP reef-building coral taxa—species in the genera Pocillopora, Porites, Pavona, Gardineroseris—are conspecific with those found west of the ETP filter bridge.
Within the eastern Pacific region, four subtropical/tropical biogeographic provinces were early recognized: (1) Cortez Province (middle to upper Gulf of California), (2) Mexican Province (lower Gulf of California to the Gulf of Tehuantepec), (3) Panamanian Province (Tangola-Tangola Bay, Gulf of Tehuantepec to the Gulf of Guayaquil), and (4) the Galápagos Islands (Briggs 1974). More recent analyses of coral distributions have recognized an eastern Pacific oceanic islands fauna, with coral affinities shared among Clipperton Atoll, Malpelo Island, Easter Island, and Tabuaeran (=Fanning Atoll) in the Line Islands group (Glynn and Ault 2000; Glynn et al. 2007). Another recent biogeographic analysis, based on the distributions of 1135 resident shore fishes, also recognized the oceanic islands as a distinct faunal subdivision, but concluded that the continental coast is best represented by two provinces, namely (1) the Cortez, comprising the Gulf of California and lower Pacific Baja California areas, and (2) the Panamic, southward to the Gulf of Guayaquil, Ecuador (Robertson and Cramer 2009).
With an increasing knowledge of zooxanthellate scleractinian distributions, particularly during the last two decades, several workers have examined patterns of coral faunal distributions along the eastern tropical Pacific (Glynn 1997; Glynn and Ault 2000; Glynn et al. 2007; Alvarado et al. 2011), and within specific eastern Pacific countries and locations (Reyes-Bonilla 1993a; chapters in Cortés 2003; Reyes-Bonilla and López-Pérez 1998, 2009). The existence of a dispersal barrier within the eastern Pacific, the long-recognized Pacific Central American faunal gap (PCAFG), has been supported mainly by the absence of corals (Glynn and Ault 2000) and reef-associated fishes (Springer 1958; Briggs 1974) between Tangola Tangola (southern Mexico) south to the Gulf of Fonseca (El Salvador/Honduras/Nicaragua). However, the faunal similarity of newly discovered coral communities and reef-associated fishes in Nicaragua and El Salvador with central Mexican coastal areas (Michoacán, Colima) is very high, suggesting some degree of genetic connectivity across the PCAFG (Robertson and Cramer 2009; Alvarado et al. 2011).
5.2.1 Zooxanthellate Scleractinian Fauna
Here we present an updated biogeographic analysis of zooxanthellate scleractinian coral faunas from 15 ETP localities and four central/south Pacific localities. In the present data-base, the listed ETP fauna consists of 47 species in 11 genera, and the central/south Pacific localities 225 species in 40 genera (Appendix). The central/south Pacific localities include the Hawaiian Islands, Johnston Atoll, Pitcairn Islands, and Palmyra Atoll, and will be employed for analysis of and comparison with the ETP fauna. The species in this analysis were delineated chiefly from gross morphometric characteristics; the sources of this information are listed in the caption to Fig. 5.7. In light of current molecular genetic studies (Flot et al. 2010; Pinzón and LaJeunesse 2011; Pinzón et al. 2013; Boulay et al. 2014; see Chap. 14, Pinzón), and evidence from a combination of genetic and morphological analyses—including both macro- and micromorphometric characteristics (Schmidt-Roach et al. 2014)—it is evident that species delineations across the Indo-Pacific region are still unresolved. On the one hand, genetic studies offer evidence of fewer species of Pocillopora in the ETP than recognized morphometrically, three instead of seven species (Pinzón et al. 2013; see Chap. 14, Pinzón). On the other hand, new previously unrecognized cryptic species have come to light in the Pocillopora damicornis complex on the Great Barrier Reef (Schmidt-Roach et al. 2014).
A major three-way grouping of coral faunas is evident from an agglomerative hierarchical cluster analysis: (a) dominantly equatorial eastern Pacific (EEP) localities (PAN-GLN), (b) ETP oceanic islands (MAL-REV), and (c) central/southern Pacific localities (Fig. 5.2). [Locality acronyms, e.g. PAN = Panama, follow those employed in Fig. 5.2.] A notable east to west increase in species richness is apparent with eastern Pacific localities demonstrating impoverished faunas compared with potential source areas to the west of Ekman’s filter bridge, a long recognized pattern (e.g., Stehli and Wells 1971; Veron 1995). All ETP reef communities consist of fewer than 30 species , whereas central/south Pacific localities exhibit from 44 to 163 species. At Palmyra Atoll, which is in the path of the North Equatorial Counter Current (Maragos et al. 2008a, b), 163 coral species have been recorded. The North Equatorial Counter Current has been considered a likely means of transport across the filter bridge (e.g., Dana 1975; Glynn and Ault 2000), and species-rich Palmyra is a potential source locality. In the EEP cluster, the low species occurrences in the southern Galápagos Islands (GLS) and Gulf of California (GOC) are likely due, in large part, to their subtropical locations. Two species-poor mainland sites (NIC, ELS) cluster with the ETP oceanic islands. The recently-discovered coral communities in Nicaragua may reveal additional species upon further study; El Salvador, however, is a marginal coral area with highly turbid waters and is not expected to show a significant increase in species richness . Additional comments on ETP faunal relationships are noted below, in site-specific sections.
a Relationships of zooxanthellate scleractinian coral faunas at 15 eastern Pacific and four central and south Pacific localities. “Spatial patterns” of an agglomerative, hierarchical cluster analysis were portrayed using a Euclidean distance measure and group-average method for cluster formation. Constructed from presence/absence data in Appendix. b Geographic pattern of coral species richness across EP filter bridge in relation to NECC eastward flow. Circle areas are proportional to species richness. GOC, Gulf of California; TMM, tropical Mexican mainland; REV, Revillagigedo Islands; CLP, Clipperton Atoll; ELS, El Salvador; NIC, Nicaragua; CRM, Costa Rican mainland; COC, Cocos Island; PAN, Panama; COL, Colombia; MAL, Malpelo Island; ECD, Ecuador; GLS, southern Galápagos Islands; GLN, northern Galápagos Islands; RPN, Rapa Nui (Easter) and Salas y Gómez Islands; HAW, Hawaiian Islands; JOH, Johnston Island; PIT, Pitcairn (Henderson, Oeno, Ducie Islands); PAL Palmyra Atoll
Eight species in this updated inventory are likely ETP endemics, suggesting a moderate level of speciation in this region (Table 5.1). This number represents 19.5 % of the total ETP fauna reported to date. Latitudinally, all eight coral endemics are concentrated in Mexico, in the northern-most eastern Pacific region. Three Porites endemics, P. arnaudi, P. sverdrupi, and P. baueri, demonstrate restricted distributions, known from only one or two localities. All eastern Pacific endemic species are absent from Rapa Nui (Easter Island). Three endemics, Pavona gigantea, Pocillopora effusus, and Porites panamensis, are widely distributed throughout the eastern Pacific, presently reported from eight to 14 localities.
5.2.2 Millepora
Zooxanthellate hydrocorals in the genus Millepora can be important contributors to reef frameworks and coral reef growth. Millepora has a very restricted distribution in the ETP. Three species of Millepora were present on reefs in the Gulf of Chiriquí (Panama) before the 1982–83 El Niño event: Millepora intricata Milne-Edwards 1857, Millepora boschmai Weerdt and Glynn 1991, and Millepora platyphylla Hemprich and Ehrenberg 1834. All three species bleached and experienced high mortality, during and immediately following the period of elevated sea temperatures (Glynn and de Weerdt 1991). Living colonies of M. platyphylla could no longer be found in Panama after 1983 (as of 2015), and are now apparently absent from the ETP (Glynn 2011). Millepora boschmai also disappeared from survey sites at Uva Island after 1983, but a small population was found nearby in 1992 (Glynn and Feingold 1992). All colonies from this remnant population disappeared in early 2000, as well as a few known colonies at Coiba Island (Brenes et al. 1993), and none have been observed in the eastern Pacific since that time (Maté 2003). This species was recognized in collections made from two localities in Indonesia by Razak and Hoeksema (2003), including South Sulawesi in 1994 and Sumba in 1984. In Panama, Millepora intricata re-appeared a few years after 1983, ostensibly by sexual recruitment from deep refuge populations (Smith et al. 2014; see Chap. 17, Smith et al.); it is again an important member of coral communities in the Gulf of Chiriquí, Panama (Fig. 5.3). Unlike M. platyphylla and M. boschmai, with populations restricted to shallow habitats (usually ≤6–8 m depth), M. intricata occurs in deep (>11 m depth) as well as shallow reef zones. This allowed M. intricata in deep-water habitats to survive two of the strongest El Niño events on record (1982–83, 1997–98), and then to re-populate shallow reef sites (Smith et al. 2014).
Millepora exaesa is known only from Clipperton Atoll in the eastern Pacific, and colonies are uncommon there (Glynn et al. 1996a, b; Carricart-Ganivet and Reyes-Bonilla 1999; Flot and Adjeroud 2009; Glynn, unpublished observation). The nearest reported central Pacific record of M. exaesa is at Wake Island (167°E), over 5500 km west of Clipperton (Table 5.2).
Early surveys showed that Millepora spp. are widespread throughout the Indo-Pacific region, and this has been confirmed by recent surveys. However, their relatively infrequent reproduction and short-lived dispersive phase (gonochoric medusae) appear inconsistent with their widespread global occurrence (Lewis 1989; see also Chap. 15, Glynn et al.). Veron (2000) shows Millepora present in all parts of the Indian Ocean and the western and southern Pacific (Fig. 5.4). Some early and more recent surveys show that various Millepora species also occur widely across the central Pacific region, e.g. Johnston Atoll (Maragos and Jokiel 1986), the Phoenix Islands (Maragos et al. 2008a, b), Tabuaeran (formerly Fanning, Maragos 1974) and other Line Islands (Maragos et al. 2008a, b); Marquesas (Chevalier 1978), Tuamotus (Ricard 1985; Harmelin-Vivien 1985; Montaggioni 1985), Society Islands (Galzin and Pointier 1985), and the Pitcairn Islands (Irving and Dawson 2012; USNM collection records) (Fig. 5.4; Table 5.2). Subfossil fragments of Millepora intricata recovered from reef cores revealed radiocarbon dates of ~5000 cal year BP, indicating the presence of this hydrocoral species in Panama for much of the Holocene Epoch (Smith et al. 2014). The highly restricted occurrence of Millepora in the ETP is mirrored in the eastern Atlantic where it is known from only two island groups, the Canary and Cape Verde Islands (Clemente et al. 2011). It is hypothesized to be a recent introduction to the eastern Atlantic region (Clemente et al. 2011).
Known global distribution of Millepora spp. as of 2014. In the eastern Pacific: Me, M. exaesa; Mp, M. platyphylla; Mb, M. boschmai; Mi, M. intricata. Mp and Mb now regionally extinct. Eastern Atlantic, Canary and Cape Verde Islands, Millepora sp., possibly M. alcicornis. Cross hatching in south-central Pacific denotes records of Millepora occurrences in Line Islands and Pitcairn Islands
5.3 Intra-regional Précis
Coral faunas and coral communities within the eastern tropical Pacific region are reviewed below, from northern Mexico to the southern-most mainland assemblages of Ecuador. The eastern Pacific oceanic islands—Revillagigedo Islands, Clipperton Atoll, Isla del Coco, Galápagos Islands, and Rapa Nui—are also considered along a N–S latitudinal gradient. This overview will hopefully provide basic coral community-level information to help evaluate intra-regional similarities and differences. We recognize that these brief intra- and inter-regional treatments would reveal only approximate comparisons at best due to the often great differences in available habitat area and habitat diversity. Regional Holocene reef development, including the coral taxa that construct frameworks, times of reef initiation, framework thickness, and accumulation rates are reviewed by Toth et al. in Chap. 6.
5.3.1 Gulf of California (Mexico)
The northern-most limit of zooxanthellate corals is in the upper reaches of the 1000 km-long Gulf of California (Fig. 5.5). Corals occur commonly over most of the length of the gulf, with increasing species richness in the southerly warmer waters. The higher thermal conditions in the southern gulf are exemplified by satellite imagery of sea surface temperatures (SSTs) in January 2006, with upper gulf SSTs of 14.5–18.3 °C and lower gulf SSTs of 20.1–22.8 °C (Ledesma-Vázquez et al. 2009). Corals are virtually absent from the western shores of the Baja California peninsula due to the southerly-flowing, cool California Current (see Chap. 3, Fiedler and Lavín). Distributional analyses based on presence/absence data have revealed two coral faunas within the Gulf of California, one in the northern and one in the southern gulf (Reyes-Bonilla and López-Pérez 2009). The northern fauna ranges from Punta Peñasco (31.3°N) to Punta Prieta (27°N), and consists predominantly of encrusting, monospecific Porites panamensis populations. Occasional colonies of Porites sverdrupi are also present on rocky substrates, and Cycloseris curvata, the latter in deeper rhodolith communities. The southern fauna ranges from Loreto to Cabo San Lucas. This fauna is more diverse, consisting of 20 species with a close affinity with tropical mainland Mexico (Fig. 5.2). According to recent assessments, the gulf coral fauna has demonstrated an increase from 16 to 20 species (e.g., Reyes-Bonilla et al. 2005, 2010; Reyes-Bonilla and López-Pérez 2009) (Fig. 5.6). Sixteen of these species belong to the Indo-Pacific fauna, three to the eastern Pacific fauna, and one (P. sverdrupi) is endemic to the gulf and mainland Mexico.
Differences in zooxanthellate scleractinian coral species richness at eastern and central Pacific localities before (blue) and after (red) major surveys. Most ‘previous’ records were published in 2003 (Cortés 2003), but some are from major works as early as the latter half of the 1980s. Single bars (ELS, NIC, PAL) denote a lack of data from before the time periods examined. Sources Previous/Current GOC 1/2; TMM 1/2,3; REV 1/2; CLP 4/3,4; ELS -/5; NIC -/6; CRM 7,8,9/3,10; COC 8/3,10; PAN 11, 12/3,12; COL 13/14; MAL 10,13/12,14; ECD 15/16; GLS 15/17; GLN 15/3,4,18; RPN 19/4,20; HAW 21/22,23; JOH 24/25,26; PIT 27/28; PAL -/29. (1) Reyes-Bonilla (2003); (2) Reyes-Bonilla et al. (2010); (3) Boulay et al. (2013); (4) Glynn et al. (2007); (5) Reyes-Bonilla and Barraza (2003); (6) Alvarado et al. (2010), (2011); (7) Cortés and Guzmán (1998); (8) Cortés and Jiménez (2003); (9) Jiménez (2007a, b); (10) Cortés (updated checklist); (11) Holst and Guzmán (1993), Maté (2003); (12) Maté (updated checklist); (13) Zapata and Vargas-Ángel (2003); (14) Zapata (updated checklist); (15) Glynn (2003); (16) Rivera and Martínez (2011); (17) Glynn (updated checklist); (18) Hickman (2008); (19) Glynn et al. (2003); (20) Wieters and Navarrete (updated checklist); (21) Maragos (1995); (22) Maragos (updated checklist); (23) Maragos et al. (2004); (24) Maragos and Jokiel (1986); (25) Lobel and Lobel (2008); (26) Maragos (updated checklist); (27) Paulay (1989); (28) Irving and Dawson (2012); (29) Williams et al. (2008), Maragos (updated checklist)
The coral assemblages and associated biota in the gulf were classified into four biotopes by Reyes-Bonilla and López-Pérez (2009): (1) isolated colonies or patches, (2) corals in rhodolith communities and other soft bottom habitats, (3) coral communities, and (4) coral reefs. Biotopes 1 and 2 occur in the northern gulf, and coral communities and coral reefs in the southern gulf. Corals, predominantly Porites panamensis, are highly dispersed in type 1 assemblages, usually amounting to <1 % cover where present. Even at low abundance, however, Halfar et al. (2005) demonstrated a potentially high carbonate sediment production rate for P. panamensis at Bahía de Los Ángeles (Fig. 5.5). Only a single rhodolith community, with associated fungiid corals, was reported in the northern gulf, while five were reported in the southern gulf. Three of these communities occurred at 25–30 m depth between La Paz and Monserrat Island near Loreto. Diaseris distorta is predominant with the occasional presence of Cycloseris curvata and mobile species of Psammocora, Porites, and rarely Pavona (Table 5.3). Present taxonomic evidence indicates that the coralline flora of rhodolith communities consists of four species in four genera (Steller et al. 2009). This biotope provides shelter and trophic resources for a diverse assemblage of non-coral invertebrates (bryozoans, molluscs, crustaceans, and echinoderms), fishes, and cryptic biota (Steller et al. 2003, 2009).
Coral community biotopes occur commonly at shallow depths (≤10 m) along the southwestern peninsular gulf coast. The predominant coral genera in these communities are Pocillopora and Pavona. While buildups can attain several meters in thickness (height), framework structures per se are not evident. Particularly robust coral communities, e.g. at Cabo Pulmo and La Paz, demonstrated mean coral cover values of 15–20 % with 4–6 species present. Both Pocillopora damicornis and Pocillopora verrucosa contribute substantially to these communities. The structural complexity of these coral communities offers numerous shelter sites that greatly increase the diversity of macroinvertebrates, fishes, and cryptic biota. In addition, metazoan coral symbionts and diverse coral bioeroding organisms inhabit these communities. Gastropod, echinoderm, and fish corallivores are ubiquitous in the gulf and actively prey on all coral species (e.g., Reyes-Bonilla 2003; Hendrickx et al. 2005; Álvarez-Filip et al. 2006; Reyes-Bonilla and López-Pérez 2009). Acanthaster planci is widespread, especially in the lower half of the gulf where corals are in high abundance (Dana and Wolfson 1970; Barham et al. 1973). Reyes-Bonilla (1993a, b, 2003) reported relatively high population densities of A. planci at Cabo Pulmo, 16 to >40 ind ha−1 over a 13-year period, but with no indication of local coral depletion.
True coral reefs, i.e. those with wave-resistant framework structures, were reported by Reyes-Bonilla and López-Pérez (2009) to be present at five sites in the extreme southern sector of the Baja California peninsula. These reefs are built predominantly by Pocillopora spp., with coral frameworks reaching 2 m in height. The largest and most thoroughly studied reef is located at Cabo Pulmo (Brusca and Thomson 1975; Reyes-Bonilla et al. 1997a, b). It is often cited as the northern-most eastern Pacific coral reef. It covers 150 ha and contributes to its surroundings an estimated 13,500–31,000 tons of CaCO3 annually (Reyes-Bonilla and López-Pérez 2009). This reef has formed on intrusive dikes and rocky hardgrounds. Even though the carbonate production rate of this reef is high, the coarser, reef-derived sediments are transported off-reef, especially during the passage of tropical cyclones, with no appreciable sediment retention. This loss greatly limits vertical reef accretion. Riegl et al. (2007) concluded that the Cabo Pulmo formation, sedimentologically, functions only in a limited way like a coral reef.
Compared to natural biotic effects, such as predation and bioerosion, extreme physical conditions have demonstrated the greatest negative influence on coral communities in the Gulf of California. Hurricanes and tropical storms regularly frequent the southern-most sector of the gulf (see Chap. 3, Fiedler and Lavín), and can limit reef development as noted above at Cabo Pulmo. Storm-generated turbulence and sediment transport are responsible for local coral breakage and the burial of fungiid populations (Reyes-Bonilla and López-Pérez 2009). During the past few decades, ENSO-related high and low seawater temperature extremes have caused significant coral bleaching and mortality in the lower gulf (Reyes-Bonilla 2001; Iglesias-Prieto et al. 2004; Paz-García et al. 2012). These thermal-bleaching events can lead to widespread and spatially uniform mortality of Pocillopora spp., approaching 20 %, over 10s–100s of km of coastline. While noteworthy, this level of mortality is significantly lower than that reported in other ETP localities. Reyes-Bonilla (2001) hypothesized that the relatively low level of thermal bleaching/mortality is possibly a result of local upwelling during the summer season, and proximity of lower gulf waters to the California Current system that contributes to the formation of a cool front at the entrance to the gulf.
5.3.2 Tropical Mainland Mexico
Beyond the Gulf of California, the tropical mainland coast of Mexico extends toward the southeast from southern Sinaloa to Chiapas states, a distance of nearly 2000 km (Fig. 5.7). Only two poritid species have been reported from Sinaloa (Porites panamensis, Porites sverdrupi) and, excluding extensive Miocene-Pliocene fossil reefs, no zooxanthellate corals are known from Chiapas. Both of these regions are dominated by coastal lagoons, sandy beaches, estuaries and mangrove ecosystems (Flores-Verdugo et al. 2001). The greatest reef development occurs in Nayarit and Oaxaca, each with 20 and 16 coral species , respectively (Reyes-Bonilla 2003; Reyes-Bonilla et al. 2005). Both of these areas are near upwelling centers; the northern center is located at Banderas Bay, Nayarit (López-Sandoval et al. 2009), and the southern upwelling area occurs along the Huatulco coast, Oaxaca (Steenburgh et al. 1998), which borders the Gulf of Tehuantepec (see Chap. 3, Fiedler and Lavín). Pocilloporid fringing reefs occur in both of these areas, usually at sheltered sites; reef frameworks attain vertical heights of up to 6 m, live coral cover commonly ranges between 20 and 50 %, and extends over several ha at several sites (Carriquiry and Reyes-Bonilla 1997; Reyes-Bonilla 2003). Coral communities and small patch reefs are also present in other west Mexican areas, particularly along coastal Guerrero and Michoacan states. Recent exploration has dramatically increased the species richness of zooxanthellate corals over a relatively brief period, from 19 (Reyes-Bonilla 2003) to 26 (Reyes-Bonilla et al. 2005, 2010; Glynn et al. 2007; Boulay et al. 2014), due mainly to continued exploration (Fig. 5.6; Appendix).
The coral faunas of tropical mainland Mexico (TMM) and the Gulf of California (GOC) are very similar (Fig. 5.2a), however, mainland Mexico is more speciose (26 vs. 20 species), with several reef-building species (in the genera Pocillopora, Porites, Gardineroseris) that have not been reported from the Gulf.
Information on coral community development at the Marías Islands, Nayarit, was lacking until relatively recently, with the delayed publication of field studies conducted in 1997 (Pérez-Vivar et al. 2006). All 10 species of zooxanthellate corals collected there are known from other Mexican mainland areas. Porites baueri, long considered an endemic species restricted to the Marías Islands and elsewhere on the Nayarit coast (Reyes-Bonilla 1993b), was not found. This species may be synonymous with Porites lobata, which was abundant in the islands in 1997. Including museum collections, the presently known coral fauna of the Marías Islands consists of 16 species. Coral zonation was characterized by shallow populations of Pocillopora, and deeper occurrences, to 10 m depth and deeper, of species of Porites, Pavona, and Psammocora. This is a frequent pattern observed throughout Mexico (Reyes-Bonilla and López Pérez 1998) and elsewhere in the eastern Pacific (Glynn 1976; Wellington 1982). The community of algal endosymbionts (Symbiodinium) inhabiting particular coral host species was found recently to be a critical factor in defining this depth zonation. From a combination of genetic and photo-physiological analyses of Pocillopora verrucosa and Pavona gigantea, Iglesias-Prieto et al. (2004) demonstrated that the physiological performance of host-specific zooxanthellae were adapted to different depth-related light regimes. Thus, pocilloporid holobionts exhibited optimal growth at shallow depths, whereas the growth rates of massive colonies were highest in deeper waters.
It is now recognized that the notable coral development of the Huatulco reef tract is subject to a variety of relatively frequent and severe natural disturbances with high inter-annual variability (Glynn and Leyte-Morales 1997; López-Pérez et al. 2002; López-Pérez and Hernández-Ballesteros 2004). These disturbances range from seasonal upwelling events to plankton blooms and high turbidity conditions, hurricanes, ENSO high and low temperature extremes, and sea urchin outbreaks leading to intense grazing and bioerosion (e.g., Leyte-Morales 2000; Lirman et al. 2001; Herrera-Escalante et al. 2005; López-Pérez et al. 2007; Benítez-Villalobos et al. 2008). López-Pérez and Hernández-Ballesteros (2004) have proposed that coral mortality caused by such random spatio-temporal disturbances are likely to produce a mosaic of patches at different stages of recovery, thus contributing to dynamic and diverse coral community structures. It would be of interest to study the coral-rich Nayarit area, also subject to upwelling and severe ENSO activity, to determine if disturbance regimes there similarly influence coral community structure and dynamics.
Several recent molecular genetic studies have helped to elucidate the connectivity between coral populations and their biogeographic relationships in Mexican waters and beyond. Most of these studies indicate a high degree of genetic structure in local populations and, presumably, limited gene flow. For example, significant geographic differentiation was reported for Pocillopora damicornis, a broadcast spawning species (Chavez-Romo et al. 2008), and Porites panamensis, a brooder with a brief larval life (Paz-García et al. 2008). Limited gene flow among most sampled localities was also reported for Pavona gigantea, a wide-ranging broadcast spawning species (Saavedra-Sotelo et al. (2011). However, the largest estimated migration rate indicated moderate and unidirectional gene flow from Huatulco to the Marietas Islands in Banderas Bay (Nayarit) and to La Paz. Blancas-López (2009) also observed weak genetic differentiation (i.e. connectivity) among populations of Pocillopora verrucosa, suggesting some level of gene flow among populations of this broadcast spawning species. Among the various physical and biotic factors invoked to explain these patterns, the surface circulation of west Mexico is critical. There is a general northerly set of surface currents, involving the Costa Rica Coastal Current (CRCC) moving along the Huatulco coast and the West Mexican Current that flows from Oaxaca to the entrance of the Gulf of California (see Chap. 3, Fiedler and Lavín). This would potentially allow certain source populations in the south to supply larvae that could recruit to downstream Mexican mainland areas and eventually as far north as the Gulf of California. Since the CRCC originates in the equatorial eastern Pacific, it could conceivably serve as a vehicle of transport for coral larvae to reach Mexico from Costa Rica and Panama.
5.3.3 Revillagigedo Islands (Mexico)
The volcanic islands making up the Revillagigedo Islands group are dispersed between 19° to 20°N latitude, and range from 750 km (San Benedicto and Socorro Islands) to 1200 km (Clarión Island) to the west of the Mexican mainland coast (Fig. 5.8). Roca Partida, consisting of two small rock peaks with a very limited shallow shelf, is located to the NE of Clarión Island. The number of coral records in the Revillagigedo Islands increased dramatically in the early 1990s, thanks to scuba surveys conducted by Mexican workers. This increase in species richness , from 12 to 21 then to 25, was due mostly to the discovery of Indo-Pacific corals not present on the west Mexican coast or elsewhere in the eastern tropical Pacific (Ketchum and Reyes-Bonilla 1997, 2001). Several of these species are shared with Clipperton Atoll, located nearly 1000 km to the south (at 10°N), which is occasionally in the path of the North Equatorial Counter Current (Glynn et al. 1996a, b). The current species list for the Revillagigedos totals 25 zooxanthellate scleractinians (Appendix). The strong coral faunal similarities between the Revillagigedos and Clipperton, also involving brachyuran crabs (Garth 1992) and reef fishes (Robertson and Allen 1996), prompted Ketchum and Reyes-Bonilla (1997, 2001) to suggest that this oceanic region should be recognized as a unique biogeographic subregion of the eastern tropical Pacific. The latter workers noted that surface currents would allow dispersal from Clipperton to the Revillagigedos, especially during the summer season, and that both island areas could serve as stepping stones for the migration of inshore marine species from the central to the more northern eastern tropical Pacific region. Traditionally, the W to E biotic migration route was assumed to be closer to the equator, from the Line Islands to Isla del Coco and Central America (Costa Rica, Panama) via the NECC (Dana 1975; Cortés 1997; Glynn and Ault 2000). Based on distributional evidence, it appears that there may be two dispersal routes, one just north of the equator and one leading to northern Mexican waters via oceanic island stepping stones.
The principal fringing reefs of Socorro and Clarión Islands are generally present in bays on western and southern shores in the lee of westerly-moving cyclones, which frequent this region (Fig. 5.8; Reyes-Bonilla 2003; see Chap. 3, Fiedler and Lavín). Pocillopora spp. predominate at shallow depths and massive corals (Porites, Pavona) increase in abundance from 5 to 30 m depth; coral rubble also increases with depth. Relatively few coral species were observed at Roca Partida and San Benedicto Island, likely due to the small size of these islands and restricted shelf space. In addition, the Bárcena volcano on San Benedicto Island was active as recently as 1952, and the resulting lava flows, debris, and fine sediments probably devastated nearshore communities and interfered with recovery. The six coral species observed there in 1994 were found principally on large basalt blocks and not on unstable volcanic sediments (Glynn et al. 1996a, b).
Moderate numbers of Acanthaster planci were observed at Clarión and San Benedicto Islands in 1994 (Glynn, unpublished observation). On 29 and 30 April, 36 individuals were counted on each of two occasions by a single diver between 6 and 17 m near Roco del Cuervo (18° 21′ 13.7″; 114° 41′ 06.2″), off the southeast shore of Clarión Island. The sea stars were feeding mostly on crustose coralline algae, but also on massive Porites lobata colonies, and large and small Pocillopora eydouxi colonies as well as broken branches of Pocillopora spp. Several A. planci also were observed feeding on P. eydouxi in coral communities on the northwestern end of San Benedicto Island on 3 May, 1994.
5.3.4 Clipperton Atoll (France)
Clipperton Atoll, the only atoll present in the eastern Pacific region, is located about 1300 km SW of the Mexican mainland and 950 km SSE of the Revillagigedo Islands (Fig. 5.1). Clipperton is volcanic in origin, part of a chain of guyots on the Clipperton fault, bordering the Cocos (south) and Pacific (north) plates. It is an ‘almost atoll’, with a 29 m high trachyte rock on the SE shore (Rocher Clipperton, Fig. 5.9), and an enclosed meromictic lagoon (Charpy et al. 2009; Trichet 2009). Present-day shallow, stratified brackish waters and H2S-enriched bottom waters prevent coral growth inside the lagoon. However, before the mid-1800s at least two channels communicated with the open ocean, thus allowing for coral framework construction; fossilized coral deposits are still evident today inside the lagoon (Carricart-Ganivet and Reyes-Bonilla 1999). It is likely that a tectonic uplift event (Trichet 2009) closed off the lagoon between 1839 and 1849 (Charpy et al. 2010). Bottom profiling to 200 m depth around the perimeter of the atoll revealed a prominent 20 m terrace that is generally present at all seaward exposures (Glynn et al. 1996a, b). The 200 m isobath is located relatively close to shore, from 200 to 500 m seaward of the reef flat.
Clipperton Atoll (CLP), modified after Glynn et al. 1996a. 50 and 200 m isobaths are based on the 21 bottom-profiling transects shown
With recent coral studies supported by scuba, the known coral fauna has increased from 16 or 17 species (Carricart-Ganivet and Reyes-Bonilla 1999; Flot and Adjeroud 2009) to 21 species (Fig. 5.7; Appendix). Contributing to this increase is the recognition of additional species of Pocillopora (e.g., P. elegans and P. verrucosa), the naming of a new poritid species (Porites arnaudi), and a molecular genetic study revealing the presence of Porites evermanni (Boulay et al. 2014). These new species’ records are not considered recent introductions, but resident species present for some undetermined time. Pavona gigantea was listed from Clipperton by Durham and Barnard (1952), but has not been observed at the atoll for several decades since. The zooxanthellate sceractinian fauna of Clipperton, consisting of mainly (~90 %) Indo-Pacific species is most closely allied with the Revillagigedo Islands in the EP (Fig. 5.2).
In 1994, live coral cover was exceptionally high (10–100 %) at all insular shelf depths from 8 to 60 m, and crustose coralline algae were abundant (5–40 % cover) between 0.5 and 7 m depth (Glynn et al. 1996a, b). Porites lobata and Pavona varians contributed prominently to live cover, the former up to 50 % of cover between 10 and 30 m, and the latter commonly as high as 100 % between 25 and 60 m and deeper. Monospecific patches of Pavona varians were present at 70 m. Porites arnaudi, noted as Porites sp., was commonly observed, but P. lobata predominated in most areas. Porites arnaudi was often more common on the near-vertical faces of the 20 m terrace (Fig. 5.10). Pocillopora sp. patches, 10s to 100s m2 in area, were highly dispersed and usually present between 5 and 20 m depth. The corals in most of these patches were dead and encrusted by the coralline alga Porolithon onkodes. The pocilloporid species making up these patches, later identified by Carricart-Ganivet and Reyes-Bonilla (1999), was Pocillopora meandrina. More recent workers have not commented on whether the Pocillopora patches were dead or recovering. Pavona minuta, Pavona maldivensis, and Leptoseris scabra were generally minor components of the coral communities. Surveys conducted 10 years later indicated no significant change in the overall robust state of Clipperton’s coral communities (Flot and Adjeroud 2009). Two permanently-marked monitoring sites were established in 2005 at 10–12 m depth on the north and south reef slopes, thus allowing an opportunity to track coral community condition over time (Salvat et al. 2008).
Like most ETP localities, the coral reef biota of Clipperton exhibits a strong affinity with the central/south Pacific region. In addition to the zooxanthellate coral fauna, high proportions of reef algae (Payri et al. 2009), crustaceans (Poupin et al. 2009), molluscs (Kaiser 2009), echinoderms (Solís-Marín and Laguarda-Figueras 2009), and fishes at Clipperton (Robertson and Allen 1996; Robertson et al. 2004; Béarez and Séret 2009) exhibit transpacific distributions. For coral reef fishes, Robertson et al. (2004) noted that pelagic spawners with strong dispersal ability were highly represented among transpacific species . Also, many reef-associated Indo-Pacific invertebrate taxa inhabiting eastern Pacific reefs possess teleplanic larvae (Scheltema 1988; Glynn and Ault 2000). Molecular studies demonstrate gene flow and recent invasions between the Central Pacific and Clipperton Atoll of coral, echinoderm, and fish species (Lessios et al. 1998; Lessios and Robertson 2006; Baums et al. 2012). Some of these studies have shown east to west gene flow, and connectivity of reef species among eastern Pacific oceanic islands (see Chap. 16, Lessios and Baums). Thus, it is necessary to know the flow regimes and transit times of the North Equatorial Counter Current (NECC), the primary eastward-flowing current in the central and eastern tropical Pacific.
Satellite-tracked drifting buoy data analyzed over a 15 year period (1979–1993) revealed a mostly eastward flow of the NECC across the E Pacific ‘barrier’ between 6° and 10°N, occasionally reaching 12–14°N (Glynn et al. 1996a, b). Travel times from the Line Islands in the Central Pacific to Clipperton Atoll were commonly 200 days, although one record during an ENSO event showed the NECC travelling across 46° of longitude in about 60 days. A comparison of west to east distances travelled during non-El Niño and El Niño years indicated a significant increase in NECC velocity during the latter periods. Accordingly, the NECC could offer an effective means of larval transport to Clipperton, which in turn could serve as a stepping stone to other ETP localities, e.g. the Revillagigedo Islands as suggested by Ketchum and Reyes-Bonilla (1997).
5.3.5 El Salvador
The recently described coral assemblages of El Salvador and Nicaragua are populated by a combined total of 14 zooxanthellate species . Notable differences, however, are the occurrences of Porites lobata at El Salvador only (Reyes-Bonilla and Barraza 2003), and Pavona clavus, Pavona varians, and Gardineroseris planulata in Nicaragua but not at El Salvador (Alvarado et al. 2011). No structural coral reef development has been reported from these areas (see Chap. 6, Toth et al.), although coral community patches are present.
The coastal stretch between Guatemala and northwestern Nicaragua (Gulf of Fonseca) is known as the “Pacific Central American Faunal Gap (PCAFG)”, originally named due to the absence of rocky shore fishes (Springer 1958). The Guatemalan coast and the northern sector of El Salvador consist of sandy beaches and mangrove forests. Until recently the PCAFG was also understood to separate the coral faunas of southern Mexico and Costa Rica (Glynn and Ault 2000). At Los Cóbanos (El Salvador), there is an abrupt change in the orientation of the coastline with the appearance of low rocky outcrops and platforms (Fig. 5.11a). The remainder of the Salvadoran coast consists of high energy sandy beaches or muddy estuaries. The littoral fringe of the Gulf of Fonseca consists of rocky outcrops and one of the most extensive mangrove ecosystems of Central America. The southern Nicaraguan coast consists of a combination of rocky headlands and sandy beaches (Cortés 2007). The occurrence of coral populations within the PCAFG could serve as a dispersal corridor, and explain the close association of southern and northern ETP coral faunas (Fig. 5.2).
The rocky outcrops of Los Cóbanos support the best developed and most diverse coral communities in El Salvador (Reyes-Bonilla and Barraza 2003). Small pocilloporid coral communities occur on basalt outcrops, most no larger than 30 m2, that occupy a total of 159 km2 of a large terrace (8000 ha) that borders the coast. Thirteen coral species (eight zooxanthellate and five azooxanthellate) were reported at this site. Lemus et al. (1994) performed the first quantitative coral surveys at Los Cóbanos in 1993 and 1994; these workers sampled Porites lobata and reported highly divergent live cover values between 0.41 and 62 % for this species. The mean diameter of the sampled Porites colonies was ~50 cm. At Punta Remedios (Fig. 5.11a), they reported 0.42 % live coral cover, 34.6 % algae, and 44.8 % sand and rock. Only dead Pocillopora fragments were noted at this site.
New sites at Los Cóbanos (Playa El Faro, Playa Salinitas or Decameron Beach) were sampled in 2006 by Segovia-Prado and Navarrete-Calero (2007). They reported high live cover of large Porites colonies: El Faro—33.7 % cover, 161 colonies, mean diameter = 121 cm; Salinitas—65.4 % cover, 75 colonies, mean diameter = 146 cm. Algal cover also was high: El Faro—41.7 %; Salinitas—29.5 %. These mixed coral/algal communities were classified into five distinct assemblages, based on generic prevalence: (1) Padina-Halimeda-Codium, (2) Porites-Padina-Halimeda, (3) Porites-Acanthophora-Padina, (4) Porites-Padina-Hypnea, and (5) Porites-Dictyota-Galaxaura. Concerns were raised over possible threats to corals by the invasive alga Acanthophora spicifera, and a high incidence of bioeroders (Lithophaga, Cliona) and potential space competitors (vermetid gastropods). Reyes-Bonilla and Barraza (2003) noted other macroalgae, invertebrates (e.g., octocorals, molluscs) and reef-associated fishes observed at Los Cóbanos. A potentially significant physical threat noted by Reyes-Bonilla and Barraza (2003) is the seasonally high rainfall period from May to October with associated severe coastal erosion and transport of suspended sediments.
At Playa el Zope (Los Cóbanos), López and Jiménez (2008) found healthy colonies of Psammocora obtusangula and Psammocora stellata interspersed with Porites lobata. They reported 81 colonies of P. obtusangula (mean length × width = 10.8 × 7.2 cm) and two small colonies of P. stellata (<6 cm2). Several P. obtusangula colonies bore small wounds resembling fish bite marks.
The most recent near-shore surveys at Los Cóbanos, in 2009 and at the same sites sampled by Lemus et al. (1994), and Segovia-Prado and Navarrete-Calero (2007), failed to reveal living or dead Pocillopora and reported only low Porites lobata cover of 0.1 and 5.5 % (Alvarado, unpublished data). A live Pocillopora community was reported in the early literature at Los Cóbanos, the Punta Remedios site (Orellana-Amador 1985; Gotuzzo 1996), but has not been re-located in recent surveys (Reyes-Bonilla and Barraza 2003). All Pocillopora spp. collected in this study were identified from dead beach fragments (Appendix). At relatively deep (10–15 m) offshore sites, only azooxanthellate corals were observed, with Tubastraea coccinea predominant. Considering the high variability of live coral cover reported over the years by different workers, and the uncertain location of sampling sites, this coastal sector is in need of a systematic and technologically advanced survey protocol.
5.3.6 Nicaragua
Before 2010, coral surveys along the Pacific coast of Nicaragua indicated the possibility of isolated colonies of Pocillopora close to the Costa Rican border in the area of San Juan del Sur (Durham and Barnard 1952; Glynn and Ault 2000; Spalding et al. 2001; Ryan and Zapata 2003; Cortés 2007). The presumed meagre occurrence of corals in this area may be related to severe and prolonged upwelling (Glynn et al. 1983).
In July 2009, Alvarado et al. (2010, 2011) quantitatively assessed the composition of ten coral communities along the southern coast of Nicaragua, including sites in the Department of Rivas, Municipalities of San Juan del Sur, and Tola (11° 23′N; 86° 02′W and 11° 07′N; 85° 47′W; Fig. 5.11b). They reported the presence of 39 species of macroalgae, 13 scleractinian corals (nine zooxanthellate and four azooxanthellate species), two crustaceans, five molluscs, 11 echinoderms, and 52 reef fishes. Mean coral cover across all sites was 9.0 ± 1.9 (SD)%, with the highest cover at Guacalito-La Anciana (18.5 ± 8.8 %) and Punta Pie de Gigante (16.7 ± 5.3 %). The most common species were Gardineroseris planulata and Pavona gigantea with notably high cover at Punta Gigante and La Anciana (Fig. 5.11b). A relatively large and vibrant Pocillopora incipient reef was present in the Punta El Toro embayment with mean live cover of 14.5 ± 7.9 %.
The Rivas coral biotopes, such as those present at Punta Gigante, La Anciana, and Punta El Toro, were small, patchy, and confined to a series of small coves and islets protected from wave assault. These communities were dominated by Pavona gigantea. The coral reef fish assemblages were less diverse than those reported for more developed eastern Pacific reef areas elsewhere, but were similar to those in marginal environments, such as upwelling centers (Alvarado et al. 2011). The coral-rich area between Punta Gigante and La Anciana, a 13-km coastal stretch, was touted by Alvarado and co-workers as a hotspot—despite the lack of reef development—and in urgent need of protection and management.
5.3.7 Mainland Costa Rica
A dramatic increase in coral species richness , from 10 to 26 species , is evident in faunal inventories when passing from Nicaragua to Costa Rica (Table 5.4; Appendix). This disparity is likely due to a combination of factors: (a) the recent discovery of coral communities in Nicaragua, (b) a concentrated research effort on coral reefs in Costa Rica since the 1980s, (c) the relatively large area of varied coastal habitats along Costa Rica’s coast, and (d) the stressful conditions associated with upwelling along the southeastern Nicaraguan coast. The mainland coastline of Costa Rica consists of diverse marine ecosystems as well as islets and islands of various sizes, and differences in proximity to the coast (Denyer and Cárdenas 2000; Cortés and Wehrtmann 2009). The presently recognized tally of 26 zooxanthellate coral species from mainland Costa Rica has been relatively stable during the past decade, thanks to the sustained studies of local workers (e.g., Guzmán and Cortés 1989a; Cortés 1990; Jiménez 1997, 2001a, b; Cortés and Guzmán 1998). This inventory takes into account a single new record for Costa Rica, Porites evermanni, revealed in a molecular genetic study (Boulay et al. 2014), and Porites rus, not observed again since its discovery at Bahía Sámara (sector 5, Fig. 5.12) in the early 1980s (Cortés and Murillo 1985). Pavona chiriquiensis, initially described from populations in Panama, is now known to occur at several sites along the Costa Rican coast. New distributional records for P. chiriquiensis in the central and south Pacific include the Tokelau Islands (~172°W), American Samoa (~170°W), the northern and southern Phoenix Islands (~170°W), and the Line Islands (155°–160°W) (Maragos, unpublished records). A cluster analysis of presence/absence data demonstrates a close affinity with the coral faunas of Panama and Colombia (Fig. 5.2).
The northwestern Pacific sector of Costa Rica is characterized by a marked dry season (January–April), with strong easterly trade winds that promote seasonal upwelling (Cortés 1996/1997; Jiménez 2001b; Alfaro et al. 2012). Upwelling is most pronounced further north, toward the Nicaraguan border. The dry climate extends south to Golfo de Nicoya, the largest estuarine environment in Costa Rica. The central coastal section is a transitional zone with an increasing humid climate to the south. The southeastern coast is subject to year-round high rainfall, which supports a tropical rain forest ecosystem (Jiménez and Soto 1985; Herrera 1986; Kappelle et al. 2002).
For ease of discussion, mainland Costa Rica is divided into 10 sectors where coral communities and reefs occur, from the Nicaraguan border to Golfo Dulce near Panama (Fig. 5.12). Oceanic Isla del Coco (Cocos Island) is assigned to sector 11, and is considered below in Sect. 5.3.8.
The northern-most area, Bahía Salinas (sector 1), is heavily influenced by seasonal upwelling in the Gulf of Papagayo (Alfaro et al. 2012). Water temperature in this area can be as low as 12 oC with low oxygen levels and low pH (recorded by Rixen et al. 2012 in Bahía Culebra; see also Jiménez 2001a; Alfaro and Cortés 2012; Alfaro et al. 2012). Because of these extreme conditions, reef and coral community development are limited; Pavona gigantea is predominant with isolated colonies of Porites panamensis. Upwelling effects generally decrease to the south and southeast towards the Nicoya Peninsula. At Península de Santa Elena (sector 2), relatively large pocilloporid reefs are present; one of these structural reefs is built mainly by Pocillopora eydouxi (Cortés 1996/1997; Bassey-Fallas 2010). Immediately south of Santa Elena, lie the Islas Murciélago (sector 3). Several coral reefs occur in this area; one is constructed of massive Gardineroseris planulata colonies. An extensive pocilloporid reef, consisting of large areas of Pocillopora inflata, was killed during a phytoplankton bloom in 2007 (Jiménez et al., in preparation). Coral recovery, including sexual and asexual recruitment, is very slow in this cool upwelling zone (Cortés, unpublished data). Bahía Culebra (sector 4) is one of the most intensely studied areas in Pacific Costa Rica (Cortés 2012a). Coral reefs and coral communities have been surveyed and monitored in this large bay and surrounding areas since the early to mid-1980s (Cortés and Murillo 1985; Jiménez 1997, 2001b; Jiménez et al. 2001, 2010; Bezy et al. 2006; Cortés et al. 2010; Cortés 2012a).
The Matapalo fringing reef in the southern part of sector 4, 1.4 km in length, is possibly the largest coral reef bordering mainland Costa Rica (Jiménez 2007a; Fig. 5.13). This reef, first surveyed quantitatively in 1978, was largely degraded at that time; it consisted of large tracts of dead pocilloporid corals, and at most 0.1 % live cover of Psammocora stellata (Glynn et al. 1983). [An aerial view of this reef in 1973, with the name Punta Gorda, is shown in Fig. 5.4a in Glynn et al. 1983.] The death of the reef at that time was attributed to cold-water stress due to intense upwelling near the end of the Little Ice Age (150–300 years BP). The overall live cover of pocilloporid corals on this reef amounted to >40 % when surveyed in 2007. Species richness was high, consisting of 16 living species, and 19 species total, including freshly dead coralla of three species (Cycloseris curvata, Diaseris distorta, and Leptoseris papyracea). Carbonate buildups or bioherms of Leptoseris papyracea are unique to this area. The Matapalo reef has again experienced recent high coral mortality first observed in 2007, and coincidental with extensive and prolonged harmful algal blooms (Jiménez 2007b). It is likely that recent declines in coral cover are due, at least in part, to coastal development activities and poor waste management promoting algal blooms, which are now nearly monthly occurrences.
Overall, much of the reef building in the extreme northwestern area of Costa Rica (sectors 1–4) has been achieved by massive instead of branching pocilloporid species. For example, the major reef builders north of Islas Murciélago are Pavona gigantea and Gardineroseris planulata. At Bahía Culebra, Pavona clavus is one of the major reef builders (Cortés and Jiménez 2003).
Extensive growths of the invasive green alga Caulerpa sertularioides have been reported in northwestern Costa Rica at Bahía Culebra (Fernández and Cortés 2005; Fernández-García 2007; Fernández-García et al. 2012), and at the Matapalo reef (Jiménez 2007a). Since 2001, C. sertularioides cover has been expanding with negative impacts on coral reefs and communities. At La Penca, Bahía Culebra, a Psammocora reef lost 95 % live cover at the expense of this alga during a 6 year period (Bezy et al. 2006). It is a strong competitor for space, overgrowing and smothering low-profile coral colonies. Caulerpa racemosa blooms are also known to cause coral mortality in Panama (see Fig. 8.31 in Chap. 8, and Fong et al., Chap. 11).
Little studied and isolated reefs occur in sector 5 along the outer coastline of Península de Nicoya (Cortés and Jiménez 2003; Bezy et al. 2006; Jiménez et al. 2010). A few small colonies of Porites rus, first observed in the eastern Pacific at Bahía Sámara, about 10 km SE of Punta Guiones, disappeared a few years after their discovery (after 1983) and have not been observed anywhere in the EP since. Porites evermanni was recently reported in coral communities on the Península de Nicoya, in other areas of Costa Rica, and elsewhere in the equatorial eastern Pacific (Boulay et al. 2014). The identity of this species was determined from a molecular genetic study; judging from its widespread occurrence and large population sizes it is probably a long-term resident of the equatorial eastern Pacific. Reef-building no longer occurs in the Gulf of Nicoya due to high suspended sediment loading and freshwater dilution. Currently only dead pocilloporid frameworks are present in the gulf, usually in sheltered environments.
The central Pacific coastline (sector 6) extends from the east margin of the outer Gulf of Nicoya to Punta Dominical. Isolated reefs and coral communities also have been reported in this area (Cortés and Jiménez 2003). Parque Nacional Marino Ballena (sector 7) is exceptional in the central Pacific coastal area because of its well-developed reefs (Alvarado et al. 2005). These reefs have been impacted by El Niño warming events (Jiménez and Cortés 2001) and excessive terrigenous sedimentation resulting from coastal development (Alvarado et al. 2005, 2009).
The Reserva Biológica Isla del Caño (Caño Island Biological Reserve, sector 8) is located about 15 km west of Península de Osa. Well-developed coral reefs are present at Isla del Caño, from drying reef flats (unusual in the ETP) with abundant Pocillopora spp., crustose coralline algae and Porites microatolls to deep patch reefs constructed of Porites lobata and Pavona clavus (Guzmán and Cortés 1989a; Macintyre et al. 1993; Cortés and Jiménez 2003). These coral communities have been intensely studied, including corallivore activity (Guzmán 1988a, b), coral growth rates (Guzmán and Cortés 1989b), zooplankton ecology (Guzmán and Obando 1988), and meiofaunal populations of reef sediments (Guzmán et al. 1987a). Also, coral mortality associated with algal blooms (“red tides”) was documented at Isla del Caño following the 1982–83 El Niño warming event (Guzmán et al. 1987b, 1990). Finally, Glynn et al. (1991, 1994, 1996a, b, 2000, 2008, 2011, 2012; see Chap. 15, Glynn et al.) have completed long-term studies on the reproductive activities of eleven reef-building coral species and one abundant azooxanthellate coral at Isla del Caño. This work has demonstrated a potentially important role of sexual reproduction in recovery processes in Costa Rica (Guzmán and Cortés 2001), and at several other areas in the equatorial ETP (Glynn and Colley 2008; Glynn et al. 2012).
Coral communities and reefs occur on the seaward side of the Península de Osa (sector 9), but are generally only present in sheltered locations due to high wave assault (Cortés and Jiménez 1996, 2003). Golfo Dulce (sector 10), a fjord-like embayment (Hebbeln and Cortés 2001), is protected from high seas; numerous coral communities and a large fringing reef are present in this gulf (Cortés 1990; Macintyre et al. 1993; Cortés and Jiménez 2003). One of the upper gulf reefs (Punta Islotes), constructed primarily of Porites lobata, initiated growth over 5000 years ago (Cortés et al. 1994). Highest accretion occurred between 2500 and 500 years BP, and then slowed due to changes in the local geological setting that increased freshwater input. Reef growth ceased during the last 60 years due to high terrigenous sediment loads.
5.3.8 Isla del Coco (Costa Rica)
Isla del Coco (sector 11), is located over 500 km offshore of Costa Rica, about half way between the Pacific coast of Central America and the Galápagos Islands (Cortés 2008). It is a volcanic island situated on the northeastern-trending Coco Volcanic Cordillera (Rojas and Alvarado 2012). The island and surrounding waters are a marine protected area—Parque Nacional Isla del Coco—the largest MPA in Costa Rica, and is now imbedded in the Área Marina de Manejo de Montes Submarinos (Sea Mounts Marine Management Area).
Twenty-two zooxanthellate scleractinians make up the Isla del Coco coral fauna, compared to 25 species total for all 10 Costa Rican mainland sectors (Table 5.4; Appendix). Of all eastern tropical Pacific coral localities, the Isla del Coco coral fauna demonstrates its closest affinity with the Costa Rican mainland (Fig. 5.2; Cortés 2012b). Pavona xarifae, once considered a unique Isla del Coco record in the eastern tropical Pacific, has been synonymized with Pavona minuta by Veron (2000). Veron’s synonymy is recognized here; therefore P. minuta is now more broadly distributed, considered present at Clipperton Atoll, in the Revillagigedo Islands, and at Palmyra Atoll (Appendix). Verification is necessary, however, ideally from specimens collected at their type localities and subjected to the same genomic and morphological analyses.
Coral reefs are best developed on the northern island shelf (from Punta María to Wafer Bay, and at Weston and Chatham bays), which is protected from southern swells (Guzmán and Cortés 1992; Cortés 2014). The largest Costa Rican coral reefs occur at Isla del Coco, with one reef exceeding 2 km in length (Fig. 5.14). Porites lobata is the chief framework species, with colonies of Pavona clavus, Pavona gigantea, and Gardineroseris planulata also contributing to reef building; some reef areas have extensive cover of Pocillopora spp. as well (i.e., P. elegans, P. damicornis, and P. eydouxi). Severe coral bleaching and high levels of coral mortality occurred at Isla del Coco during the 1982–83 El Niño warming event (Guzmán and Cortés 1992; Macintyre et al. 1993). Pre-El Niño coral cover of 80-90 % on some reefs declined to 3-26 % after the event. However, by 2002 recovery had been impressive and continues to the present (as of 2015). Since the mid- to late-1990s, Leptoseris scabra, a wide-ranging Indo-Pacific species, appeared for the first time on Isla del Coco reefs and now plays a role there in reef consolidation. The appearance of other species not reported from Isla del Coco before the mid-1990s include Pavona maldivensis, Pavona frondifera, Pavona chiriquiensis, and Pocillopora inflata. All three Pavona species are known to occur at Palmyra Atoll, Line Islands (Appendix), and Pocillopora inflata may be present in the Phoenix Islands (Obura 2002). The sudden appearance of these species at Isla del Coco, in the downstream path of the North Equatorial Counter Current (NECC), prompted Guzmán and Cortés (1992, 2007) to suggest that long-distance dispersal may be an important factor in the recovery of these eastern Pacific reefs following ENSO disturbances.
Isla del Coco (COC), Costa Rica. Red patches denote location of coral reefs (modified after Cortés and Jiménez 2003)
Summarizing for all Costa Rican reef sites subject to disturbance and long-term monitoring, a gradient of coral recovery is apparent that is related to increasing anthropogenic impacts and levels of protection (Cortés et al. 2010). The emerging pattern, from coral reefs exhibiting rapid to slow recovery, is as follows: Isla del Coco, Isla del Caño, Parque Nacional Marino Ballena, and Bahía Culebra. Coral cover at oceanic Isla del Coco, the most remote of the monitored sites, exhibited higher levels of recovery than the reefs at Isla del Caño, a moderate distance off-shore and relatively un-disturbed by humans. The lowest levels of coral recovery occurred at Parque Nacional (PN) Marino Ballena and Bahía Culebra, both on-shore areas subject to diverse coastal stresses. The PN Marino Ballena is protected whereas the coral reefs at Bahía Culebra are not. The degree of protection of these four areas is greatest at Isla del Coco and least at Bahía Culebra. A similar pattern was reported by Edgar et al. (2010) who found that eastern Pacific MPAs with higher controls and regulation had greater fish biomass and more coral cover than areas with limited or no protection.
5.3.9 Panama
Panama has been the focus of coral reef ecological studies since the early 1970s. The initial research in Panama during the 1970s was directed toward documenting the presence of structural coral reefs in the eastern Pacific region (see Chap. 1 for a historical perspective). With the discovery of numerous reefs, interests then turned to investigation of the physical and biotic controls of their distributions, particularly in relation to the different oceanographic conditions in the Gulfs of Panama and Chiriquí (Fig. 5.15). As in southern Mexico (Gulf of Tehuantepec), and the border region between Nicaragua and Costa Rica (Gulf of Papagayo), the low elevation of the Central American Cordillera in Panama, at 80°W, allows seasonal (January–April) NE Trade Wind flow across the isthmus, thus causing upwelling in the Gulf of Panama and further south across the Panama Bight and along coastal Colombia. Upwelling, with accompanying low water temperatures and high nutrient conditions, promotes phytoplankton blooms that can interfere with coral growth. The high Tabasará mountain range in western Panama, reaching 3000 m elevation and more in some areas, reduces by more than two-thirds the Trade wind flow, thus preventing upwelling in the Gulf of Chiriquí, but allowing brief SST shoaling and cooling of the upper surface layer more commonly than previously suspected (D’Croz and O’Dea 2007). The extent of reef building is notable in this warm and more thermally stable enclave. Structural coral reefs are also present in the seasonally variable Gulf of Panama, but are not so well developed as in the Gulf of Chiriquí (see Chap. 6, Toth et al.). Coral community development, and the presence of coral reefs, is greatest on the numerous islands present on the isthmian shelf.
With the exception of the hydrocoral Millepora intricata, which often contributes importantly to coral communities and reef frameworks in the Gulf of Chiriquí (Fig. 5.3), the chief frame-building scleractinian species in both gulfs are Pocillopora damicornis, Pocillopora elegans, Porites lobata, Pavona clavus, Pavona gigantea, and Gardineroseris planulata. Zooxanthellate scleractinian species richness is virtually the same in both gulfs (Maté 2003). Three uncommon transitory species, Leptoseris papyracea, Diaseris distorta and Cycloseris curvata, are sometimes found in deeper (12–25 m), offreef, sedimentary habitats. A single, small, living population of C. curvata is presently known in the Gulf of Chiriquí; only dead skeletons of the other two species have been reported from Panama.
Like Costa Rica, the Panamanian coral fauna has remained relatively stable during the past decade, with 25 presently recognized zooxanthellate scleractinian species. Recent additions are Porites evermanni, Pavona minuta, Pavona duerdeni, and Siderastrea glynni (Fig. 5.7; Appendix). The validity of S. glynni is in question; possibly it is not a resident endemic, but a human-introduced Caribbean species (Forsman et al. 2005). Panama’s coral species richness is equal to that of Costa Rica, with both faunas demonstrating a strong affinity (Fig. 5.2). It is not inconceivable that the high species richness of Indo-Pacific corals in Panama, Costa Rica, and Colombia is at least in part associated with the inflow of the NECC into this equatorial region (see Chap. 3, Fiedler and Lavín). The NECC may also be responsible for the sporadic occurrence of Indo-Pacific cnidarian vagrants in equatorial eastern Pacific waters, e.g., Porites rus (Costa Rica), Millepora platyphylla (Panama), and Acropora valida (Colombia) as well as ephemeral occurrences of non-cnidarian invertebrate and fish species (Glynn and Ault 2000; see Chap. 16, Lessios and Baums).
Perhaps the most influential environmental driver affecting Panama’s coral populations is ENSO. The ENSO warm phase (El Niño) in particular, but also the cool phase (La Niña) can cause coral bleaching and mortality. Mean overall coral mortality in Panama following the 1982–83 El Niño disturbance ranged from 76 to 90 % (Wellington and Glynn 2007). Coral mortality during the 1997–98 El Niño was much lower, amounting to only ∼13 % in the Gulf of Chiriquí, and no detectable mortality in the Gulf of Panama. The low coral mortality in the Gulf of Chiriquí was attributed to endosymbiont resistance (see Chap. 13, Baker et al.), and the absence of coral mortality in the Gulf of Panama to upwelling, which prevented high stressful temperatures (Glynn et al. 2001).
Biotic disturbances may follow thermally-induced coral mortality. For example, bioerosion of dead corals can accelerate with increasing sea urchin population densities, which could lead to coral framework loss and the cessation of reef growth (Glynn 1988; Eakin 1992, 1996, 2001). Acanthaster planci, where abundant in the Gulf of Chiriquí in the 1970s and early 1980s, posed a threat to some uncommon corals that survived El Niño bleaching. However, this corallivore’s numbers decreased dramatically on reefs after the mid-1990s and is now mostly present in coral communities where it is no longer a threat (see Chap. 10, Enochs and Glynn). Past ENSO impacts can be detected from changes in coral colony morphology, increases in non-coral benthic cover, eroded reef structures, and increases in coral rubble accumulations. ENSO-related coral mortality has not been observed in Panama during the last 18 years, and significant recovery of live coral cover has occurred on reefs in the Gulfs of Panama and Chiriquí (Baker et al. 2008).
The demonstrated capacity for sustained high levels of sexual reproduction as well as asexual regeneration (Glynn and Fong 2006) has likely figured importantly in the recovery of coral communities. Continued study of coral sexual reproduction has demonstrated that the reproductive modes and spawning patterns of Pavona clavus, Psammocora stellata and Psammocora profundacella (Glynn et al. 2011, 2012) are similar to those of seven previously investigated species (Pocillopora, two spp; Porites lobata; agariciids, 4 spp), namely (a) broadcast spawning of minute ova at or a few days following full moon, (b) high annual fecundity due to frequent and prolonged spawning, and (c) reproduction most active during periods of high but not stressful sea water temperature (see Chap. 15, Glynn et al.). Year-round gametogenesis occurred in non-upwelling Gulf of Chiriquí, but ceased during the 3-month upwelling season in the Gulf of Panama. Gametogenesis continued during mild El Niño warming events (Colley et al. 2006). Glynn and Colley (2008) hypothesized that broadcast spawning coral species can better tolerate and survive unstable environmental conditions in the eastern Pacific than brooding species. Tubastraea coccinea, a ubiquitous azooxanthellate brooder in Panama (and elsewhere in the eastern Pacific), releases planulae during new and full lunar phases, mostly during high thermal periods (Glynn et al. 2008). This species also is highly fecund, and is reproductively mature when colonies possess as few as 2 to <10 polyps.
During the past decade (early to mid 2000s) detailed mapping of the presence and abundances of coral and octocoral communities has been undertaken in two of Panama’s largest MPAs, the Pearl Islands (Gulf of Panama), and Coiba Island (Gulf of Chiriquí) and surroundings (Guzmán et al. 2004, 2008). [As a cautionary note, the live coral cover on these maps (1) includes corals and octocorals, (2) none of the islands supports a continuous fringing reef, and (3) coral cover on the relatively small structural reefs (not shown) often exceeds 40 %. The reader is referred to Guzmán et al. (2004, 2008) for details on the sampling methodology.] Moderate to high coral cover, 20 to >40 %, was reported in both of these archipelagos (Figs. 5.16 and 5.17) with the highest coral cover recorded on the smaller islands (Table 5.5). The high coral cover category in the Coiba Islands (18.4 %) was slightly over twice as high as that recorded in the Pearl Islands (8.1 %). Nearly 80% of the surveyed areas in the Pearl Islands supported low coral cover (<20 %) compared with ∼35 % in the Coiba Islands. It is necessary to consider at least three factors contributing to this difference: (a) deforestation, land use and sedimentation, all of which have been greatest in the Pearl Islands, (b) upwelling versus non-upwelling environments, the latter generally promoting more robust coral growth, and (c) phytoplankton blooms causing copious mucus production that can smother corals (Guzmán et al. 1990).
Live coral cover in Pearl Islands MPA (168,800 ha), Gulf of Panama, Panama. The ~7 km wide Trollope Bank is shown to the SE of El Rey Island. MPA boundaries in blue. Modified after Guzmán et al. (2008)
Live coral cover in Coiba MPA (270,125 ha), Gulf of Chiriquí, Panama. MPA boundary in blue. Modified after Guzmán et al. (2004)
In these island-wide surveys a distinction was made between coral communities (CC) and coral reefs (CR), which revealed some intriguing differences: (a) scleractinian corals and octocorals demonstrated higher species richness in CC compared to CR, (b) coral cover was higher in CC than CR in the Coiba Islands preserve, but higher in CR than CC in the Pearl Islands, (c) uncommon, rare or endangered cnidarians were encountered more frequently in CC than CR, and (d) a negative relationship between live coral cover and species richness emerged in the Coiba Islands, with CC consisting of a greater variety of cnidarian species than CR. A similar trend to that in ‘a’ above was reported by Benfield et al. (2008) for reef fish assemblages in the Pearl Islands, which were significantly more diverse and species rich in CC than CR. These results prompted Guzmán et al. (2004, 2008) to hypothesize that CC may serve as refugia and source areas for larvae that could repopulate CR that become degraded during large-scale disturbances such as El Niño bleaching/mortality events.
Building on Abele’s (1976) demonstration of a species-rich community of decapod crustaceans associated with living pocilloporid corals (55 species), several recent studies have revealed an impressive number of motile, cryptic invertebrates and fishes that seek refuge in living and dead coral frameworks and rubble. For example, the biomass and abundances of various invertebrate taxa (Polychaeta, Gastropoda, Crustacea, Holothuroidea, Ophiuroidea) associated with live corals are generally higher than those sheltering among dead corals and coral rubble (Enochs and Hockensmith 2009; Enochs 2012). Species richness , however, is significantly higher in dead and degraded coral rubble (261–370 species) compared with live coral habitats (112–219). Among some of the several key reasons for this are (a) the inimical defenses of live corals (nematocysts, mucus secretions), (b) the agonistic and competitive nature of obligate coral metazoan symbionts, and (c) the greater habitat heterogeneity and niche diversity of rubble substrates. These results show that an abundant and speciose cryptic fauna is still present on highly degraded reefs that can continue to function in various reef processes, e.g. providing prey for reef fishes, scavenging, biodegradation, remineralization, bioerosion, interalia (Enochs et al. 2011; Enochs 2012; Enochs and Manzello 2012a, b).
Recent studies of the diversity, community structure and trophic relationships of coral-associated fishes have increased our understanding of their diverse and pivotal ecological roles in the ETP (Dominici-Arosemena and Wolff 2006; Benfield et al. 2008; Glynn 2008; Robertson and Allen 2008; Glynn et al. 2014). A large number of facultative corallivores, and cryptic fishes residing in coral rubble, many of the latter invertivore and piscivore predators, have been identified (Glynn 2006, 2008). Some of these taxa may play critical roles in limiting the settlement and survival of recruiting species.
5.3.10 Mainland Colombia
In spite of the adverse climatic and oceanographic conditions (Glynn and Ault 2000; Zapata and Vargas-Ángel 2003), isolated colonies of scleractinian corals occur along most of the 1450 km of the Colombian Pacific coast. Corals are most common in the northern half of the coast from the Panama-Colombia border south to Cabo Corrientes, a stretch dominated by igneous rocky shores. South of Cabo Corrientes to the Ecuador-Colombia border the coast is composed of alluvial sediments and dominated by soft bottoms and mangroves intermixed with some stretches of soft sedimentary rock from the Tertiary. The Colombian Pacific coastal region is one of the rainiest regions of the world, with annual precipitation exceeding 10,000 mm at some localities. High freshwater discharge from the western slopes of the Andes and the adjacent lowlands into the Pacific Ocean significantly reduces salinity and increases turbidity and sedimentation, particularly in the southern half of the region (Restrepo and Kjerfve 2000; Alory et al. 2012). Yet, the best developed coral reefs and coral communities of the Colombian Pacific are located in this southern portion at Gorgona Island, which is adjacent to this stretch of coastline, but located sufficiently far offshore to reduce the limiting influence of the coastal environment. Accordingly, Kleypas et al. (1999) listed Gorgona Island and Ensenada de Utría, also in Colombia (see below), as the two lowest salinity localities with coral reefs in the eastern Pacific. Therefore, coral communities and true reefs (carbonate frameworks) are few, small, patchily distributed and poorly developed. Consequently, coral communities and reefs in this area of the eastern Pacific are species-poor (≤12 scleractinian coral species per site) and the entire coral fauna is composed of about 28 species, although taxonomic difficulties, particularly within the genus Pocillopora, make this number somewhat uncertain (Table 5.6).
Four localities along Colombian Pacific shores have been reported to have significant coral communities or coral reefs: Cabo Marzo and Punta Cruces, Punta Tebada, Ensenada de Utría, and Gorgona Island (Fig. 5.18). The northernmost area is a prominent rocky point located at Cabo Marzo (Fig. 5.19) where a significant coral formation occurs. Known locally as El Acuario, the shallow (≤3 m) substrate here is composed of a solid rock bed with boulders dominated by filamentous algae (47 % mean cover), scleractinian corals (33 % mean cover) and octocorals. Branching corals of the genus Pocillopora are the most common (27 % mean cover) followed by massive colonies of Pavona gigantea. There is, however, little indication of significant reef framework development at this site. Six species of zooxanthellate scleractinian corals occur at El Acuario (Zapata et al. 2008; Table 5.6). A small coral patch consisting of sparse Pocillopora damicornis colonies and associated rubble is also located in neighboring Bahía Aguacate. Further south at Punta Cruces, in a cove in front of the hamlet known as Piñas (Fig. 5.19), there also is a coral community dominated by massive and encrusting corals covering nearly 38 % of the rocky substrate.
Coral communities and coral reefs at Punta Tebada, Ensenada de Utría and Gorgona Island, Pacific Colombian (COL) coast. Red patches at Punta Tebada and Ensenada de Utría denote high coral cover. Gorgona Is legend: red = structural coral reef (arrecife); orange = coral community (comunidad coralina); yellow = coral reef/community in need of confirmation (por confirmar)
Coral assemblages characterized by high colony densities, but lacking a framework buildup are common in the Utría and Tebada region. Largely, these communities develop on rocky banks, shoals, and vertical walls, particularly in small embayments and coves, ranging in depth from about 1 to 10 m; moderately strong swells and vigorous water circulation are prevalent in these habitats, however. Despite the high energy of the environment, the dominant taxa are branching Pocillopora species, particularly P. elegans, and massive colonies of Porites lobata, Pavona clavus, and Pavona gigantea; less abundant to rare are Gardineroseris planulata, Pocillopora damicornis, Psammocora stellata, and Pavona sp. aff. frondifera (Vargas-Ángel 2001, unpublished data). Together with the scleractinian corals, coralline and turf algae, and other sessile invertebrates including non-zooxanthellate corals, barnacles, sea fans, and clams, inhabit these shallow basalt substrates. Generally high levels of grazing from sea urchins and herbivorous fishes are in part responsible for the limited macroalgal development in these communities. Coral recruitment from sexual propagules or fragmentation is also noticeably low on both the grazed basalt surfaces or the rubble and sand-covered bottom surrounding corals.
Of the coastal coral reefs, La Chola (also known as La Aguada) fringing reef, located at Ensenada de Utría (Fig. 5.18), is the largest, best developed, and probably the most thoroughly studied in this part of Colombia. The reef is located on the central, eastern shoreline of a narrow embayment, characterized by calm and relatively clear waters. Elevated turbidity and runoff events following heavy rainfall, which is common in this region, can reduce visibility to about 1.0–1.5 m, demonstrating that these corals are able to tolerate periods of elevated turbidity and reduced salinity. Thinly-branched ecomorphs of Pocillopora are prevalent on the reef, which contrast with the more stout and stubby congeneric colonies developing in the non-framework building communities of outer wave-exposed habitats. When the reef was surveyed in 1988–89 by Vargas-Ángel (1996), the predominant coral species were Pocillopora damicornis (80 %) and Psammocora stellata (16 %), which formed a terrace-like framework, covering approximately 10.5 ha; uncommon to rare species included Pocillopora capitata, Pavona varians, and Pavona gigantea. The reef at Utría lacks the typical geomorphological zones (i.e., backreef, reef flat, forereef); it is a relatively broad reef flat followed by a seaward slope, where coral development gradually decreases to a depth of approximately 10 m. Reef coring and probing revealed that the uncemented Pocillopora framework was at least 2.0–4.5 m in height, with uninterrupted growth throughout the last 2000–3000 years, and mean vertical accretion rates of 2.47 m kiloyear−1 ±0.15 (SE) (Vargas-Ángel 2001; see Chap. 6, Toth et al.). Community structure surveys conducted later in 1996–98 (Vargas-Ángel 2001) indicated that mean percent live coral cover was 41 %. A temporal comparison of permanent transects placed since 2002 indicates that coral cover on this reef has declined on average by 65 % from 57 % in 2002 to 37 % in 2013 (M. López-Victoria and F. A. Zapata, unpublished data). In all of these surveys, coral composition was relatively homogeneous throughout the shallow reef flat, with intermingled turf algal patches and pockets of rubble and bioclastic sand. Absence of ecological zonation has been regarded as an indicator of “instability”, whereby chronic stress and environmental disturbances may offset the competitive interactions and biological processes leading to species habitat partitioning (Karlson 1999). In addition to chronic siltation and turbidity stress, this reef is subject to subaerial exposure during extreme low tides, recurrent ENSO events, and periodic intense upwelling episodes (Vargas-Ángel 2001; Zapata and Vargas-Ángel 2003).
Located approximately 70 km north of Utría, the Tebada reef is a small structure, covering roughly 4.5 ha, and situated in the southern end of the Gulf of Cupica. When surveys were conducted in 1996–1998 mean live coral cover was 39 %. At that time the living coral community was dominated by a mixture of Psammocora stellata and Psammocora profundacella, which together amounted to nearly 90 % of the live reef cover (Vargas-Ángel 2001), in contrast with the reefs farther south at Ensenada de Utría and Gorgona Island, which were dominated by pocilloporid species (Zapata and Vargas-Ángel 2003). Four more coral species were present at Tebada than further south, including a few small colonies of Pocillopora damicornis, Pavona varians, Pavona gigantea, and Gardineroseris planulata; the latter is relatively uncommon on coastal reefs and in coral communities. The evident structural pattern of the Tebada reef was an increase of live coral cover with increasing distance from shore, related to a decrease in scleractinian species richness (Vargas-Ángel 2001, 2003). Reef probing on the reef flat in 1996 revealed that the Tebada reef framework was at least 4 m thick, and coring to a depth of approximately 1 m revealed an uncemented framework composed mainly of Psammocora rubble (Vargas-Ángel, unpublished data). Based on its geographic location, it is reasonable to assume that the Tebada reef is subject to similar environmental stressors as those at Utría, particularly ENSO and upwelling stress events. The reef flat at Tebada, however, is approximately 1 m deeper than that at Utría; therefore, it is not subject to subaerial exposure during extreme low tides. Recent inspection of this site in April 2014 revealed that both Pocillopora and Psammocora were important members of this coral formation and that framework construction was modest (C.G. Muñoz and M. Rodríguez-Moreno, personal communication). While Vargas-Ángel et al. (2001) reported minimal coral bleaching at this reef during June 1998, local fishermen recalled that noticeable coral bleaching occurred here at some time during the 1997–1998 El Niño event.
The coral reefs and coral communities of Gorgona Island are the largest and best developed in the Colombian Pacific region (Glynn et al. 1982; Zapata and Vargas-Ángel 2003). The largest structural coral reefs of Gorgona Island, La Azufrada and Playa Blanca reefs, are located on the eastern, leeward side of the island (Fig. 5.18). These reefs exhibit high coral cover, are more or less similar in size (~10 ha), structure and zonation, and are composed mainly of pocilloporid species with a significant presence of massive corals of the genera Pavona and Gardineroseris at the seaward reef base. A total of 24 species has been recorded for Gorgona Island (Table 5.6), but some of these records may have to be confirmed with more detailed work. The report of Acropora valida at Gorgona Island, a species known previously from the western Pacific but known today to be widespread across the Indo-Pacific region (Veron 2000), is intriguing: three colonies were found at 10 m depth on a rocky outcrop at the northernmost tip of Gorgona Island in 1983 (von Prahl and Mejía 1985). While the biogeographical implications of this report were potentially major given competing hypotheses regarding the origin of the eastern Pacific coral fauna (Dana 1975; Heck and McCoy 1978), despite repeated efforts to find new specimens, this species has never again been observed at Gorgona Island or any other locality in the eastern Pacific.
Live coral and algal cover on these reefs appear to be highly dynamic, particularly on shallow areas due to recurrent subaerial exposures during extreme low tides (Zapata et al. 2010). While recurrent disturbances on the reef flat can at times reduce live coral cover to very low levels and increase algal turfs, high densities of herbivorous sea urchins and fishes readily remove filamentous turf algae leaving an exposed reef matrix composed of coral rubble cemented by calcareous algae that sexually-produced coral recruits eventually colonize. Coral recovery is further enhanced by asexual recruitment via colony fragmentation, which is very common and facilitated by the feeding activities of some fishes, particularly triggerfishes (Balistidae) and puffers (Tetraodontidae) (Palacios et al. 2014).
Coral formations of Gorgona Island have been studied since the 1970s and have been described in several accounts (von Prahl et al. 1979; Glynn et al. 1982; von Prahl and Erhardt 1985; von Prahl 1986a, b; Zapata 2001; Zapata et al. 2001; Zapata and Vargas-Ángel 2003). Nonetheless, significant coral formations continue to be found around the island. While Glynn et al. (1982) had previously described an incipient reef formation at La Camaronera, located on the western, wave-exposed coast, as “linear pocilloporid buildups or spurs and hillocks of various sizes”, a different formation was found further offshore. This is a coral community that extends 8 ha and is made up of 11 species of corals, which cover 49 % of the substrate. Massive species of the genera Pavona, Gardineroseris and Porites are dominant, forming high topographic relief that provides habitat for a distinct fish community (Palacios and Zapata 2014). This coral formation contrasts with the incipient coral reef of La Ventana, also mentioned briefly by Glynn et al. (1982). This is a pocilloporid reef located on the south-eastern side of the island that remained undescribed until recently. While this is a small reef covering 1 ha, it exhibits very high live coral cover (73 %) because its low-lying reef framework is never subaerially exposed during extreme low tides. However, this dense pocilloporid stand provides low relief and constitutes a relatively monotonous habitat for a distinct but less equitable fish community than that observed at La Camaronera (Palacios and Zapata 2014).
5.3.11 Malpelo Island (Colombia)
Malpelo Island, the easternmost and least remote of the five oceanic islands or archipelagos in the ETP, is a very small (1.6 km long) volcanic island located nearly 380 km off the Colombian continental coast, 630 km from Isla del Coco and 996 km from San Cristóbal, the nearest of the Galápagos Islands (Fig. 5.20). Malpelo’s marine environment is most similar to that of its closest oceanic neighbor, Isla del Coco, but in contrast to the latter, only a total of 13 zooxanthellate scleractinian species has been recorded at Malpelo (Table 5.6). A particularly interesting and possibly new undescribed species of Pocillopora, which exhibits an unusual morphology with a tightly submassive, hemispherical corallum (Fig. 5.21), was reported by Rodríguez-Ramírez and Zapata (2012). This rare coral, if a valid new species, would represent a possible endemic species for the island. Nonetheless, in terms of coral species composition, Malpelo is more similar to continental localities in El Salvador and Nicragua than to the neighboring oceanic islands of Isla del Coco or the Galápagos (Fig. 5.2). However, this may be a result of poor species richness at Malpelo, El Salvador and Nicaragua rather than a reflection of similar environments with similar faunas. This interpretation is supported by results of similar analyses with the fish fauna in which oceanic islands in general, and Isla del Coco, Isla Malpelo and the Galápagos Islands in particular, were always most similar in species composition, and distinct from continental localities (Robertson and Cramer 2009).
While oceanographic conditions appear to be favorable for vigorous coral growth (but see below), steep rocky walls prevent the buildup of significant reef structures, and corals often exhibit unusual growth forms. For instance, some pocilloporids display horizontally-oriented colony growth with flattened branches, likely the result of strong wave action (Fig. 5.22), while Porites lobata very commonly forms shingle-like structures on steeply inclined substrates (Fig. 5.23). Besides small, isolated coral patches and scattered coral colonies of Pocillopora spp. and Porites lobata along the rocky sublittoral, several sites around the island harbor coral communities, the largest of which is known as El Arrecife (The Reef). This community demonstrates a marked zonation pattern with pocilloporid colonies in shallow areas, Porites lobata and Pavona clavus at mid-depths and Gardineroseris planulata in the deepest areas. Strong oceanic swells and occasional rockfalls appear to upturn and break coral colonies affecting the development of coral formations around the island (Birkeland et al. 1975; Garzón-Ferreira and Pinzón 1999). Clear evidence of the effects of an unknown major disturbance was observed in July 2011 at El Arrecife, suggesting that the lack of reef building at this site may be due to recurrent sediment export during strong turbulence. Bastidas-Salamanca et al. (2014) reported a reduction in coral cover from ~45 % in 2011 to ~35 % in 2012 at El Arrecife and speculated that this may have been caused by the mechanical action of a tsunami-like disturbance during a seismic event. However, the noticeable increase in variance around the mean coral cover values reported in 2012 does not lend support to this hypothesis because a major wave would probably have caused more uniform damage and suggests instead the action of a much more localized factor that affected some sites more than others.
Like other localities in the eastern Pacific in general and within Panama Bight in particular, Malpelo Island is exposed to high thermal variability. First, there is marked interannual thermal variability associated with ENSO events that occur at irregular intervals every few years. However, little is known about the effects of the 1982–83 El Niño warming event on Malpelo’s coral formations, although they may have been affected as much as other reefs in the region (Glynn et al. 1988). During the 1997–98 El Niño, coral bleaching and mortality were low at Malpelo Island, but such effects were also relatively low everywhere else in the Colombian Pacific compared to levels of bleaching observed in 1982–83 (Vargas-Ángel et al. 2001). Second, there is a markedly seasonal annual cycle with lower and more variable water temperature between January and March than during the rest of the year, as in much of Panama Bight (Forsberg 1969; Stevenson et al. 1970; D’Croz and Robertson 1997; Zapata et al. 2011). During this period, the Intertropical Convergence Zone migrates from ~8–10°N to ~2°N and the north-east trade winds blow more strongly over the bight, producing a cyclonic pattern of water circulation and cold water upwelling in the middle of the bight, with a consequent shallowing of the thermocline (Rodríguez-Rubio et al. 2003; Devis-Morales et al. 2008). While cooling episodes may be common at Malpelo Island, in April 2009 an unusually cold event caused extensive bleaching of Porites lobata and several other coral species (Zapata et al. 2011). Thus, coral formations at Malpelo Island may also be limited by extreme temperature variations over several temporal scales.
5.3.12 Mainland Ecuador
Coral reef formations of relatively high diversity occur along the mainland coast of Ecuador from Esmeraldas to Santa Elena. At Esmeraldas, (El Faro point) near Cabo San Francisco, small patches of Porites lobata are present in the low intertidal zone. A P. lobata patch reef, about 1250 m2 between 15 to 20 m depth, is present on the Surrones Bank, located approximately 6 km north of Pedernales (Fig. 5.24). Another small P. lobata patch reef occurs at 7 m depth at Ligüique in the Pacoche Wild Life Refuge. Coral diversity and reef development are greatest in the Machalilla National Park (Fig. 5.24). The Machalilla MPA lies within the coastal stretch from 2° 15′S to 1°00′N (Glynn 2003). A fringing reef located near the fishing village of Machalilla, at 1°28′S, is the southern-most known coral reef on the American Pacific coast. A mixed and diverse octocoral/coral community is present at Islote los Ahorcados, just south of the Machalilla reef. Pavona clavus, Pavona gigantea, Pocillopora verrucosa, and Pocillopora damicornis are the chief corals at this site. Small pocilloporid patches are also present at Pelado Islet, a new MPA located about 40 km south of the Machalilla MPA. And most recently (personal communication, 2015) Jesenia Zambrano has reported the presence of Pocillopora in the Puntilla Santa Elena MPA (see Fig. 21.7 in Chap. 21, Alvarado et al.).
Over the last decade, coral species richness has increased from 18 to 23 species (Fig. 5.6; Appendix). This increase is due to the recognition of four additional species of Pocillopora and Porites evermanni. These new records probably represent long-term resident species and not recent arrivals. The relatively high species count of 23 for coastal Ecuador and no authenticated coral records for Peru underlines the limiting influence of the cool Peru Coastal Current, which reaches Talara just south of the Gulf of Guayaquil (Fig. 5.1; see Chap. 3, Fiedler and Levín). Pocilloporid corals have been reported (René Espinoza, personal communication), but unconfirmed, at Santa Clara Island, near 3°S, at the entrance to the Gulf of Guayaquil. The species composition of the coral fauna of mainland Ecuador is most similar to that of the southern Galápagos Islands (Fig. 5.2).
As generally observed across the equatorial eastern Pacific, coastal Ecuadorian coral communities were severely impacted by El Niño-induced bleaching in 1982–83 and 1997–98 (Glynn 2003). Due to the remote locations of the coastal coral sites it has not been possible to monitor community responses or recovery after these disturbances. Thanks to the comprehensive cnidarian surveys and inventories of two MPAs compiled by Rivera and Martínez (2011), with the collaboration of Odalisca Breedy, it is possible to infer recovery since 1997–98, which appears to have been significant and widespread. Their photographic guide illustrates numerous, robust, zooxanthellate species at several localities and depths.
Coral formations are best developed and most numerous at Isla de La Plata, about 30 km west of the mainland. Several patch and fringing coral reefs are present between 1 and 15 m depth along La Plata’s east coast (Fig. 5.25a). Most of these formations are isolated and relatively small, ≤200 m in length; however, the largest fringing reef at Acuario is about 500 m long. Pavona clavus, some colonies 3–5 m in diameter, is the chief framework-building species at this locality. Farther south, at Palo Santo, Pocillopora meandrina is the principal reef-building species. A bathymetric assessment of zooxanthellate corals performed by Martínez et al. (2011) at five sites (Palo Santo, Acuario, Bahía Drake, Tres Piedra, El Faro) showed that Pavona and Psammocora exhibited a broad depth distribution with marked differences between species. Pavona clavus predominated from 1 to 6 m depth, whereas Pavona gigantea was present primarily from 6 to 15 m, with a few isolated colonies as shallow as 4 m. Psammocora superficialis colonies were found from 2 to 15 m, while Psammocora stellata occurred from 6 to 10 m only. Pocillopora species occupied mostly shallow depths, especially Pocillopora ligulata and Pocillopora meandrina, which were not observed deeper than 5 m. Pocillopora damicornis, Pocillopora verrucosa, and Pocillopora eydouxi, however, reached depths of 10–11 m. The only colonies of Porites lobata observed at La Plata Island were present at the Tres Piedras site at 5 m depth.
a La Plata Island, Machalilla National Park, Ecuador. Substrate types are color coded (see legend), isobaths are shown at 3 m intervals. Modified after Rivera and Martínez (2011). b Satellite image of La Plata Island (2014 Digital Globe, Google Maps, April 2007)
The presence of six species of azooxanthellate scleractinians was also reported by Rivera and Martínez (2011). One of these (Cladopsammia gracilis), was especially abundant on the sides and underneath Pavona clavus colonies at the Acuario site. As in Panama, numerous gorgonian species were observed, but these generally were not common in coral reef habitats. Especially diverse and densely populated gorgonian communities were present at sites with strong currents, such as the shoals off Puntilla de Santa Elena reserve and south of Machalilla (Fig. 5.24).
Eastern Pacific coral reef development is generally confined to sheltered areas; the satellite image in Fig. 5.25b shows the prevailing swell direction from the SW and high wave assault on the western and southern island shores. Encrusting coralline algal communities are well developed along the exposed rocky coastal areas. The extent of coral reef development on mainland Ecuador now rivals and may exceed that in the Galápagos Islands. This was not the case before the ENSO activity of the past few decades, which has resulted in a marked reduction of coral communities and coral reefs in the southern Galápagos Islands. Since 2010, managers of the Machalilla National Park have installed nine permanent moorings in the MPA to prevent anchor damage from increasing tourism.
5.3.13 Galápagos Islands (Ecuador)
The Galápagos Islands are bathed in a confluence of westerly flowing surface currents that vary seasonally in magnitude and in latitude and are collectively referred to as the South Equatorial Current (SEC) in their passage towards the central Pacific. During years considered ENSO “neutral” the northerly island pinnacles of Wenman and Darwin (Fig. 5.26) are typically bathed in constitutively warmer waters from the southwesterly deviation of the NECC extending from the Panama Bight. It strengthens its southerly extension as trade winds from the SE weaken during the equatorial warm season months (Dec-May). The southerly and central islands, however, are more exposed to the northerly extent of the South Pacific gyre, which transports surface water from the SE Peruvian oceanic and coastal systems, often referred to as the Humboldt Current. Separated by 1–2° of latitude from the aforementioned northwestern pinnacles, the central islands are as a result on average 1–2 °C cooler throughout the year (Banks 2002) harboring a greater relative proportion of coral genera such as Pavona spp. that persist under a wider range of fluctuating and cooler temperatures compared to Porites spp. that dominate the coral communities of warmer northerly islands (Banks et al. 2009).
The northeast to southwest SST gradient is exaggerated during the cold season months (Jun–Nov) and is reduced to less than 1 °C during the warm season (Dec–May) (Figs. 5.27 and 5.28). These regional differences notably disappear during strong El Niño years when surface temperatures are elevated, sustained and homogenized across space and seasons, and conversely emphasized during La Niña episodes when trade winds and Humboldt transport are strengthened.
Monthly patterns in mean SST for near-shore environments across each major island (calculated areas up to 5 km from island coastline using NASA MODIS-Aqua 1 km2 remotely sensed data 2003–2008). Islands and respective monthly SST plots are color coded. Lowest and highest monthly SSTs occurred at Floreana in September (20–21 °C) and at Darwin in March (27–28 °C)
Coral species richness of the southern and northern Galápagos Islands is similar in spite of the large difference in habitable substrate areas (Dawson et al. 2009). This is likely a result of high connectivity between islands. Nineteen and 21 zooxanthellate scleractinian species are recognized as members of the coral faunas of the southern and northern islands respectively (Appendix). The coral fauna of the southern islands demonstrates its strongest affinity with the Ecuadorian mainland, and that of the northern islands with mainland Colombia (Fig. 5.2). Three species seemingly restricted to the southern islands, Pavona chiriquiensis, Cycloseris curvata, and Diaseris distorta, are not important in reef building. Four species confined to the northern islands, Leptoseris scabra, Pavona maldivensis, Pocillopora woodjonesi, and Porites evermanni, are widespread Indo-Pacific species. All of these species except P. woodjonesi, which is rare, do contribute minimally to reef building in different areas of the eastern Pacific.
Development of coral communities and structural reefs is best expressed at Darwin (Fig. 5.29) and Wenman (Fig. 5.30), the two northern-most islands (Glynn et al. 2015). The largest known coral reef in the Galápagos Islands, located on the insular shelf between Darwin Arch and the eastern shore of Darwin Island, is nearly one km in length, and consists of vertically consolidated poritid stacks towering from 2 to nearly 4 m in height. While the chief frameworks are at 12–16 m depth, live coral cover extends to 28 m depth along the NW/SE trending isobath. This reef has demonstrated notable recovery since the 1982–83 El Niño with live cover now exceeding 25 % over most of its length. The high cover of Pocillopora spp. before 1982–83 has virtually disappeared (Glynn, unpublished observation). We propose naming this impressive structure Wellington Reef, in honor of Gerard M. Wellington who performed the first island-wide marine resource surveys in the Galápagos Islands.
Unusually high coral abundances and incipient reef development characterize the narrow insular shelf along a 1 km stretch at Bahía Tiburón, Wenman Island (Fig. 5.30). As at Darwin Island, massive Porites colonies predominate; however, large branching Pocillopora spp. colonies are numerous on rock boulders at shallow depths (<8 m), and Pavona spp. increase in abundance toward the deep (20–30 m) reef base.
Biogeographic patterns across the archipelago are greatly influenced by meso- and small-scale oceanography. Surface waters mix significantly with subsurface inputs from bathymetrically- and wind-driven upwelling processes across the archipelago. The eastward flowing Equatorial Undercurrent (EUC or Cromwell Current) shoals to 40–80 m depth over and around the Galápagos platform. The EUC brings 13–18°C water into the surface and is considered the most important oceanographic feature in maintaining extraordinary plankton productivity and unique cold water habitats, particularly in the western exposed islands of Fernandina and Isabela (Fig. 5.26).
In contrast to the enabling Panamic influence in the north, the EUC favors solitary azooxanthellate corals and rather restricts the development of reef-building corals in the western regions, generating small scale patterns of cold water mixing around western shores of the central islands. These areas are relatively spatially consistent yet quite ephemeral where EUC filaments shoal around the bathymetry and mix into the upper euphotic zone (Shaeffer et al. 2008). Since widespread mortalities during the El Niño event of 1982–83, the patterning of suitable coral habitat in the southern islands is more heterogeneous compared to that of Wolf and Darwin. Although small patches of coral communities are common across the central isles (Pinta, Marchena, Genovesa), the largest extensions of zooxanthellate corals are found across the relatively sheltered northern leeward shores of Floreana Island, protected bays in eastern Santiago, Punta Pitt at the eastern end of San Cristóbal and the southerly extent of Gardner Bay at Española Island (Fig. 5.26).
An interesting feature of the western islands of Isabela and Fernandina is that they show historical evidence of large reef-forming corals in sheltered bays, despite the prevailing EUC upwelling and near absence of reef-building corals today. For example, there are large Pavona clavus colonies in Urvina Bay, Isabela, which was uplifted in the 1950s (Glynn and Wellington 1983; Colgan 1990, 1991; Dunbar et al. 1994). There are also reports of Pocillopora spp. persisting in shallow coastal pools such as those at Punta Espinosa, Fernandina, and Concha y Perla lagoon near Puerto Villamil, Isabela (Baums et al. 2014; Feingold and Glynn 2014). It seems possible that the strong stratification through solar heating at the surface in sheltered pools with low current could create a sub-habitat suitable for sporadic hermatypic coral growth. The downstream oceanic connectivity between coralline communities in the east and possible incursions of warmer water during strong El Niño years may also present opportunities for new recruitment over decadal scales.
Other unique coralline environments in the islands include extensive beds of free-living Psammocora stellata and Porites lobata composed of 3–14 cm diameter fragments across sand substrates at Punta Espejo, southeast Marchena Island (Feingold 2001). The fungiid free-living coral Diaseris distorta also persisted with a large population (>100,000 individuals) localized in patches across the sand sediment platform at 15–20 m depth east of Corona del Diablo (a small volcanic offshore crater at the northern tip of Floreana Island). Another fungiid, Cycloseris curvata, was present there in small numbers (30–40 individuals) (Feingold 1996).
Internal waves also influence local coral ecology. Kelvin waves that propagate to the east and pass in sets from the western Pacific shoal and deepen the thermocline over week-long periods in their passage across the islands. Tropical Instability Waves also form large scale front features with eddies that extend out from the upwelled equatorial cold tongue passing over the archipelago towards the west (Sweet et al. 2009). Both produce short-term changes in the water-column structure within larger seasonal signals. Kelvin waves being part of the El Niño trigger, and wider ENSO dynamic, are usually responsible for anomalous periods of alternating cool and heated surface waters that can last several weeks regardless of the season. Given strong surface heating of the upper mixed layer under the equatorial sun this can cause up to 10–12 °C thermal anomalies at 10 m depth over very short periods (4–6 days) as cold water extends into the shallow coastal zone.
Whereas warm-water stress upon corals in the region was largely evident in the widespread mortalities of the 1982–83 and 1997–98 El Niño events, cold water anomalies are pronounced and also impact coral health when combined with La Niña episodes (see Chap. 8, Glynn et al.). Significant mortalities of Pocillopora spp. were observed during the onset of the 2007–08 La Niña event. The upwelling phase of a set of Kelvin waves coincided with the transition into the cold season, further exaggerated by La Niña conditions both exacerbating and prolonging cold-water shock to shallow water communities across the southerly islands. The gradual recovery observed since 1995 of Pocillopora spp. colonies monitored in Corona del Diablo (Floreana Island) was greatly impacted between 2007 and 2009 with numbers falling from 167 colonies to just 20 (Feingold and Glynn 2014) and similar patterns were observed across the south. Visual surveys along the northeast coast of Pinta in 2010, however, showed encouraging survivorship of large Pocillopora colonies, likely as a result of their positioning in transitory warmer water to the north.
These effects were also observed as far north as Wolf (Wenman) and Darwin islands, which harbor the greatest remaining massive coral frameworks (Glynn et al. 1983, 2003, 2008, 2015; Banks et al. 2009). Those species that persisted after the 1982–83 and the compounding 1997–98 El Niño event exhibited an interesting potential for redundancy in functional roles between alternating periods of warm water and cold-water stress. Pavona clavus colonies adjacent to Porites lobata, for example, remained healthy during heavy bleaching of Porites during cold water incursons in February 2008. This may have allowed for the maintenance of key ecological services (e.g., structure, refuge, and productivity) until the recovery of the more abundant Porites as warmer conditions returned. Although not yet observed directly, the reverse is likely to be true under strong heating stress for P. clavus. These observations also lend themselves to the possibility of selection for temperature-resistant zooxanthellae clades or “disaster” endosymbionts (Correa and Baker 2011; see Chap. 13, Baker et al.) persisting in those corals that survived temperature shock during recent strong ENSO events in northerly communities.
A reduced and clustered population following El Niño mortalities also presents other concerns. During surveys from 2006–2008 the average prevalence of coral tissue abnormalities across eight sites at Wenman and Darwin was over 23 % (Vera and Banks 2009), which is at least twice as high as that reported in Caribbean and Indo-Pacific regions. The species Porites lobata was most affected with 35 % showing symptoms of parasitism or illness. The condition identified as Porites trematodiasis was most evident. These findings support observations from other areas correlating the frequency of strong bleaching events and high host densities with disease outbreaks (Bruno et al. 2007; Cróquer and Weil 2009). Background climate-change scenarios of increased ocean acidification in a region already subjected to low pH-upwelled waters also presents unqualified risks for reduced coral calcification, increased infiltration of bioeroders, and diminished reef accretion (Manzello 2010; see Chap. 18, Manzello et al.).
Nonetheless a gradual seven-year recovery trend across the northerly coral frameworks over the 12–25 m platform between Darwin Arch and the east coast of Darwin Island (Glynn et al. 2009, 2015) is also corroborated by local observations of tissue regeneration and coral recruits close to existing colonies at other sites (Banks, Personal Communications). Wenman and Darwin, despite their small geographic size, make a disproportionately large contribution to the overall Panamic and Indo-Pacific subtidal biodiversity in the islands. Galápagos park authorities, in concert with local NGOs and the Ecuadorian Navy, have begun implementing low-impact tourism anchorages to complement zoned-protected areas physically signposted in 2006 (Merlen et al. 2009). Best-practice guidelines for tourism dive operations would help reduce physical damage by inexperienced divers to reefs, while fixed bouy anchorages should help minimize the significant damage caused by anchors and chains to slow growing colonies and encourage their future recovery.
The effect of the increased human footprint in the Galápagos Marine Reserve since the 1980s and its impact on habitat-forming corals is a topic of continuing speculation. Observations since the strong El Niño events suggest that climate disturbance when coupled with certain human disturbances (such as unsustainable extraction of resource species or chronic anchor damage) can cause broad shifts in ecosystem composition and biodiversity state that further inhibit natural recovery processes (Sonnenholzner et al. 2009; Edgar et al. 2010). Significant interactions between overfishing and ENSO may be particularly important in determining extinction risk given that since 1982–83 several azooxanthellate coral species have greatly reduced populations (e.g., Tubastrea floreana) or are even possibly already extinct (e.g., Rhizopsammia wellingtoni). Significant increases in the proportion of degraded corals and the bioerosion of weakened coral frameworks by the slate pencil sea urchin Eucidaris galapagensis have been linked to coral degradation and observed both in situ and upon tectonically uplifted colonies (Colgan 1990, 1991). The recovery of coral populations is further compounded by overgrazing scenarios inhibiting recruits through the notable proliferation of the sea urchins Tripnuestes depressus and Lytechinus semituberculatus; these sea urchins may create barrens if there is a significant reduction in their natural reef predators (Sonnenholzner et al. 2009; Edgar et al. 2010). Any such biotic and indirect cascade effects will influence coral recovery intervals, population viability, and recruitment between strong El Niño events.
The 1997–98 El Niño event coincided with rapid extraction of lobster species Panulirus gracilis and Panulirus penicillatus, both candidate predators for coral burrowing molluscs such as Lithophaga spp. and the corallivore Coralliophila violacea at Wenman and Darwin Islands. Targeted and incidental fishing of intermediate-sized predatory fish with regulatory roles such as the leather bass Dermatolepis dermatolepis, demersal labrids and other serranids (including the exported insular endemic bacalao Mycteroperca olfax) also accompany both lobster and sea cucumber fishing seasons. Multivariate reconstructions of benthic coverage (Edgar et al. 2010) from early (pre-1982–83 El Niño) photographic records and subsequent photoquadrat and visual census monitoring indicate little change in benthic community composition between 1984 immediately after the strong El Niño event and some 20 years later in 2004. The footprint when removing natural biological controls of grazers through fisheries appears to be widespread in the archipelago and has resulted in high abundance of encrusting calcareous algae such as Lithothamnium sp. indicative of sea urchin barrens. Observations of increased Eucidaris sea urchin densities near to fishing ports (Sonnenholzner et al. 2009; Edgar et al. 2010) appears to support this hypothesis with analogies reported in other regions such as Hong Kong (Qui et al. 2014).
With new coastal-zoning reforms being considered, GMR coral communities present a curious dichotomy. They are particularly vulnerable before acidification and cascade effects after removal of regulatory species in their vicinity, yet are also a naturally resilient habitat subject to intense changes conditioned by a unique oceanography and millennia of ENSO fluctuations in the region. Moderation and mitigation of these effects through monitoring and feedback into management certainly deserve careful consideration given that novel climate stress is likely to be deterministic in the future survivorship of remaining Galápagos corals and the diverse communities they support.
5.3.14 Rapa Nui (Easter) and Salas y Gómez Islands (Chile)
Rapa Nui (Easter) and Salas y Gómez islands (both included under RPN, Appendix), the latter slightly more than 400 km east of Rapa Nui, share a common and relatively species poor shallow marine biota (Fig. 5.31). Two comprehensive reports by Castilla and Rozbaczylo (1987) and DiSalvo et al. (1988) offer excellent introductions to the marine habitats and biota of Rapa Nui. Studies focusing on the coral faunas and communities became available in the early to mid-2000s (Wellington et al. 2001; Hubbard and Garcia 2003; Glynn et al. 2003, 2007).
Rapa Nui (Easter Island), Chile. Inset, Salas y Gómez Island, ~500 km east of Easter Island. Red dot west of Cape O’Higgins denotes location of coral reef framework (Hubbard and García 2003)
Only 15 species of zooxanthellate corals are known from the larger and more speciose Rapa Nui with nearly half of these in the genus Pocillopora. Pocillopora verrucosa exhibits high cover over mid-depths at several sites on the west coast and at Motu-Iti and Motu-Nui at the southern tip of Rapa Nui (Fig. 5.32). Porites lobata is the single poritid species present, and makes up the bulk of the coral cover across all depths. Unlike its colony growth form elsewhere in the eastern Pacific, which is smooth to nodular and dome-shaped, at Rapa Nui the corallum takes on a variety of forms—columns, blades, spikes, cones, and marginal tabulae (Glynn et al. 2003). Pavona species, so prominent at other eastern Pacific sites, are absent from the Rapa Nui islands group. However, two species of faviid corals, Leptastrea purpurea and Leptastrea transversa, are present but they are minor components of RPN coral communities. These are the only members of the Faviidae known from the eastern Pacific region. While endemism is high in some groups (e.g., molluscs, 42 %; fishes, 77 %; Friedlander et al. 2013), all zooxanthellate scleractinian corals, except for the two faviid species, are widely distributed throughout the eastern Pacific and central/south Pacific regions (Appendix). Ongoing molecular genetic studies, however, suggest re-classification of Pocillopora verrucosa as a separate endemic species (D.A. Paz-García, E. Wieters, F.J. García-de-León, E.F. Balart, unpublished data).
Traditionally, Rapa Nui has been considered as the eastern-most outpost of the Polynesian or southeast Pacific region (e.g., Veron 1995). However, an analysis of coral species similarities (presence/absence records) between the Pitcairn Island group and Rapa Nui indicates that this locality is more closely aligned with the eastern Pacific faunal group (Glynn et al. 2007). In support of this result, a dispersal barrier between Rapa Nui and the Pitcairn Islands was revealed through satellite-tracked surface drifter buoys. Surface currents were found to flow from east to west, forming an effective barrier to the eastward dispersal of planktonic larvae that occupy the upper surface of the ocean. Most recently, Veron et al. (2015) also concluded that Rapa Nui has its strongest affinity with the far eastern scleractinian zooxanthellate fauna. Reef-building coral species richness is notably higher in the Pitcairn’s Islands than at Rapa Nui. Veron (1995) reported 61 species in the Pitcarins; recent surveys have increased this number to 87 (Appendix).
Vibrant coral communities with high coral cover (40–80 %) characterize most sites along the now well-sampled western and northeast coasts of Rapa Nui, whereas corals appear to be scarcer on the less-studied, wave-battered southeast coast that is directly exposed to major oceanic swells (DiSalvo et al. 1988). Considering the high live coral cover at favorable sites, it is not surprising that incipient reef development has been observed. Massive Porites colonies in particular have relatively high skeletal extension rates, about 1 cm year−1 (Glynn et al. 2003), are longevous, and attain enormous dimensions (Figs. 5.33, 5.34 and 5.35). Porites frameworks were observed by Hubbard and Garcia (2003) on the western and northern sides of the island. The largest of these is found northeast of Poike Peninsula, off Cape O’Higgins. Some coral buildups on elevated basalt substrates appear deceptively like true structural reefs (Fig. 5.34). These shelf locations are protected from severe wave assault from the southeast, originating in high latitude southern seas. Even though pocilloporid corals often occur as dense aggregations at sheltered sites, they do not form interlocking frameworks as at other ETP localities.
In general, two species, Porites lobata and Pocillopora verrucosa, account for the vast majority (95 %) of total coral cover across the island. While typically scarce, encrusting colonies of Leptastrea spp. can reach up to 10 % cover at some locations near Ovahe and Tahai. There they occur intermixed with Porites and Pocillopora in large patches (10s of square meters) on flat, mid-depth (10–12 m) platforms. Although still nominal in terms of their contribution to total coral cover, a notable increase in the prevalence of Pocillopora eydouxi has been observed over the past five years, particularly along the west coast (E. Wieters and H. Garcia, personal observation).
Generally, weak depth zonation patterns characterize changes in the relative abundances of the dominant coral community functional groups (Friedlander et al. 2013; Wieters et al. 2014). Total coral cover is lowest at shallow depths (<5 m), however, where branching Pocillopora spp. are nearly absent, massive Porites are predominant (average 38 % cover). Below this, Pocillopora increase in abundance to peak at mid depths (15–20 m), where their relative contribution to total coral cover is similar to that of Porites at most sites (average 38 and 36 %, respectively). Massive Porites again dominate in deeper habitats (>20 m), and Pocillopora become less abundant, but still reach 10–20 % cover. Abundant live coral extends well below the 30 m depth limit with Pocillopora and Porites known to occur frequently to 60 m depth.
Across the northeast and west coasts, shifts in the relative abundance of species cause spatial differentiation in the functional structure of coral-dominated benthic communities and fish assemblages (Wieters et al. 2014). Though changes are best described by multivariate characterization of communities based on functional groups, some of the most apparent differences in terms of species are due to relatively higher abundances of Pocillopora and some macroalgal groups along the northwestern coast and tip of the island, as well as variable presence and abundances of the reef fish Centropyge hotumatua, Acanthurus leucopareius and Chrysiptera rapanui. Further studies have also identified contrasting communities at similar depths, with a preponderance of either corals or macroalgae (predominantly Padina). These alternative, coral/algal community configurations can exist side-by-side (within hundreds to thousands of meters), and commonly present sharp boundaries between them, which remain unaltered for relatively long periods of time.
Published studies concerning ecological processes maintaining coral-dominated communities at Rapa Nui are virtually nonexistent. Ongoing experimental and comparative studies at a few locations suggest that, like elsewhere, grazing is a critical process involved in preventing macroalgae from overgrowing live corals. Evidence thus far also suggests grazing is especially effective when corals have been previously damaged and weakened by thermally-induced bleaching (Wieters, unpublished data). At Rapa Nui, where herbivorous fishes are generally small-bodied and in low abundance, sea urchins (Diadema) appear to be particularly important. While no previous studies have documented ecological effects or even the diet of benthic herbivores at Rapa Nui, comparisons with nearby Salas y Gomez reveal between-island differences in abundances of sea urchins and macroalgae (Friedlander et al. 2013), further suggesting the potential for a critical top-down role by these benthic herbivores. Whether the effects and relative importance of sea urchins are currently magnified at Rapa Nui, due to the very apparent overfishing that seems responsible for reduced abundances of carnivorous fishes and other large predators, is not known. Obvious sea urchin-induced erosion of dead corals and limestone deposits is limited to a few specific locations of particularly high sea urchin densities (mean 19.0 ind. m−2 ± 2.6 SE), but there is no evidence that sea urchins cause mortality of established live colonies (Wieters, personal observation).
Severe, island-wide coral bleaching at Rapa Nui is known to have occurred twice in recent years: (1) following torrential rains and associated increased sedimentation in 1980 (Egaña and DiSalvo 1982), and (2) following prolonged sea warming associated with La Niña in 2000 (Wellington et al. 2001). This exceptionally strong La Niña event, centered at and above the equator in the northern hemisphere, resulted in two month-long elevated SSTs in the southern ocean, that extended over 73° of latitude from Rapa Nui to Fiji. During this event the bleaching threshold at Rapa Nui (26.1 °C) was exceeded for 9–10 weeks in February and March. This thermally-driven disturbance led to high mortality of Pocillopora spp. to 20 m depth throughout the island. Within one year after the disturbance, greater than 80 % of Pocillopora colonies inhabiting shallow habitats (<9 m) were dead and a clear pattern of decreased mortality with increasing depth was documented (Fig. 5.36). While Porites experienced generally similar levels of bleaching compared with Pocillopora in 2000, the former appeared much more resistant with far fewer colonies having succumbed a year later. Initial recovery of Pocillopora was uneven spatially, with increased prevalence of coral tissue abnormalities and continued mortality observed up to 5 years after the massive bleaching at some sites, particularly in mid- to deep habitats (>13 m; compare mortality levels in 2001 and 2005, Fig. 5.36). The factors inhibiting or delaying recovery and causing the continued declines of Pocillopora at some sites are unknown, but preliminary evidence suggests that depth-dependent resistance and other disturbances can be important. Despite the extensive bleaching, large scale mortality of one of the dominant species, and among-site variability in recovery, overall recovery of coral communities to date has been impressive, with total coral cover now similar to (deep habitats) or above (shallow habitats) the pre-bleaching levels (Wieters et al. 2014).
While no massive bleaching event similar to that documented in 2000 has been observed recently (as of 2014), regular surveys over the past 5 years show that bleaching of Pocillopora and Porites occurs every year. While the intensity/prevalence varies greatly among sites and years, the regular decline in zooxanthellae densities and/or degraded pigmentation suggests that these corals are frequently subjected to stressful conditions. This relatively mild bleaching rarely appears to cause coral mortality directly, but does appear to have consequences for other species in the community. Bleached colonies of both Pocillopora and Porites are more likely to harbor associated fauna than their neighboring “healthy” (fully pigmented) counterparts. Moreover, the abundance and species richness of fauna are higher on bleached (but live) corals (Fig. 5.37). Increase in macroalgal abundance also occurs during years of more prevalent bleaching, though susceptibility of corals to overgrowth varied among sites and appears to be associated with local coral energy reserves (lipid concentration, Fig. 5.38). These results suggest that disruptive stress that causes coral bleaching changes the habitat-modifying role of branching and massive corals as well as the intensity of competition with algae under natural conditions in the field. These hypotheses are currently being tested.
Percentage of healthy and bleached Porites and Pocillopora colonies with diverse associated species of invertebrate functional groups. ‘Others’ include infrequently observed taxa such as sponges, polychaete worms, turrid snails, caridean shrimp, and non-trapeziid brachyuran crabs. A minimum of 50 colonies of each species and condition was assessed at mid-depth (10–15 m) across four sites on the west coast of Rapa Nui
Relationship between mean percentage cover of macroalgae and mean lipid concentration of Pocillopora verrucosa colonies at mid-depth (10–15 m) habitats during years of mild bleaching (>30 % cover bleached corals) across western and northeastern shores of Rapa Nui. Symbols are sites 1 to 10s of km apart
5.4 Conclusions
Coral reef-building potential exists across the entire eastern tropical Pacific region, from the Gulf of California to Rapa Nui, regardless of low species richness at the northern and southern latitudinal limits of coral distribution. With the exception of newly discovered ETP coral formations, e.g. El Salvador and Nicaragua, and small habitable sites, e.g. Malpelo Island, coral species richness is relatively uniform at 20–25 species per locality across the eastern Pacific region. The presently recognized total species count of zooxanthellate corals in the ETP region is 47.
Concentrated reef development occurs chiefly on islands of the continental shelf and on distant oceanic islands, but also at some environmentally suitable coastal areas such as Huatulco (Mexico), along the shores bordering the southern Gulf of Papagayo and Golfo Dulce (Costa Rica), and at Machalilla (Ecuador). Massive colonies of Porites, Pavona, and Gardineroseris are usually the dominant reef-building corals in ETP oceanic island environments such as Clipperton Atoll, Isla del Coco, the northern Galápagos Islands, and Rapa Nui.
Coral species richness is relatively high in the northern latitudes of mainland Mexico and offshore in the Revillagigedo Islands, and farther south in the equatorial eastern Pacific, from Costa Rica to Ecuador. Reef building occurs in upwelling centers (Huatulco, Gulf of Papagayo, Gulf of Panama), and in several areas of the equatorial eastern Pacific, which experience aperiodic severe ENSO disturbances.
Thomas Dana’s hypothesis of the dispersal of central/west Pacific corals into the ETP via the NECC is indirectly supported by analyses of faunal affinities and molecular genetic evidence. Species-rich Palmyra Atoll in the northern Line Islands is in the path of the easterly flowing NECC and could likely serve as a source of coral propagules to be transported to the equatorial eastern Pacific. Surface current transport from the Line Islands via the NECC to Clipperton Atoll (a stepping stone) is also possible.
Potentially important corallivore and bioeroder species have caused greatest damage to corals after El Niño warming/bleaching events. The crown-of-thorns seastar Acanthaster planci is moderately abundant in only three ETP areas—Gulf of California and Revillagigedo Islands (Mexico), and Gulf of Chiriquí (Panama), and has not been reported to overwhelm coral cover anywhere in the ETP. Population outbreaks of the black long-spined sea urchin, Diadema mexicanum, have been reported at several localities, usually following disturbances and high coral mortality, resulting in extensive bioerosion of reef frameworks and massive coral colonies. The slate pencil sea urchin Eucidaris galapagensis occurs at high population densities in the southern Galápagos Islands, where it has eroded reef frameworks and prevented the recovery of coral communities devastated by the severe 1982–83 El Niño event.
Competition for space among corals and macroalgae has been documented locally in El Salvador (Acanthophora spicifera vs. Porites lobata), Costa Rica (Caulerpa sertularioides vs. Pocillopora spp., Psammocora stellata) and Panama (Caulerpa racemosa, C. sertularioides vs. Psammocora stellata). Coral patches are sometimes overgrown and killed by algae, but such competitive dominance is usually ephemeral, with plant cover eventually subsiding and corals re-populating previously occupied substrates.
Despite diverse natural physical and biotic disturbances at both local (e.g., upwelling centers, bioeroder outbreaks) and regional (severe ENSO episodes) scales, ETP coral communities have generally demonstrated a high degree of resilience, with robust recovery at several localities in recent years.
References
Abele LG (1976) Comparative species richness in fluctuating and constant environments: coral-associated decapod crustaceans. Science 192:461–463
Alfaro EJ, Cortés J (2012) Atmospheric forcing of cold subsurface water events in Bahía Culebra, Costa Rica. Rev Biol Trop 60(suppl 2):173–186
Alfaro EJ, Cortés J, Alvarado JJ, Jiménez C, León A, Sánchez-Noguera C, Nivia-Ruiz J, Ruiz-Campos E (2012) Clima y variabilidad climática de la temperatura subsuperficial del mar en Bahía Culebra, Guanacaste, Costa Rica. Rev Biol Trop 60(Suppl 2):159–171
Alory G, Maes C, Delcroix T, Reul N, Illig S (2012) Seasonal dynamics of sea surface salinity off Panama: the far Eastern Pacific Fresh Pool. J Geophys Res 117:C04028. doi:10.1029/2011JC007802
Alvarado JJ, Cortés J, Fernández C, Nivia J (2005) Coral communities and reefs of Ballena Marine National Park, Pacific coast of Costa Rica. Cienc Mar 31:641–651
Alvarado JJ, Fernández C, Cortés J (2009) Water quality conditions on coral reefs at the Marino Ballena National Park, Pacific Costa Rica. Bull Mar Sci 84:137–152
Alvarado JJ, Reyes-Bonilla H, del Castillo Á, Cárdenas PA, Buitrago F, Aguirre-Rubí J (2010) Coral reefs of the Pacific coast of Nicaragua. Coral Reefs 29:201
Alvarado JJ, Ayala A, Álvarez del Castillo-Cárdenas PA, Fernández C, Aguirre-Rubí J, Buitrago F, Reyes-Bonilla H (2011) Coral communities of San Juan del Sur, Pacific Nicaragua. Bull Mar Sci 87:129–146
Álvarez-Filip L, Reyes-Bonilla H, Calderón-Aguilera LE (2006) Community structure of reef fishes in Cabo Pulmo reef, Gulf of California. Mar Ecol 27:253–262
Asher J, Maragos J, Kenyon J, Vargas-Ángel B, Coccagna E (2012) Range extensions for several species of Acropora in the Hawaiian Archipelago and the Papahānaumokuākea Marine National Monument. Bull Mar Sci 88:337–338
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:435–471
Banks SA (2002) Ambiente físico. In: Danulat E, Edgar GJ (eds) Reserva Marina de Galápagos. Línea Base de la Biodiversidad. Fundación Charles Darwin/Servicio Parque Nacional Galápagos, Santa Cruz, Galápagos, Ecuador, pp 22–37
Banks SA, Vera M, Chiriboga Á (2009) Characterizing the last remaining reefs: establishing reference points to assess long term change in Galápagos zooxanthellate coral communities. Galapagos Res 66:43–64
Banta WC (1991) The Bryozoa of the Galápagos. In: James MJ (ed) Galápagos marine invertebrates: taxonomy, biogeography, and evolution in Darwin’s islands. Plenum Press, New York, pp 371–389
Barham EG, Gowdy RW, Wolfson FH (1973) Acanthaster (Echinodermata, Asteroidea) in the Gulf of California. Fish Bull (Seattle) 71:927–942
Bassey-Fallas G (2010) Evaluación ecológica de los arrecifes y comunidades coralinas de las Islas Murciélago y sección norte de la Península de Santa Elena en el Pacífico de Costa Rica. MSc thesis, Universidad Nacional Heredia, Costa Rica
Bastidas-Salamanca M, Rangel-Buitrago N, Morales-Giraldo D, Ricaurte C, Gomez-López DI, Navas-Camacho R, Navarrete SM, Alonso DA, Vivas-Aguas LJ, Obando-Madera PS, Rodríguez-Rodríguez JA, Licero-Villanueva LV, Perdomo LV (2014) Estado del ambiente y los ecosistemas marinos y costeros. In: INVEMAR. Informe del estado de los ambientes y recursos marinos y costeros en Colombia: Año 2013. Serie de Publicaciones Periódicas No. 3, Santa Marta, pp 23–71
Baums IB, Boulay JN, Polato NR, Hellberg ME (2012) No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol Ecol 21:5418–5433
Baums IB, Durante MD, Laing AA, Feingold J, Smith T, Bruckner A, Monteiro J (2014) Marginal coral populations: the densest known aggregation of Pocillopora in the Galápagos Archipelago is of asexual origin. Front Mar Sci 1:59. doi:10.3389/fmars.2014.00059
Béarez P, Séret B (2009) Les poisons. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Mus National d’Hist Naturelle, Paris IRD, Marseille, pp 143–154
Benfield S, Baxter L, Guzmán HM, Mair JM (2008) A comparison of coral reef and coral community fish assemblages in Pacific Panama and environmental factors governing their structure. J Mar Biol Assoc UK 88:1331–1341
Benítez-Villalobos F, Domínguez y Gómez MT, López-Pérez RA (2008) Temporal variation of the sea urchin Diadema mexicanum population density at Bahías de Huatulco, western Mexico. Rev Biol Trop (Int J Trop Biol) 56(Suppl 3):255–263
Benzoni F, Stefani F, Pichon M, Galli P (2010) The name game: morpho-molecular species boundaries in the genus Psammocora (Cnidaria, Scleractinia). Zool J Linnean Soc 160:421–456
Bezy MB, Jiménez C, Cortés J, Segura A, León A, Alvarado JJ, Gillén C, Mejía E (2006) Contrasting Psammocora-dominated coral communities in Costa Rica, tropical eastern Pacific. In: Proceedings of 10th International Coral Reef Symposium, vol 1, Okinawa, pp 376–381
Birkeland C, Meyer DL, Stames JP, Buford CL (1975) Subtidal communities of Malpelo Island. Smithson Contrib Zool 176:55–68
Blancas-López AV (2009) Conectividad demográfica de Pocillopora verrucosa (Anthozoa: Scleractinia) en el Pacífico mexicano. MSc thesis CICESE, p 83
Boulay JN, Hellberg ME, Cortés J, Baums IB (2014) Unrecognized coral species diversity masks differences in functional ecology. Proc R Soc B 281:20131580
Brenes R, Cuadras J, Durbán M, Fernández-López A, González LM, Miranda A (1993) Plan de manejo del Parque Nacional Coiba (1a fase). Informe inédito, AECI, ICONA, INRENARE, Panamá, 213 p +19 maps
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York p 475
Bruno J, Selig E, Casey K, Page C, Willis B, Harvell C, Sweatman HY, Melendy A (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. doi:10.1371/journal.pbio.0050124
Brusca RC, Thomson DA (1975) Pulmo Reef: the only “coral reef” in the Gulf of California. Cienc Mar 2:37–52
Cantera JR (1983) Distribution des peuplements des scléractiniaires sur un récif de l’Ille de Gorgona (Côte Pacifique de Colombie). Tethys 11:25–31
Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks SA, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, DeVantier L, Edgar G, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron J, Wallace C, Weil E, Wood E (2008) One third of reef-building corals face elevated extinction risk from climate change and local impact. Science 321:560–563
Carricart-Ganivet JP, Reyes-Bonilla H (1999) New and previous records of scleractinian corals from Clipperton Atoll, eastern Pacific. Pac Sci 53:370–375
Carriquiry JD, Reyes-Bonilla H (1997) Estructura de la comunidad y distribución geográfica de los arrecifes coralinos de Nayarit. Cienc Mar 23:227–248
Castilla JC, Rozbaczylo N (1987) Invertebrados marinos de Isla de Pascua y Sala y Gómez. In: Castilla JC (ed) Islas Oceánicas Chilenas: Conocimiento científicos y necesidades de investigaciones. Ediciones Univ Católica de Chile, Santiago, pp 191–215
Cea Egaña A, DiSalvo LH (1982) Mass expulsion of zooxanthellae by Easter Island corals. Pac Sci 36:61–63
Charpy L, Rodier M, Sarazin G (2009) Biogéochimie du lagon. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Muséum national d’Histoire naturelle, Paris, IRD, Marseille. Patrimoines naturels 68:67–79
Charpy L, Rodier M, Couté A, Perrette-Gallet C, Bley-Loëz C (2010) Clipperton, a possible future for atoll lagoons. Coral Reefs 29:771–783
Chavez-Romo HE, Correa-Sandoval F, Paz-Garcia DA, Reyes-Bonilla H, López-Pérez RA, Medina-Rosas P, Hernández-Cortés MP (2008) Genetic structure of a scleractinian coral, Pocillopora damicornis, in the Mexican Pacific. Proceedings of 11th International Coral Reef Symposium, vol 1, Ft Lauderdale, pp 429–433
Chevalier J-P (1978) Les coraux des Iles Marquises. In: Les Iles Marquises, geómorphologie, climatologie, faune et flore. Cah Pac No 21 Paris, pp 243–283
Clemente S, Rodríguez A, Brito A, Ramos A, Monterroso Ó, Hernández JC (2011) On the occurrence of the hydrocoral Millepora (Hydrozoa: Milleporidae) in the subtropical eastern Atlantic (Canary Islands): is the colonization related to climatic events? Coral Reefs 30:237–240
Coates AG, Obando JA (1996) The geologic evolution of the Central American isthmus. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. University of Chicago Press, Chicago, pp 21–56
Colgan MW (1990) El Niño and the history of eastern Pacific reef building. In: Glynn PW (ed) Global ecological consequences of the 1982–83 El Niño-Southern Oscillation. Elsevier oceanography series 52. Elsevier, Amsterdam, pp 183–232
Colgan MW (1991) El Niño and coral reef development in the Galápagos Islands: a study of the Urvina Bay uplift. In: James MJ (ed) Galápagos marine invertebrates: taxonomy, biogeography, and evolution in Darwin’s Islands. Plenum Press, New York, pp 99–120
Colley SB, Glynn PW, May AS, Maté JL (2006) Species-dependent reproductive responses of eastern Pacific corals to the 1997–1998 ENSO event. In: Proceedings of 10th International Coral Reef Symposium, vol 1, pp 61–70
Correa AMS, Baker AC (2011) Disaster taxa in microbially mediated metazoans: how endosymbionts and environmental catastrophes influence the adaptive capacity of reef corals. Glob Change Biol 17:68–75
Cortés J (1986) Biogeografía de corales hermatípicos: El Istmo Centro Americano. An Inst Cienc del Mar y Limnol, Univ Nal Autón Mexico 13:297–304
Cortés J (1990) The coral reefs of Golfo Dulce, Costa Rica: distribution and community structure. Atoll Res Bull 344:1–37
Cortés J (1996/1997) Comunidades coralinas y arrecifes del Área de Conservación Guanacaste, Costa Rica. Rev Biol Trop 44/45:623–625
Cortés J (1997) Biology and geology of coral reefs of the eastern Pacific. Coral Reefs 16(suppl):S39–S46
Cortés J (2003) Latin American coral reefs. Elsevier, Amsterdam, p 497
Cortés J (2007) Coastal morphology and coral reefs. In: Bundschuh J, Alvarado GE (eds) Central America: geology, resources, and hazards, vol 1. Taylor & Francis, Milton Park, pp 185–200
Cortés J (2008) Historia de la investigación marina de la Isla del Coco, Costa Rica. Rev Biol Trop 56(suppl 2):1–18
Cortés J (2011) Eastern Tropical Pacific coral reefs. In: Hopley D (ed) The Encyclopedia of Modern Coral Reefs: Structure, Form and Process. Springer, Berlin pp 351–358
Cortés J (2012a) Historia de la investigación marina en Bahía Culebra, Guanacaste, Costa Rica. Rev Biol Trop 60 (Suppl 2):19–37
Cortés J (2012b) Marine biodiversity of an Eastern Tropical Pacific oceanic island, Parque Nacional Isla del Coco, Costa Rica. Rev Biol Trop 60 (Suppl 3):131–185
Cortés J (2014) Cocos Island marine ecosystems. In: Kappelle M (ed) Costa Rican ecosystems. University of Chicago Press, Chicago (in press)
Cortés J, Guzmán HM (1998) Organismos de los arrecifes coralinos de Costa Rica: descripción, distribución geográfica e historia natural de los corales zooxantelados (Anthozoa: Scleractinia) del Pacífico. Rev Biol Trop 46:55–92
Cortés J, Jiménez CE (1996) Coastal-marine environments of Parque Nacional Corcovado, Puntarenas, Costa Rica. Rev Biol Trop 44(suppl 3):35–40
Cortés J, Jiménez C (2003) Corals and coral reefs of the Pacific of Costa Rica: history, research and status. In: Cortés J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 361–385
Cortés J, Murillo MM (1985) Comunidades coralinas y arrecifes del Pacífico de Costa Rica. Rev Biol Trop 33:197–202
Cortés J, Wehrtmann IS (2009) Diversity of marine habitats of the Caribbean and Pacific of Costa Rica. In: Wehrtmann IS, Cortés J (eds) Marine biodiversity of Costa Rica, Central America. Springer, Berlin, pp 1–45
Cortés J, Macintyre IG, Glynn PW (1994) Holocene growth history of an eastern Pacific fringing reef, Punta Islotes, Costa Rica. Coral Reefs 13:65–73
Cortés J, Jiménez CE, Fonseca AC, Alvarado JJ (2010) Status and conservation of coral reefs in Costa Rica. Rev Biol Trop 58(Suppl 1):33–50
Cróquer A, Weil E (2009) Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Dis Aquat Org 87:33–43
D’Croz L, O’Dea A (2007) Variability in upwelling along the Pacific shelf of Panama and implications for the distribution of nutrients and chlorophyll. Estuar Coastal Shelf Sci 73:325–340
D’Croz L, Robertson DR (1997) Coastal oceanographic conditions affecting coral reefs on both sides of the Isthmus of Panama. In: Proceedings of 8th International Coral Reef Symposium, vol 2, Panama, pp 2053–2058
Dana TF (1975) Development of contemporary eastern Pacific coral reefs. Mar Biol 33:355–374
Dana TF, Wolfson A (1970) Eastern Pacific crown-of-thorns starfish populations in the lower Gulf of California. San Diego Soc Nat Hist Trans 16:83–90
Darwin C (1880) The origin of species by means of natural selection or the preservation of favored races in the struggle for life. John Murray, London, p 458
Dawson TP, Jarvie F, Reitsma F (2009) A habitat suitability model for predicting coral community and reef distributions in the Galapagos Islands. Galapagos Res 66:20–26
Delesalle B (1985) Atoll de Mataiva: archipel des Tuamotu. In: Delesalle B, Galzin R, Salvat B (eds) French Polynesian coral reefs. Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 269–307
Denyer P, Cárdenas G (2000) Costas marinas. In: Denyer P, Kussmaul S (eds) Geología de Costa Rica. Editorial. Tecnología de Costa Rica, Cartago, pp 185–218
Devis-Morales A, Schneider W, Montoya-Sánchez RA, Rodríguez-Rubio E (2008) Monsoonlike winds reverse oceanic circulation in the Panama Bight. Geophys Res Lett 35:L20607. doi:10.1029/2008GL035172
DiSalvo LH, Randall JE, Cea A (1988) Ecological reconnaissance of the Easter Island sublittoral marine environment. Nat Geogr Res 4:451–473
Dominici-Arosemena A, Wolff M (2006) Reef fish community structure in the tropical eastern Pacific (Panamá): living on a relatively stable rocky reef environment. Helgol Mar Res 60:287–305
Dunbar RB, Wellington GM, Colgan MW, Glynn PW (1994) Eastern Pacific sea surface temperature since 1600 AD: the δ18O record of climate variability in Galápagos corals. Paleoceanography 9:291–315
Durham JW (1966) Coelenterates, especially stony corals, from the Galápagos and Cocos Islands. In: Bowman RI (ed) The Galápagos, Proceedings of the symposia of the Galápagos international scientific project. University of California Press, Berkeley, pp 123–135
Durham JW, Barnard JL (1952) Stony corals of the eastern Pacific collected by the Velero III and Velero IV. Allan Hancock Pac Expeditions 16:1–110
Eakin CM (1992) Post-El Niño Panamanian reefs: less accretion, more erosion and damselfish protection. In: Proceedings of 7th International Coral Reef Symposium, vol 1, Guam, pp 387–396
Eakin CM (1996) Where have all the carbonates gone? A model comparison of calcium carbonate budgets before and after the 1982–1983 El Niño. Coral Reefs 15:109–119
Eakin CM (2001) A tale of two ENSO events: carbonate budgets and the influence of two warming disturbances and intervening variability, Uva Island, Panama. Bull Mar Sci 69:171–186
Edgar GJ, Banks SA, Brandt M, Bustamante RH, Chiriboga Á, Earle SA, Garske LE, Glynn PW, Grove JS, Henderson S, Hickman CP, Miller KA, Rivera F, Wellington GM (2010) El Niño, grazers and fisheries interact to greatly elevate extinction risk for Galápagos marine species. Global Change Biol 16:2876–2890
Ekman S (1953) Zoogeography of the sea. Sidgwick and Jackson, London p 417
Emerson WK (1991) First records for Cymatium mundum (Gould) in the eastern Pacific Ocean, with comments on the zoogeography of the tropical trans-Pacific tonnacean and non-tannacean prosobranch gastropods with Indo-Pacific faunal affinities in west American waters. The Nautilus 105:62–80
Enochs IC (2012) Motile cryptofauna associated with live and dead coral substrates: implications for coral mortality and framework erosion. Mar Biol 159:709–722
Enochs IC, Hockensmith G (2009) Effects of coral mortality on the community composition of cryptic metazoans associated with Pocillopora damicornis. In: Proceedings of 11th International Coral Reef Symposium, vol 2, Ft Lauderdale, pp 1368–1372
Enochs IC, Manzello DP (2012a) Responses of cryptofaunal species richness and trophic potential to coral reef habitat degradation. Diversity 4:94–104
Enochs IC, Manzello DP (2012b) Species richness of motile cryptofauna across a gradient of reef framework erosion. Coral Reefs 31:653–661
Enochs IC, Toth LT, Brandtneris VK, Afflerbach JC, Manzello DP (2011) Environmental determinants of motile cryptofauna on an eastern Pacific coral reef. Mar Ecol Prog Ser 438:105–118
Feingold JS (1996) Coral survivors of the 1982–83 El Niño-Southern Oscillation, Galápagos Islands, Ecuador. Coral Reefs 15:108
Feingold JS (2001) Responses of three coral communities to the 1997–98 El Niño-Southern Oscillation: Galápagos Islands, Ecuador. Bull Mar Sci 69:61–77
Feingold JS, Glynn PW (2014) Coral research in the Galápagos Islands, Ecuador. In: Walsh SJ, Mena CF (eds) The Galápagos marine reserve: a dynamic social-ecological system. Springer Science and Business Media, New York, pp 3–22
Fernández-García C (2007) Propagación del alga Caulerpa sertularioides (Chlorophyta) en Bahía Culebra, Golfo de Papagayo, Pacífico norte de Costa Rica. M.Sc. thesis, Universidad de Costa Rica, San José, Costa Rica p 92
Fernández C, Cortés J (2005) Reef site: Caulerpa sertularioides, a green alga spreading aggressively over coral reef communities in Culebra Bay, North Pacific of Costa Rica. Coral Reefs 24:10
Fernández-García C, Cortés J, Alvarado JJ, Nivia-Ruiz J (2012) Physical factors contributing to the benthic dominance of the alga Caulerpa sertularioides (Caulerpaceae, Chlorophyta) in the upwelling Bahía Culebra, north Pacific of Costa Rica. Rev Biol Trop 60(Suppl 2):93–107
Flores-Verdugo FJ, de la Lanza-Espino G, Contreras-Espinosa F, Agraz-Hernández CM (2001) The tropical Pacific coast of Mexico. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Ecol Stud 144:307–314. Springer, Berlin
Flot JF, Adjeroud M (2009) Les coraux de Clipperton. In: Charpy L (ed) Clipperton: environnement et biodiversité d’un microcosme océanique. Muséum national d’Histoire naturelle, Paris, IRD, Marseille (Patrimoines naturels; 68), pp 155–162
Flot J-F, Couloux A, Tillier S (2010) Haplowebs as a graphical tool for delimiting species: a revival of Doyle’s “field for recombination” approach and its application to the coral genus Pocillopora in Clipperton. BMC Evol Biol 10:372
Forsbergh ED (1969) On the climatology, oceanography and fisheries of the Panama Bight. Inter-Am Trop Tuna Comm Bull 14:49–385
Forsman ZH, Guzmán HM, Chen CA, Fox GE, Wellington GM (2005) An ITS region phylogeny of Siderastrea (Cnidaria: Anthozoa): is S. glynni endangered or introduced? Coral Reefs 24:343–347
Friedlander AM, Ballesteros E, Beets J, Berkenpas E, Gaymer CF, Gorny M, Sala E (2013) Effects of isolation and fishing on the marine ecosystems of Easter Island and Salas y Gómez, Chile. Aquatic Conserv: Mar Freshw Ecosyst 23:515–531
Galzin R, Pointier J-P (1985) Ile de Moorea: Archipel de la Société. In: Delesalle B, Galzin R, Salvat B (eds) French Polynesian coral reefs. In: Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 75–101
Garth JS (1991) Taxonomy, distribution, and ecology of Galápagos Brachyura. In: James MJ (ed) Galápagos marine invertebrates: taxonomy, biogeography, and evolution in Darwin’s islands. Plenum Press, New York, pp 123–145
Garth JS (1992) The brachyuran crabs of the Revillagigedo Islands, Colima, Mexico with remarks on insular endemism in the eastern tropical Pacific. Proc San Diego Soc Nat Hist 24:1–6
Garzón-Ferreira J, Pinzón J (1999) Evaluación rápida de estructura y salud de las formaciones coralinas de la Isla de Malpelo (Pacífico colombiano). Bol Invest Mar Cost 28:137–154
Glynn PW (1976) Some physical and biological determinants of coral community structure in the eastern Pacific. Ecol Monogr 46:431–456
Glynn PW (1988) El Niño warming, coral mortality and reef framework destruction by echinoid bioerosion in the eastern Pacific. Galaxea 7:129–160
Glynn PW (1997) Eastern Pacific reef coral biogeography and faunal flux: Durham’s dilemma revisited. In: Proceedings of 8th International Coral Reef Symposium, vol 1, Panama, pp 371–378
Glynn PW (2001) Eastern Pacific coral reef ecosystems. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Springer, Berlin, pp 281–305
Glynn PW (2003) Coral communities and coral reefs of Ecuador. In: Cortés J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 449–472
Glynn PW (2006) Fish utilization of simulated coral reef frameworks versus eroded rubble off Panama, eastern Pacific. In: Proceedings of 10th International Coral Reef Symposium, vol 10, Okinawa, pp 250–256
Glynn PW (2008) Food-web structure and dynamics of eastern tropical Pacific coral reefs: Panama and Galápagos Islands. In: McClanahan TR, Branch GM (eds) Food webs and the dynamics of marine reefs. Oxford University Press, Oxford, pp 185–208
Glynn PW (2011) In tandem reef coral and cryptic metazoan declines and extinctions. Bull Mar Sci 87:767–794
Glynn PW, Ault JS (2000) A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19:1–23
Glynn PW, Colley SB (2008) Survival of brooding and broadcasting reef corals following large scale disturbances: is there any hope for broadcasting species during global warming? In: Proceedings of 11th International Coral Reef Symposium, Ft. Lauderdale (session 11), pp 1–5
Glynn PW, de Weerdt WH (1991) Elimination of two reef-building hydrocorals following the 1982–83 El Niño warming event. Science 253:69–71
Glynn PW, Feingold JS (1992) Hydrocoral species not extinct. Science 257:1845
Glynn PW, Fong P (2006) Patterns of reef coral recovery by the regrowth of surviving tissues following the 1997–98 El Niño warming and 2000, 2001 upwelling cool events in Panama, eastern Pacific. In: Proceedings of 10th International Coral Reef Symposium, Okinawa, pp 624–630
Glynn PW, Leyte-Morales GE (1997) Coral reefs of Huatulco, west Mexico. Reef development in upwelling Gulf of Tehuantepec. Rev Biol Trop 45:1033–1047
Glynn PW, Wellington GM (1983) Corals and coral reefs of the Galápagos Islands. University of California Press, Berkeley, p 297
Glynn PW, von Prahl H, Guhl F (1982) Coral reefs of Gorgona Island, with special reference to corallivores and their influence on community structure and reef development. An Inst Invest Mar Punta Betín 12:185–214
Glynn PW, Druffel EM, Dunbar RB (1983) A dead Central American coral reef tract: possible link with the Little Ice Age. J Mar Res 41:605–637
Glynn PW, Cortés J, Guzmán HM, Richmond RH (1988) El Niño (1982–83) associated coral mortality and relationship to sea surface temperature deviations in the tropical eastern Pacific. In: Proceedings of 6th International Coral Reef Symposium, vol 3, Brisbane, pp 237–243
Glynn PW, Gassman NJ, Eakin CM, Cortés J, Smith DB, Guzmán HM (1991) Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galápagos Islands (Ecuador), Part I—Pocilloporidae. Mar Biol 109:355–368
Glynn PW, Colley SB, Eakin CM, Smith DB, Cortés J, Gassman NJ, Guzmán HM, Rosario JB, Feingold J (1994) Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galápagos Islands (Ecuador) II. Poritidae. Mar Biol 118:191–208
Glynn PW, Veron JEN, Wellington GM (1996a) Clipperton Atoll (eastern Pacific): oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs 15:71–99
Glynn PW, Colley SB, Gassman NJ, Black K, Cortés J, Maté JL (1996b) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). III. Agariciidae (Pavona gigantea and Gardineroseris planulata). Mar Biol 125:579–601
Glynn PW, Colley SB, Ting JH, Maté JL, Guzmán HM (2000) Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galápagos Islands (Ecuador)—IV. Agariciidae, recruitment and recovery of Pavona varians and Pavona sp.a. Mar Biol 136:785–805
Glynn PW, Maté JL, Baker AC, Calderón MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Glynn PW, Wellington GM, Wieters EA, Navarrete SA (2003) Reef-building coral communities of Easter Island (Rapa Nui), Chile. In: Cortés J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 473–494
Glynn PW, Wellington GM, Riegl B, Olson DB, Borneman E, Wieters EA (2007) Diversity and biogeography of the scleractinian coral fauna of Easter Island (Rapa Nui). Pac Sci 61:67–90
Glynn PW, Colley SB, Maté JL, Cortés J, Guzmán HM, Bailey RL, Feingold JS, Enochs IC (2008) Reproductive ecology of the azooxanthellate coral Tubastrea coccinea in the Equatorial Eastern Pacific: Part V. Dendrophylliidae. Mar Biol 153:529–544
Glynn PW, Riegl B, Romanski AMS, Baums IB (2009) Rapid recovery of a coral reef at Darwin Island, Galapagos Islands. Galapagos Res 66:6–13
Glynn PW, Colley SB, Guzmán HM, Enochs IC, Cortés J, Maté JL, Feingold JS (2011) Reef coral reproduction in the eastern Pacific: Costa Rica, Panama and the Galápagos Islands (Ecuador). VI. Agariciidae, Pavona clavus. Mar Biol 158:1601–1617
Glynn PW, Colley SB, Maté JL, Baums IB, Feingold JS, Cortés J, Guzmán HM, Afflerbach JC, Brandtneris VW, Ault JS (2012) Reef coral reproduction in the equatorial eastern Pacific: Costa Rica, Panama, and the Galápagos Islands (Ecuador). VII. Siderastreidae, Psammocora stellata and Psammocora profundacella. Mar Biol 159:1917–1932
Glynn PW, Enochs IC, Afflerbach JA, Brandtneris VW, Serafy JE (2014) Eastern Pacific reef fish responses to coral reef recovery following El Niño disturbances. Mar Ecol Prog Ser 495:233–247
Glynn PW, Riegl BM, Purkis S, Kerr J, Smith T (2015) Coral reef recovery in the Galápagos Islands: the northern-most islands (Darwin and Wenman). Coral Reefs 34:421–436. doi:10.1007/s00338-015-1280-4
Gotuzzo R (1996) Celenterados. In: Serrano F (ed) Historia natural y ecología de El Salvador, vol II. Ministerio de Educación, San Salvador, El Salvador, pp 51–64
Grigg RW, Hey R (1992) Paleoceanography of the tropical eastern Pacific Ocean. Science 255:172–178
Guzmán HM (1988a) Distribución y abundancia de organismos coralívoros en los arrecifes coralinos de la Isla del Caño, Costa Rica. Rev Biol Trop 36:191–207
Guzmán HM (1988b) Feeding behavior of the gastropod corallivore Quoyula monodonta (Blainville). Rev Biol Trop 36:209–212
Guzmán HM, Cortés J (1989a) Coral reef community structure at Caño Island, Pacific Costa Rica. PSZNI: Mar Ecol 10:23–41
Guzmán HM, Cortés J (1989b) Growth rates of eight species of scleractinian corals in the eastern Pacific (Costa Rica). Bull Mar Sci 44:1186–1194
Guzmán HM, Cortés J (1992) Cocos Island (Pacific of Costa Rica) coral reefs after the 1982–83 El Niño disturbance. Rev Biol Trop 40:309–324
Guzmán HM, Cortés J (1993) Arrecifes coralinos del Pacífico oriental tropical: Revisión y perspectivas. Rev Biol Trop 41:535–557
Guzmán HM, Cortés J (2001) Changes in reef community structure after fifteen years of natural disturbances in the eastern Pacific (Costa Rica). Bull Mar Sci 69:133–149
Guzmán HM, Cortés J (2007) Reef recovery 20-yr after the 1982–83 El Niño massive mortality. Mar Biol 151:401–411
Guzmán HM, Obando VL (1988) Diversidad y abundancia diaria y estacional del zooplankton marino de la Isla del Caño, Costa Rica. Rev Biol Trop 36:139–150
Guzmán HM, Obando VL, Cortés J (1987a) Meiofauna associated with a Pacific coral reef in Costa Rica. Coral Reefs 6:107–112
Guzmán HM, Cortés J, Richmond RH, Glynn PW (1987b) Efectos del fenómeno de “El Niño-Oscilación Sureña” 1982/83 en los arrecifes de la Isla del Caño, Costa Rica. Rev Biol Trop 35:325–332
Guzmán HM, Cortés J, Glynn PW, Richmond RH (1990) Coral mortality associated with dinoflagellate blooms in the eastern Pacific (Costa Rica and Panama). Mar Ecol Prog Ser 60:299–303
Guzmán HM, Guevara CA, Breedy O (2004) Distribution, diversity, and conservation of coral reefs and coral communities in the largest marine protected area of Pacific Panama (Coiba Island). Environ Conserv 31:111–121
Guzmán HM, Benfield S, Breedy O, Mair JM (2008) Broadening reef protection across the marine conservation corridor of the eastern tropical Pacific: distribution and diversity of reefs in Las Perlas Archipelago, Panama. Environ Conserv 35:46–54
Halfar J, Godinez-Orta L, Riegl B, Valdez-Holguin JE, Borges JM (2005) Living on the edge: high latitude Porites carbonate production under temperate eutrophic conditions. Coral Reefs 24:582–592
Harmelin-Vivien M (1985) Tikehau Atoll, Tuamotu Archipelago. In: Delesalle B, Galzin R, Salvat B (eds) Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 211–268
Hebbeln D, Cortés J (2001) Sedimentation in a tropical fyord: Golfo Dulce, Costa Rica. Geo-Mar Lett 20:142–148
Heck KL Jr, McCoy ED (1978) Long-distance dispersal and the reef-building corals of the Eastern Pacific. Mar Biol 48:349–356
Hendrickx ME, Brusca RC, Findley LT (2005) A distributional checklist of the macrofauna of the Gulf of California, Mexico. Part 1. Invertebrates. Tucson, Arizona-Sonora Desert Museum, p 429
Herrera W (1986) Clima de Costa Rica. Edit. UNED, San José, Costa Rica, p 118
Herrera-Escalante T, López-Pérez RA, Leyte-Morales GE (2005) Bioerosion caused by the sea urchin Diadema mexicanum (Echinodermata: Echinoidea) at Bahías de Huatulco, western Mexico. Rev Biol Trop 53(Suppl 3):263–273
Hickman PC Jr (2008) A field guide to corals and other radiates of Galápagos. Galápagos marine life series. Sugar Spring Press, Lexington, Virgina p 161
Holst I, Guzmán HM (1993) Lista de corales hermatípicos (Anthozoa: Scleractinia; Hydrozoa: Milleporina) a ambos lados del Istmo de Panamá. Rev Biol Trop 41:871–875
Hubbard DK, Garcia M (2003) The corals and coral reefs of Easter Island—A preliminary look. In: Loret J, Tanacredi JT (eds) Easter Island: scientific exploration into the world’s environmental problems in microcosm. Kluwer Academic, New York, pp 53–77
Iglesias-Prieto R, Reyes-Bonilla H, Riosmena-Rodriguez R (2003) Effects of 1997–1998 ENSO on coral reef communities in the Gulf of California, Mexico. Geofísica Int 42:1–5
Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE (2004) Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc Lond B 271:1757–1763
Irving R, Dawson T (2012) The marine environment of the Pitcairn Islands. The Pew Environment Group, Global Ocean Legacy, Dundee University Press, p 61
Jiménez C (1997) Corals and coral reefs of Culebra Bay, Pacific coast of Costa Rica: anarchy in the reef. In: Proceedings of 8th International Coral Reef Symposium, vol 1, Panama, pp 329–334
Jiménez C (2001a) Arrecifes y ambientes coralinos de Bahía Culebra, Pacífico de Costa Rica: aspectos biológicos, económico-recreactivos y de manejo. Rev Biol Trop 49(Suppl 2):215–231
Jiménez C (2001b) Seawater temperature measured at the surface and at two depths (7 and 14 m) in one coral reef at Culebra Bay, Gulf of Papagayo, Costa Rica. Rev Biol Trop 49(2):153–161
Jiménez C (2007a) Evaluación ecológica rápida del arrecife coralino de Playa Matapalo (Golfo de Papagayo), uno de los arrecifes más extensos en la costa del Pacífico de Costa Rica. Informe para Conservación Internacional, San José, Costa Rica, p 42
Jiménez C (2007b) Evaluación rápida del blanqueamiento y mortalidad de corales ocurrida en el Golfo de Papagayo (Septiembre-Diciembre 2007). Unpub report to Vicerrectoría de Investigación de la Universidad de Costa Rica, p 36
Jiménez CE, Cortés J (2001) Effects of the 1991-1992 El Niño on scleractinian corals of the Costa Rican central Pacific coast. Rev Biol Trop 49 (Supl 2):239-250
Jiménez J, Soto R (1985) Patrones regionales en la estructura y composición florística de los manglares de la costa Pacífica de Costa Rica. Rev Biol Trop 33:25–37
Jiménez C, Cortés J, León A, Ruíz E (2001) Coral bleaching and mortality associated with El Niño 1997/98 event in an upwelling environment in the eastern Pacific (Gulf of Papagayo, Costa Rica). Bull Mar Sci 69:151–169
Jiménez C, Sánchez C, Ruíz E, Buxton T (2009) Informe de la re-evaluación de algunos ambientes coralinos de las Islas Murciélagos (ACG), Mayo 2009. Unpubl report to Research Program Área de Conservación Guanacaste (ACG), p 29
Jiménez C, Bassey G, Segura Á, Cortés J (2010) Characterization of the coral communities and reefs of two previously undescribed locations in the upwelling region of Gulf of Papagayo (Costa Rica). Rev Mar Cost 2:95–108
Johnson ME, Backus DH, Riosmena-Rodríguez R (2009) Contribution of rhodoliths to the generation of Pliocene-Pleistocene limestone in the Gulf of California. In: Johnson ME, Ledesma-Vázquez J (eds) Atlas of coastal ecosystems in the western Gulf of California: tracking limestone deposits on the margin of a young sea. University of Arizona Press, Tucson, pp 83–94
Kaiser KL (2009) Les mollusques. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Mus National d’Hist Naturelle, Paris IRD, Marseille, pp 217–234
Kappelle M, Castro M, Acevedo H, González L, Monge H (2002) Ecosistemas del área de Conservación Osa (ACOSA). Edit. INBio, Sto. Domingo de Heredia, Costa Rica, p 500
Karlson RH (1999) Dynamics of coral communities. Kluwer Academic Publisher, Drodrecht, p 290
Ketchum JT, Reyes-Bonilla H (1997) Biogeography of hermatypic corals of the Archipiélago Revillagigedo, Mexico. In: Proceedings of 8th International Coral Reef Symposium, vol 1, Panama, pp 471–476
Ketchum JT, Reyes-Bonilla H (2001) Taxonomía y distribución de los corales hermatípicos (Scleractinia) del Archipiélago de Revillagigedo, México. Rev Biol Trop 49:803–848
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Ledesma-Vázquez J, Johnson ME, Gonzalez-Yajimovich O, Santamaría-del-Ángel E (2009) Gulf of California geography, geological origins, oceanography, and sedimentation patterns. In: Johnson ME, Ledesma-Vázquez J (eds) Atlas of coastal ecosystems in the western Gulf of California: tracking limestone deposits on the margin of a young sea. University of Arizona Press, Tucson, pp 1–10
Lemus LS, Pocasangre JA, Zelaya TD (1994) Evaluación del estado actual de la distribución y cobertura de los arrecifes coralinos en la zona de Los Cóbanos, Departamento de Sonsonate. Licenciatura thesis, Escuela de Biología, Facultad de Ciencias Naturales y Matemática, Univ de El Salvador, San Salvador, p 67
Lessios HA, Robertson DR (2006) Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc R Soc London B 273:2201–2208
Lessios HA, Kessing BD, Robertson DR (1998) Massive gene flow across the world’s most potent marine biogeographic barrier. Proc R Soc London B 265:583–588
Lewis JB (1989) The ecology of Millepora: a review. Coral Reefs 8:99–107
Leyte-Morales GE (2000) Perturbaciones naturales y antropogénicas en las comunidades coralinas de Oaxaca (1977–1998). Resumen. XII Congreso Nacional de Oceanografía. Huatulco, Oaxaca, p 5
Lirman D, Glynn PW, Baker AC, Leyte-Morales GE (2001) Combined effects of three sequential storms on the Huatulco coral reef tract, Mexico. Bull Mar Sci 69:267–278
Lobel PS, Lobel LK (2008) Aspects of the biology and geomorphology of Johnston and Wake Atolls, Pacific Ocean. In: Riegl BM, Dodge RE (eds) Coral reefs of the USA, Coral reefs of the world 1. Springer, Berlin, pp 655–689
López WA, Jiménez C (2008) Primer registro de Psammocora obtusangula y P. stellata (Anthozoa: Scleractinia: Siderastreidae) para la costa salvadoreña. Resúmenes XII Congreso de la Sociedad Mesoamericana para la Biología y la Conservación (SMBC), San Salvador, El Salvador. Mesoamericana 12(3):99
López-Pérez RA, Budd AF (2009) Coral diversification in the Gulf of California during the Late Miocene to Pleistocene. In: Johnson ME, Ledesma-Vázquez J (eds) Atlas of coastal ecosystems in the western Gulf of California: tracking limestone deposits on the margin of a young sea. University of Arizona Press, Tucson, pp 58–71
López-Pérez RA, Hernández-Ballesteros LM (2004) Coral community structure and dynamics in the Huatulco area, western Mexico. Bull Mar Sci 75:453–472
López-Pérez RA, Hernández-Ballesteros LM, Herrera-Escalante T (2002) Cambio en la dominancia de la comunidad arrecifal en Chachacual, Bahías de Huatulco, Oaxaca. Cienc Mar 16:33–38
López-Pérez RA, Mora-Pérez MG, Leyte-Morales GE (2007) Coral (Anthozoa: Scleractinia) recruitment at Bahías de Huatulco, western Mexico: implications for coral community structure and dynamics. Pac Sci 61:355–369
López-Sandoval DC, Lara-Lara JR, Álvarez-Borrego S (2009) Phytoplankton production by remote sensing in the region off Cabo Corrientes, Mexico. Hidrobiológica 19:185–192
Macintyre IG, Glynn PW, Cortés J (1993) Holocene reef history in the eastern Pacific: mainland Costa Rica, Caño Island, Cocos Island, and Galápagos Islands. In: Proceedings of 7th International Coral Reef Symposium, vol 2, Guam, pp 1174–1184
Manzello DP (2010) Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs 29:749–758
Maragos JE (1974) Reef corals of Fanning Island. Pac Sci 28:247–255
Maragos JE (1995) Revised checklist of extant shallow-water stony coral species from Hawaii (Cnidaria: Anthozoa: Scleractinia). Bishop Mus Occas Pap 42:54–55
Maragos JE, Jokiel PL (1986) Reef corals of Johnston Atoll: one of the world’s most isolated reefs. Coral Reefs 4:141–150
Maragos JE, Potts DC, Aeby G, Gulko D, Kenyon J, Siciliano D, Van Ravenswaay D (2004) 2000–2002 rapid ecological assessment of corals (Anthozoa) on shallow reefs of the northwestern Hawaiian Islands. Part 1: Species and distribution. Pac Sci 58:211–230
Maragos JE, Miller J, Gove J, DeMartini E, Friedlander AM, Godwin S, Musburger C, Timmers M et al (2008a) US coral reefs in the Line and Phoenix Islands, Central Pacific Ocean: history, geology, oceanography, and biology. In: Riegl BM, Dodge RE (eds) Coral reefs of the USA. Springer Science + Business Media BV, Berlin, pp 595–654
Maragos JE, Miller J, Gove J, DeMartini E, Friedlander AM, Godwin S, Musburger C, Timmers M, Tsuda R, Vroom P, Flint E, Lundblad E, Weiss J, Avotte P, Sala E, Sandin S, McTee S, Wass T, Siciliano D, Brainard R, Obura D, Ferguson S, Mundy B (2008b) U.S. coral reefs in the Line and Phoenix Islands, Central Pacific Ocean: history, geology, oceanography, and biology. In: Riegl B, Dodge RE (eds) Coral reefs of the USA. Coral Reefs of the World, vol 1. Springer, Berlin, pp 595–641
Maragos JE, Kenyon J, Aeby G, Vroom P, Vargas-Ángel B, Brainard R, Wedding L, Friedlander A, Asher J, Zgliczynski B, Siciliano D (2009) Benthic communities of the Northwestern Hawaiian Islands. In: Friedlander A, Keller, K, Wedding L, Clarke A, Monaco M (eds) A marine biogeographic assessment of the northwestern Hawaiian Islands. NOAA Tech Memorandum NOS NCCOS 84. Prepared by the NCCOS Biogeography Branch in cooperation with the Office of National Marine Sanctuaries, Silver Spring, Maryland, pp 105–154
Martínez P, Rivera F, Proaño F (2011) Ambientes coralinos del Parque Nacional Machalilla y Reserva de Producción Faunística Marino Costera Puntilla de Santa Elena: Un estudio caso para el manejo de los corales en Ecuador. Reporte Técnico del Instituto Nazca de Investigaciones Marinas y Conservación Internacional, pp 27–50
Maté JL (2003) Corals and coral reefs of the Pacific coast of Panamá. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 387–417
Merlen G, Quevedo FO, Pépolas R (2009) Protection of shallow marine ecosystems in Galápagos by permanent moorings. Galapagos Res 66:75–76
Montaggioni LF (1985) Île de Makatea: Archipel des Tuamotu. In: Delesalle B, Galzin R, Salvat B (eds) Vol 1: French Polynesian coral reefs. Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 103–157
Obura D (2002) Coral species diversity. In: Obura D, Stine GS (eds) Phoenix Island: summary of marine and terrestrial assessment. New England Aquarium, Massachusetts, pp 10–12
Orellana-Amador JJ (1985) Especies marinas de Los Cóbanos. Peces de El Salvador. Mandarin Offset International Ltd, Hong Kong, p 126
Palacios MM, Zapata FA (2014) Fish community structure on coral habitats with contrasting architecture in the Tropical Eastern Pacific. Rev Biol Trop 62(Suppl 1):343–357
Palacios MM, Muñoz CG, Zapata FA (2014) Fish corallivory on a pocilloporid reef and experimental coral responses to predation. Coral Reefs. doi:10.1007/s00338-014-1173-y
Paulay G (1989) Marine invertebrates of the Pitcairn Islands: species composition and biogeography of corals, molluscs and echinoderms. Atoll Res Bull 326:1–28
Payri C, Menon J-L, N’Yeurt A (2009) La flore marine du complexe récifal et quelques aspects de la biodiversité et de la géomorphologie de I’île. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Mus National d’Hist Naturelle, Paris IRD, Marseille, pp 129–141
Paz-García DA, Correa-Sandoval F, Chávez-Romo HE, Reyes-Bonilla H, López-Pérez RA, Medina-Rosas P, Hernández-Cortés P (2008) Genetic structure of the massive coral Porites panamensis (Anthozoa: Scleractinia) from the Mexican Pacific. In: Proceedings of 11th International Coral Reef Symposium, vol 1, Ft Lauderdale, pp 456–460
Paz-García DA, Balart EF, García-de-Léon FJ (2012) Cold water bleaching of Pocillopora in the Gulf of California. In: Proceedings of 12th International Coral Reef Symposium, vol 9A–10, Cairns, pp 1–6
Pérez-Vivar TL, Reyes-Bonilla H, Padilla C (2006) Corales pétreos (Scleractinia) de las Islas Marías, Pacífico de México. Cienc Mar 32:259–270
Pinzón JH, LaJeunesse TC (2011) Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbioses ecology. Mol Ecol 20:311–325
Pinzón JH, Sampayo EM, Cox EF, Chauka LJ, Chen CA, Voolstra CR, LaJeunesse TC (2013) Blind to morphology: genetics identifies several widespread ecologically common species and few endemics among Indo-Pacific cauliflower corals (Pocillopora, Scleractinia). J Biogeogr 40:1595–1608
Poupin J, Bouchard J-M, Albenga L, Cleva R, Hermoso-Salazar M, Solis-Weiss V (2009) Les crustacés décapodes et stomatopodes, inventaire, écologie et zoogéographie. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Mus National d’Hist Naturelle, Paris IRD, Marseille, pp 163–216
Qiu J-W, Lau DCC, Cheang C-c, Chow W-k (2014) Community-level destruction of hard corals by the sea urchin Diadema setosum. Mar Pollut Bull. doi:10.1016/j.marpolbul. 12 Dec 2013
Razak TB, Hoeksema BW (2003) The hydrocoral genus Millepora (Hydrozoa: Capitata: Milleporidae) in Indonesia. Zool Verh Leiden 345:313–336
Restrepo J, Kjerfve B (2000) Water discharge and sediment load from the western slopes of the Colombian Andes with focus on Río San Juan. J Geol 108:17–33
Reyes-Bonilla H (1993a) Estructura de la comunidad, influencia de la depredación y biología poblacional de corales hermatípicos en el arrecife de Cabo Pulmo, Baja California Sur. MSc thesis, Centro de Investigación Científica y Educación Superior de Ensenada, Ensenada, p 169
Reyes-Bonilla H (1993b) Biogeografía y ecología de los corales hermatípicos (Anthozoa: Scleractinia) del Pacífico de México. In: Salazar-Vallejo SI, González NE (eds) Biodiversidad marina y costera de México. CONABIO/CIQRO, Chetumal, pp 207–222
Reyes-Bonilla H (2001) Effects of the 1997–1998 El Niño-Southern Oscillation on coral communities of the Gulf of California, Mexico. Bull Mar Sci 69:251–266
Reyes-Bonilla H (2003) Coral reefs of the Pacific coast of México. In: Cortés J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 331–349
Reyes-Bonilla H, Barraza JE (2003) Corals and associated marine communities from El Salvador. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 351–360
Reyes-Bonilla H, Carricart-Ganivet JP (2000) Porites arnaudi, a new species of stony coral (Anthozoa: Scleractinia: Poritidae) from oceanic islands of the eastern Pacific Ocean. Proc Biol Soc Wash 113:561–571
Reyes-Bonilla H, López-Pérez RA (1998) Biogeography of the stony corals of the Mexican Pacific. Ciencias Marinas 24:211–224
Reyes-Bonilla H, López-Pérez RA (2009) Corals and coral-reef communities in the Gulf of California. In: Johnson ME, Ledesma-Vázquez J (eds) Atlas of coastal ecosystems in the western Gulf of California: tracking limestone deposits on the margin of a young sea. University of Arizona Press, Tucson, pp 43–55
Reyes-Bonilla H, Riosmena-Rodriguez R, Foster MS (1997a) Hermatypic corals associated with rhodolith beds in the Gulf of California, México. Pac Sci 51:328–337
Reyes-Bonilla H, Sinsel-Duarte H, Arizpe-Covarrubias F (1997b) Gorgonias y corales pétreos (Anthozoa: Gorgonacea y Scleractinia) de Cabo Pulmo, Mexico. Rev Biol Trop 45:1439–1443
Reyes-Bonilla H, Calderón Aguilera LE, Cruz Piñon G, Medina Rosas P, López Pérez RA, Herrero Pérezrul MD, Leyte Morales GE, Cupul Magaña AL, Carriquiry Beltrán JD (2005) Atlas de corales pétreos (Anthozoa: Scleractinia) del Pacífico mexicano. Universidad de Guadalajara, Guadalajara, TRICICLO triciclocincocinco@yahoo.com.mx, p 124
Reyes-Bonilla H, Calderón-Aguilera LE, Cruz-Piñón G, López-Pérez RA, Medina-Rosas P (2010) Evaluation of gamma diversity of reef corals (Scleractinia) in the Mexican Pacific. Rev Mexicana Biodiversidad 81:113–121
Ricard M (1985) Atoll Rangiroa, Archipel des Tuamotu. In: Delesalle B, Galzin R, Salvat B (eds) Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 159–210
Riegl BM, Halfar J, Purkis SJ, Godinez-Orta L (2007) Sedimentary facies of the eastern Pacific’s northernmost reef-like setting (Cabo Pulmo, Mexico). Mar Geol 236:61–77
Rivera F, Martínez P (2011) Guía fotográfica de corales y octocorales: Parque Nacional Machalilla y Reserva de Producción Faunística Marino Costera Puntilla de Santa Elena. With the contribution of Odalisca Breedy, NAZCA and Conservación Internacional, Ecuador, p 78
Rixen T, Jiménez C, Cortés J (2012) Impact of upwelling events on the seawater carbonate chemistry and dissolved oxygen concentration in the Gulf of Papagayo (Culebra Bay), Costa Rica: implications for coral reefs. Rev Biol Trop (Int J Trop Biol) 60 (Suppl 2):187–195. ISSN-0034-7744
Robertson DR, Allen GR (1996) Zoogeography of the shorefish fauna of Clipperton Atoll. Coral Reefs 15:121–131
Robertson DR, Allen GR (2008) Shorefishes of the tropical eastern Pacific online information system, version 1.0. www.stri.org/sftep. Accessed 23 June 2014
Robertson DR, Cramer KL (2009) Shore fishes and biogeographic subdivisions of the tropical eastern Pacific. Mar Ecol Prog Ser 380:1–17
Robertson DR, Grove JS, McCosker JE (2004) Tropical transpacific shore fishes. Pac Sci 58:507–565
Rodríguez-Ramírez A, Zapata FA (2012) An atypical corallum morphology in a species of Pocillopora. Coral Reefs 31:253
Rodríguez-Rubio E, Schneider W, Abarca del Río R (2003) On the seasonal circulation within the Panama Bight derived from satellite observations of wind, altimetry and sea surface temperature. Geophys Res Lett 30:1410. doi:10.1029/2002GL016794
Rojas W, Alvarado GE (2012) Marco geológico y tectónico de la Isla del Coco y la region marítima circunvecina, Costa Rica. Rev Biol Trop 60(Suppl 3):15–32
Ryan J, Zapata Y (2003) Nicaragua’s coral reefs: status, health and management strategies. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 203–222
Saavedra-Sotelo NC, Calderon-Aguilera LE, Reyes-Bonilla H, López-Pérez RA, Medina-Rosas P, Rocha-Olivares A (2011) Limited genetic connectivity of Pavona gigantea in the Mexican Pacific. Coral Reefs 30:677–686
Salvat B, Richard G (1985) Takapoto Atoll: Tuamotu Archipelago. In: French Polynesian coral reefs. Proceedings of 5th International Coral Reef Congress, vol 1, Tahiti, pp 323–362
Salvat B, Adjeroud M, Charpy L (2008) Les récifs coralliens de Clipperton. Rev Écol (Terre Vie) 63:179–187
Schaeffer BA, Morrison JM, Kamykowski D, Feldman GC, Xie L, Liu Y, Sweet W, McCulloch A, Banks SA (2008) Phytoplankton biomass distribution and identification of productive habitats within the Galapagos Marine Reserve by MODIS, a surface acquisition system, and in-situ measurements. Remote Sens Environ 112:3044–3054
Scheltema RS (1988) Initial edvidence for the transport of teleplanic larvae of benthic invertebrates across the east Pacific barrier. Biol Bull 174:145–152
Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N (2014) With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool J Linn Soc 170:1–33
Segovia-Prado JV, Navarrete-Calero MT (2007) Biodiversidad a nivel de ecosistema en parches de corales hermatípicos (Porites lobata, Pocillopora sp.) en la zona intermareal de la Playa Los Cóbanos, Departamento de Sonsonante, El Salvador. Licenciatura thesis, Escuela de Biología, Facultad de Ciencias Naturales y Matemática, Univ de El Salvador, San Salvador, p 68
Smith TB, Glynn PW, Maté JL, Toth LT, Gyory J (2014) A depth refugium from catastrophic coral bleaching prevents regional extinctions. Ecology 95:1663–1673
Solís-Marín F-A, Laguarda-Figueras A (2009) Les échinoderms. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Mus National d’Hist Naturelle, Paris IRD, Marseille, pp 235–247
Sonnenholzner JI, Ladah LB, Lafferty KD (2009) Cascading effects of fishing on Galapagos rocky reef communities: reanalysis using corrected data. Mar Ecol Prog Ser 375:209–218
Spalding MD, Ravilious C, Green EP (2001) World atlas of coral reefs. UNEP/WCMC, Univ California Press, Berkeley, p 424
Springer VG (1958) Systematics and zoogeography of the clinid fishes of the subtribe Labrisomini Hubbs. Pubs Inst Mar Sci Univ Texas 5:417–492
Squires DF (1959) Results of the Puritan-American Museum of Natural History Expedition to western Mexico, 7. Corals and coral reefs in the Gulf of California. Bull Amer Mus Nat Hist 118:367–432
Steenburgh WJ, Schultz DM, Colle BA (1998) The structure and evolution of gap outflow over the Gulf of Tehuantepec, Mexico. Mon Weather Rev 126:2673–2691
Stefani F, Benzoni F, Pichon M, Cancelliere C, Galli P (2008) A multidisciplinary approach to the definition of species boundaries in branching species of the coral genus Psammocora (Cnidaria, Scleractinia). Zoolog Scr 37:71–91
Stehli FG, Wells JW (1971) Diversity and age patterns in hermatypic corals. Syst Zool 20:115–126
Steller DL, Riosmena-Rodríguez R, Foster MS, Roberts CA (2003) Rhodolith bed diversity in the Gulf of California: the importance of rhodolith structure and consequences of disturbance. Aquatic Conserv: Mar Freshw Ecosyst 13:S5–S20
Steller DL, Riosmena-Rodríguez R, Foster MS (2009) Living rhodolith bed ecosystems in the Gulf of California. In: Johnson ME, Ledesma-Vázquez J (eds) Atlas of coastal ecosystems in the western Gulf of California: tracking limestone deposits on the margin of a young sea. University of Arizona Press, Tucson, pp 72–82
Stevenson MR, Guillén O, Santoro de Ycaza J (1970) Marine atlas of the Pacific coastal waters of South America. University of California Press, Berkeley, p 23
Sweet WV, Morrison JM, Liu Y, Kamykowski D, Schaeffer BA, Xie L, Banks S (2009) Tropical instability wave interactions within the Galápagos Archipelago. Deep-Sea Res Part I: Oceanogr Res Paps 56:1217–1229
Trichet J (2009) Origine, situation et traits morphologiques généraux de I’île. In: Charpy L (ed) Clipperton, environnement et biodiversité d’un microcosme océanique. Muséum national d’Histoire naturelle, Paris; IRD, Marseille. Patrimoines naturels 68:23–28
Vargas-Ángel B (1996) Distribution and community structure of the reef corals of Ensenada de Utría, Pacific coast of Colombia. Rev Biol Trop 44:643–651
Vargas-Ángel B (2001) Corals and coral reefs of the Pacific coast of Colombia with special reference to spatial and temporal patterns of environmental disturbances. PhD dissert, University of Miami, Coral Gables, Florida, p 209
Vargas-Ángel B (2003) Coral community structure off the Pacific coast of Colombia: onshore vs offshore reefs. Atoll Res Bull 499:1–21
Vargas-Ángel B, Zapata FA, Hernández H, Jiménez JM (2001) Coral and coral reef responses to the 1997–98 El Niño event on the Pacific coast of Colombia. Bull Mar Sci 69:111–132
Vera M, Banks SA (2009) Health status of the coralline communities of the northern Galapagos Islands Darwin, Wolf and Marchena. Galapagos Res 66:65–74
Veron JEN (1995) Corals in space and time: the biogeography and evolution of the Scleractinia. Comstock/Cornell, Ithaca, London, p 321
Veron JEN (2000) Corals of the world, vols 1–3. Austrlian Institute of Marine Science, Townsville
Veron J, Stafford-Smith M, DeVantier L, Turak E (2015) Overview of distribution patterns of zooxanthellate Scleractinia. Front Mar Sci 1:81. doi:10.3389/fmars.2014.00081
von Prahl H (1986a) Corales y arrecifes coralinos. In: von Prahl H, Alberico M (eds) Isla de Gorgona, Biblioteca Banco Popular. Textos Universitarios, Bogotá, Colombia, pp 59–87
von Prahl H (1986b) Crustáceos decápodos asociados a diferentes hábitas de la Ensenada de Utría. Actualidades Biológicas 15:95–99
von Prahl H (1990) Malpelo la roca viviente. Fondo FEN COLOMBIA, Editorial Presencia, Bogotá, Colombia
von Prahl H, Erhardt H (1985) Colombia: corales y arrecifes coralinos. Fondo FEN COLOMBIA, Editorial Presencia, Bogotá, Colombia, p 295
von Prahl H, Mejía A (1985) Primer reporte de un coral acropórido, Acropora valida (Dana, 1846), (Scleractinia: Astrocoeniida: Acroporidae) para el Pacífico Americano. Rev Biol Trop 33:39–43
von Prahl H, Guhl F, Grogl M (1979) Gorgona. Futura Grupo Editorial Ltd, Bogotá, Colombia, p 279
Wallace CC (1999) Staghorn corals of the world. CSIRO Publ, Melbourne, p 422
Walsh WJ, Cotton S, Jackson L, Lamson M, Martin R, Osada-D’Avella K, Preskitt L (2013) First record of Acropora gemmifera in the main Hawaiian Islands. Coral Reefs. doi:10.1007/s00338-013-1092-3
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52:223–241
Wellington GM, Glynn PW (2007) Responses of coral reefs to El Niño-Southern Oscillation sea-warming events. In: Aronson RB (ed) Geological approaches to coral reef ecology. Ecol Stud 192:342–385. Springer, New York
Wellington GM, Glynn PW, Strong AE, Navarrete SA, Wieters E, Hubbard DK (2001) Crisis on coral reefs linked to climate change. EOS, Trans Amer Geophys Union 82(1):5
Wells JW (1983) Annotated list of the scleractinian corals of the Galápagos. In: Glynn PW, Wellington GM (eds) Corals and coral reefs of the Galápagos Islands. University of California Press, Berkeley, pp 212–295
Wieters EA, Medrano A, Pérez-Matus A (2014) Functional community structure of shallow hard bottom communities at Rapa Nui, Easter Island. Lat Am J Aquat Res 42:827–844
Williams GJ, Maragos JE, Davy SK (2008) Characterization of the coral communities at Palmyra Atoll in the remote Central Pacific Ocean. Atoll Res Bull 557:1–30
Zapata FA (2001) Formaciones coralinas de Isla Gorgona. In: Barrios LM, López-Victoria M (eds) Gorgona marina: contribución al conocimiento de una isla única. INVEMAR, Serie Publicaciones Especiales No. 7, Santa Marta, pp 27–40
Zapata FA, Vargas-Ángel B (2003) Corals and coral reefs of the Pacific coast of Colombia. In: Cortés J (ed) Latin American coral reefs. Elsevier Science, Amsterdam, pp 419–447
Zapata FA, Vargas-Ángel B, Garzón-Ferreira J (2001) Salud y conservación de las comunidades coralinas In: Barrios LM, López-Victoria M (eds) Gorgona marina: Contribución al conocimiento de una isla única. INVEMAR, Serie Publicaciones Especiales No. 7, Santa Marta, pp 41–50
Zapata FA, Rodríguez-Ramírez A, Rodríguez-Moreno M, Muñoz C, López-Victoria M (2007) Confirmation of the occurrence of the coral Pavona chiriquiensis Cnidaria: Anthozoa: (Agariciidae) in the Colombian Pacific. Boletín de Investigaciones Marinas y Costeras 36:307–312
Zapata FA, García JL, Tobón A (2008) Formaciones coralinas en las localidades de Piñas (Punta Cruces) y El Acuario (Cabo Marzo). In: Giraldo A, Valencia B (eds) Chocó: paraíso por naturaleza - Punta Cruces y Cabo Marzo. Departamento de Biología, Universidad del Valle, Cali, Colombia, pp 23–32
Zapata FA, Rodríguez-Ramírez A, Caro-Zambrano C, Garzón-Ferreira J (2010) Mid-term coral-algal dynamics and conservation status of a Gorgona Island (Tropical Eastern Pacific) coral reef. Rev Biol Trop 58(Suppl. 1):81–94
Zapata FA, Jaramillo González J, Navas Camacho R (2011) Extensive bleaching of the coral Porites lobata at Malpelo, Colombia, during a cold water episode in 2009. Bol Invest Mar Cost 40(spec suppl):185–193
Acknowledgments
The following organizations facilitated research in the ETP: Mexico—Consejo Nacional de Ciencia y Tecnología (CONACYT) project numbers 108302 and 183534; Costa Rica—Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), University of Costa Rica; Panama—University of Panama, Smithsonian Tropical Research Institute (STRI); Colombia—Colciencias, Conservation International, Fundación para la Promoción de la Investigación y la Tecnología del Banco de la República, Instituto de Investigaciones Marinas y Costeras (INVEMAR), Parques Nacionales Naturales, Universidad del Valle, World Wildlife Fund, and members of the Coral Reef Research Group at Universidad del Valle; Ecuador—Charles Darwin Foundation, Galápagos National Park Service, Charles Darwin Research Station; Conservation International; Khaled bin Sultan Living Oceans Foundation, Saudi Arabia; Chile, Rapa Nui—Henri and Michel Garcia (ORCA Diving Center), H. Buck-Weise, I. Burgues, A. Medrano, T. Navarrete Fernandez, A. Perés-Matus, FONDECYT grant no. 1130167, and Center for Marine Conservation Nucleo Milenio Initiative P10-033F; research at several ETP sites was supported by the U.S. National Science Foundation (Biological Oceanography Program) and the U.S. National Geographic Society. Assistance with coral distributional records and species identifications was kindly offered by I.B. Baums, E.H. Borneman, S.D. Cairns, D. Fenner, D.K. Hubbard, and J.E.N. Veron. Joshua Levy helped with the graphics, tables, and organization of the text, and Rafael Araujo with editorial advice. The organization and clarity of this overview benefitted greatly from suggestions offered by A.C. Baker, W.M. Goldberg, and L.T. Toth. Finally, we are all indebted to the late John W. Wells, directly or indirectly, for showing us the way in the study of eastern Pacific corals.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix: Coral Species Presence (1)/Absence (0) Data Matrix
Appendix: Coral Species Presence (1)/Absence (0) Data Matrix
Presence/absence of zooxanthellate scleractinian corals at 15 eastern Pacific and four central/south Pacific localities. Recent sources for each locality are listed in the caption to Fig. 5.7. No species re-assignments were attempted for the central/south Pacific taxa listed. Pocillopora verrucosa and Pocillopora elegans are treated as separate species. GOC, Gulf of California; TMM, tropical mainland Mexico; REV, Revillagigedo Islands; CLP, Clipperton Atoll; ELS, El Salvador; NIC, Nicaragua; CRM, Costa Rican mainland; COC, Cocos Islands; PAN, Panama; COL, Colombia; MAL, Malpelo Island; ECD, Ecuador; GLS, southern Galápagos Islands; GLN, northern Galápagos Islands; RPN, Rapa Nui (Easter Island); HAW, Hawaii Is., including the NW Is.; JOH, Johnston Atoll; PIT, Pitcairn Islands, including Pitcairn, Henderson, Oeno, and Ducie Islands; PAL, Palmyra Atoll.
Species | Locality | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
GOC | TMM | REV | CLP | ELS | NIC | CRM | COC | PAN | COL | MAL | ECD | GLS | GLN | RPN | HAW | JOH | PIT | PAL | |
Acropora abrotanoides | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora aculeus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora acuminata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora aspera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora austere | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora cerealis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
Acropora cf. solitaryensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora clathrata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora cymbicyathus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora cytherea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora delicatula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora digitifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora divaricata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora elseyi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Acropora florida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora formosa a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora gemmifera b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora glauca | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora globiceps | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora granulosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora humilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora hyacinthus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora latistella | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora listeri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora longicyathus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora loripes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora lutkeni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora microclados | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora microphthalma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora monticulosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora multiacuta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora nana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora nasuta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora palmerae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Acropora paniculata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Acropora pocilloporina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora polymorpha | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora polystoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora reticulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora retusa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Acropora robusta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora samoensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora secale | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora selago | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Acropora sp. (Line and Phoenix Is.) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora sp. (slanted cones) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora sp. B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Acropora sp. C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Acropora spicifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora squarrosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora striata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora subulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora syringodes c | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Acropora tenuis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora tortuosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Acropora valida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Acropora vaughni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Acropora verweyi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Acropora yongei | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Alveopora tizardi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Alveopora verrilliana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Astreopora cf. moretonensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Astreopora expansa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Astreopora gracilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Astreopora listeri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Astreopora myriophthalma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Astreopora ocellata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Astreopora suggesta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Caulastrea cf. furcata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Coscinaraea columna | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Coscinaraea wellsi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Ctenactis echinata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Cycloseris curvata | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1i | 0 | 0 | 0 | 0 | 0 |
Cycloseris cyclolites | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Cycloseris fragilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Cycloseris sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Cycloseris tenuis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Cycloseris vaughani | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
Cyphastrea ocellina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
Cyphastrea serailia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Diaseris distorta | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1i | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
Echinophyllia aspera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Echinophyllia echinata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Echinophyllia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favia amicorum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Favia chinensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Favia favus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favia matthaii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Favia pallida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favia rotumana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Favia rotundata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favia speciosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favia stelligera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Favites abdita | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favites flexuosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favites halicora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favites pentagona | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Favites russelli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia concinna | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia fungites | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia granulosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Fungia horrida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia moluccensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia paumotensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Fungia repanda | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Fungia scutaria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Gardineroseris planulata | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
Goniastrea australensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Goniastrea edwardsi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Goniastrea pectinata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Goniastrea retiformis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Halomitra pileus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Herpolitha limax | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Hydnophora exesa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Hydnophora microconos | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Hydnophora pilosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Hydnophora rigida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Isopora brueggemanni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Isopora cuneata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Isopora palifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Leptastrea agassizi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Leptastrea bewickensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Leptastrea hawaiiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Leptastrea pruinosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
Leptastrea purpurea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
Leptastrea sp. A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Leptastrea sp. B | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Leptastrea sp. C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Leptastrea transversa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
Leptoria phrygia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Leptoseris hawaiiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
Leptoseris incrustans | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
Leptoseris mycetoseroides | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Leptoseris papyracea | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Leptoseris scabra | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
Leptoseris solida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
Leptoseris tubulifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Lobophyllia corymbosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Lobophyllia hemprichii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Merulina ampliata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montastrea annuligera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montastrea curta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Montipora aequituberculata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Montipora australiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora bilaminata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora caliculata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Montipora capitata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Montipora complanata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora crassituberculata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora danae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora dilatata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Montipora efflorescens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora elschneri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Montipora flabellata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Montipora foliosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora foveolata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Montipora grisea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora hoffmeisteri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
Montipora informis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora lobulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora millepora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora monasteriata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora nodosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora patula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Montipora peltiformis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Montipora socialis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Montipora sp. 4 cf. incrassata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
Montipora tuberculosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
Montipora venosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Montipora verrilli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Montipora verrucosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
Pachyseris sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pachyseris speciosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Parahalomitra robusta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Pavona cactus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pavona chiriquiensis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Pavona clavus | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
Pavona divaricata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Pavona duerdeni | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Pavona explanulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pavona frondifera | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pavona gigantea | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Pavona maldivensis | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
Pavona minuta | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pavona sp. cf. pollicata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Pavona sp. 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Pavona varians | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
Platygyra daedalea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Platygyra lamellina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Platygyra pini | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Platygyra ryukyuensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Platygyra sinensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Plesiastrea versipora | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Pocillopora damicornis | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Pocillopora danae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
Pocillopora effusus | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Pocillopora elegans | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
Pocillopora eydouxi | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Pocillopora inflata | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Pocillopora laysanensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Pocillopora ligulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1j | 1j | 1 | 1 | 1 | 0 | 0 |
Pocillopora meandrina | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Pocillopora molokensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
Pocillopora sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Pocillopora sp. 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Pocillopora sp. 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Pocillopora sp. 11 cf. capitata | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
Pocillopora verrucosa | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
Pocillopora woodjonesi | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
Pocillopora zelli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Porites aff. Annae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
Porites annae | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Porites arnaudi | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Porites australiensis | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Porites baueri | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Porites brighami | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites compressa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites duerdeni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites evermanni | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
Porites hawaiiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites lichen | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Porites lobata | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Porites lutea d | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
Porites murrayensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Porites panamensis | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Porites pukoensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites rus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
Porites solida e | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Porites sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Porites studeri f | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Porites superfusa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Porites sverdrupi | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Porites vaughani | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Psammocora albopicta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Psammocora brighami | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
Psammocora contigua | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Psammocora explanulata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Psammocora haimeana g | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Psammocora nierstraszi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
Psammocora obtusangula | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Psammocora profundacella | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Psammocora stellata | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
Psammocora superficialis h | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
Psammocora verrilli | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Sandalolitha robusta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Scolymia cf. vitiensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Siderastrea glynni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Stylocoeniella guentheri | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Stylophora pistillata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Symphyllia recta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Turbinaria frondens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Turbinaria reniformis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
Turbinaria sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
19 | 26 | 25 | 21 | 11 | 10 | 25 | 22 | 27 | 28 | 13 | 23 | 20 | 22 | 15 | 62 | 44 | 87 | 163 | |
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Glynn, P.W. et al. (2017). Eastern Pacific Coral Reef Provinces, Coral Community Structure and Composition: An Overview. In: Glynn, P., Manzello, D., Enochs, I. (eds) Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World, vol 8. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7499-4_5
Download citation
DOI: https://doi.org/10.1007/978-94-017-7499-4_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7498-7
Online ISBN: 978-94-017-7499-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)