Abstract
Given that global warming is the greatest threat to coral reefs, coral restoration projects have expanded worldwide with the goal of replenishing habitats whose reef-building corals succumbed to various stressors. In many cases, however, these efforts will be futile if outplanted corals are unable to withstand warmer oceans and an increased frequency of extreme temperature events. Stress-hardening is one approach proposed to increase the thermal tolerance of coral genotypes currently grown for restoration. Previous studies have shown that corals from environments with natural temperature variability experience less bleaching when exposed to thermal stress, though it remains unclear if this localized acclimatization or adaptation to variable temperatures can be operationalized for enhancing restoration efforts. To evaluate this approach, fragments from six source colonies of nursery-raised Caribbean staghorn coral (Acropora cervicornis) were treated with a variable temperature regime (oscillating twice per day from 28 to 31 °C) or static temperatures (28 °C) in the laboratory for 89 d. Following this, fragments were subjected to a heat-stress assay (32 °C) for 2 weeks. Corals treated with variable temperatures manifested signs of severe thermal stress later than static temperature laboratory controls as well as untreated field controls collected from the nursery. Furthermore, there was a stark contrast in the physiological response to heat stress, whereby the laboratory and field control groups had a significantly higher incidence of rapid tissue sloughing and necrosis, while the variable temperature-treated corals succumbed to bleaching more gradually. Overall, our data show that pre-acclimation to a variable temperature regime improves acroporid thermotolerance. As corals continue to be outplanted back onto Florida’s changing reef scape, understanding the molecular mechanisms underlying this enhanced thermal tolerance and its endurance in situ will be critical for future research and restoration applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocean warming is the most pressing stressor threatening corals and has led to an emergence of proactive efforts to combat rapid declines in reef-building coral populations. The primary method for this is coral reef restoration, which traditionally utilizes in situ or ex situ nurseries to propagate coral fragments from locally sourced populations (reviewed in Boström-Einarsson et al. 2020; Hein et al. 2020). The coral colonies are cared for by restoration practitioners until they reach a certain size, then are outplanted back onto local reefs where they can continue to grow until their populations become self-sustaining (Schopmeyer et al. 2017; Baums et al. 2019). However, if fossil fuel emissions continue at their current rates, annual coral bleaching events are projected to occur globally by mid-century (van Hooidonk et al. 2016), and reefs in the Florida Keys may experience this sooner (Manzello 2015). Even if we achieve significant reductions in global anthropogenic carbon emissions, it is imperative that coral reef restoration efforts plan for an inevitably warmer ocean.
In Florida, coral restoration projects began with the growth and propagation of two reef-building, branching coral species that were once ubiquitous on the reef tract: Acropora cervicornis (staghorn coral) and Acropora palmata (elkhorn coral). Both experienced significant declines in abundance following a disease outbreak that began in the late 1970s (Bruckner 2002) and led to their “threatened” listing on the U.S. Endangered Species Act in 2006 (National Marine Fisheries Service 2006). These species grow quickly and are easily fragmented due to their branching morphology, making them ideal candidates for coral restoration projects. In recent decades, acroporid propagation and outplanting efforts have grown in range, scale, and efficiency due to increasing investments in restoration research, methodological optimization, and collaborative efforts across research and management groups (e.g., Lirman et al. 2010; Johnson et al. 2011; Young et al. 2012; Goergen and Gilliam 2018).
Currently, tens of thousands of acroporid colonies are raised and outplanted on Florida’s coral reef every year, with > 70% survivorship observed 1 yr after outplanting (Lirman and Schopmeyer 2016; Schopmeyer et al. 2017). Recent research has begun to disentangle the effect of genotype and environment on the survivorship of nursery-propagated corals using reciprocal transplant experiments (Drury et al. 2017; Drury and Lirman 2021) and the wealth of outplant monitoring data from local coral restoration organizations (Ware et al. 2020; van Woesik et al. 2021). Long-term survivorship is more difficult to predict due to the limited duration of monitoring efforts (normally ~ 18 months based on logistical and/or funding constraints; Boström-Einarsson et al. 2020). Survivorship models using outplant monitoring data of A. cervicornis in the Florida Keys project 40% survivorship after 2 yr and just 10% survivorship after 7 yr at certain sites (Ware et al. 2020; van Woesik et al. 2021). This suggests that the standing genetic variation of Florida A. cervicornis genotypes used in restoration may be insufficient to rebuild reefs due to the intensity and complexity of environmental stressors they face. Therefore, enhancing coral resilience through human-assisted interventions may become necessary to ensure the long-term survival of outplants (Anthony 2016; National Academies of Sciences, Engineering, and Medicine 2019).
The stress-hardening technique (i.e., “pre-conditioning” or “assisted acclimatization”), defined as the deliberate pre-exposure to an acute stressor to confer stress tolerance (van Oppen et al. 2015), is a human-assisted intervention of particular interest. Following exposure, intra-generational changes may be induced in coral colonies propagated for restoration with the potential for the acclimatory effect to be passed on to future generations (Putnam and Gates 2015; Torda et al. 2017; Liew et al. 2020). The stress-hardening technique was recently attempted using sublethal temperature treatments on Montipora capitata, and the results demonstrated that acclimatization potential is influenced by the type of temperature treatment, host genotype, symbiont community composition, and historical patterns of bleaching (Dilworth et al. 2021). Additionally, pre-exposure to thermal stress has been shown to act as a protective mechanism during coral bleaching events on the Great Barrier Reef (Ainsworth et al. 2016), with similar observations made in the laboratory using heat-stress assays on Indo-Pacific acroporid species (Middlebrook et al. 2008; Bellantuono et al. 2012a, b; Bay and Palumbi 2015; Ainsworth et al. 2016). Furthermore, with more frequent warming events in recent years, there has been an opportunity to observe the heat-stress response of several coral populations in situ that experienced back-to-back thermal events exceeding the bleaching threshold. For several reefs that have experienced this phenomenon, bleaching was less prevalent during the subsequent warming event (Guest et al. 2012; Gintert et al. 2018; Fisch et al. 2019; Hughes et al. 2019; Wall et al. 2021). Overall, these studies support the idea that, in certain locations and for certain coral species, prior exposure to aberrant temperature profiles confers a degree of thermal tolerance, and that this tolerance can persist over time.
In addition, both the duration of exposure and the degree of temperature variability play roles in coral thermotolerance. For reef habitats with relatively large natural temperature fluctuations, such as in back reefs and lagoons, bleaching-resistant corals have been identified during extreme warming scenarios (Carilli et al. 2012; Rivest et al. 2017; Safaie et al. 2018; Morikawa and Palumbi 2019; Schoepf et al. 2020). On the global scale, regions with a higher variance in daily, weekly, and seasonal sea surface temperatures experience significantly less bleaching (Sully et al. 2019). To isolate the role of temperature variability alone in bleaching resistance, fragments of corals from different lagoons of American Samoa were exposed to thermal stress in the laboratory, and it was found that corals from thermally variable pools had much higher survivorship than corals from less thermally variable pools (Oliver and Palumbi 2011). Reciprocal transplantation led to higher thermotolerance of the corals moved to the highly variable pools, and phenotypic changes were estimated to be primarily driven by local acclimatization rather than fixed effects (Palumbi et al. 2014). In Florida, a similar trend was observed following a reciprocal transplant of Porites astreoides between an inshore, variable thermal environment and an offshore, stable environment, where the inshore environment promoted greater thermal plasticity (Kenkel and Matz 2016). Due to the increased thermotolerance observed across studies, temperature variability may act as a natural stress-hardening treatment.
Given the need for enhancing thermotolerance in Florida’s coral outplants, and the growing body of work supporting thermal stress-hardening and rapid acclimatization, we investigated the efficacy of a variable temperature treatment to stress-harden nursery-raised fragments of A. cervicornis in a laboratory experiment. We hypothesized that prior exposure to oscillating temperatures reaching thermally stressful conditions twice per day over the course of three months would delay the onset of coral bleaching in a simulated heat-stress event. Furthermore, we hypothesized that this variable temperature regime would stress-harden nursery-raised A. cervicornis fragments regardless of genotype, demonstrating a mechanism that has the potential to be applied across populations used in coral restoration.
Methods
Coral collection and husbandry
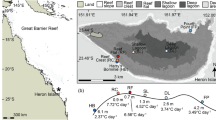
Six source colonies of A. cervicornis were collected from the University of Miami’s in situ coral nursery in Miami, FL (25.6763, − 80.0987; depth = 9 m) on February 6, 2019. At least 1 yr prior, these source colonies were collected from three different reefs in South Florida (N = 2/reef; Fig. 1a): “Broward” (the northernmost reef; 26.18298, − 80.08933 [“North”]), “Yung’s” (intermediate latitude; 25.56418, − 80.1049 [“Mid”]), or “Kelsey’s” (southernmost reef; 25.3889, − 80.1627 [“South”]). To characterize each reef’s thermal history, maximum monthly mean (MMM) temperatures were determined using the 4-km resolution monthly sea surface temperature climatology, which was derived from harmonic analysis of the advanced very high-resolution radiometer (AVHRR) Pathfinder version 5.0 temperature time series data for 1982–2008 (Casey et al. 2010). The MMM temperatures are as follows: North reef = 29.71 °C, Mid reef = 30.00 °C, South reef = 30.01 °C, and the in situ Key Biscayne coral nursery = 29.92 °C (Kaufman et al. 2021). Either “A” or “B” were assigned in this study to denote the distinct colonies from each site. Three of the six source colonies were recently genetically characterized using SNP-CHIP genotyping at Penn State University (Kitchen et al. 2019). The remaining three colonies were collected > 5 m apart from other source colonies at each original reef site to ensure genetic distinction between colonies (Drury et al. 2016). As such, each source colony will be considered a unique genet in this study.
a Map of the three original reef sites where corals were collected (Broward (North Reef), Yung’s (Mid Reef), and Kelsey’s (South Reef)) and the in situ nursery where they were maintained and propagated (Key Biscayne Nursery). b Mean temperature at each 15-min interval for the 89-d temperature treatments for the laboratory control (blue) and variable (red) corals. Outer ribbons of the mean temperature represent standard deviation. Gray shaded blocks depict diel light fluctuations as controlled by LED illumination, and the change in opacity represents the gradual ramping up and down of light levels. c The field control heat-stress assay conducted from March 15–April 10, 2019. Black line depicts the mean temperature across the multiple glass aquaria (N = 10). Dark shading around lines represents standard deviation of mean temperature across tanks. d The laboratory control and variable temperature-treated coral heat-stress assay conducted from June 17–July 9, 2019. Black line depicts the mean temperature across the multiple glass aquaria (N = 6). Dark shading around lines represents standard deviation of mean temperature across tanks
The six genets were brought back to the Experimental Reef Laboratory at the University of Miami’s Rosenstiel School for Marine and Atmospheric Science and fragmented into 128 pieces (N = 20–25 fragments/genet each ~ 5 cm in height) on February 7, 2019. Apical tips were removed to maintain consistency in growth and healing across replicates. Each fragment was glued to an acrylic pedestal using a cyanoacrylate adhesive, then placed randomly in one of two fiberglass raceways for laboratory acclimation and subsequent temperature treatments (Raceway 1: 1.83 × 0.69 × 0.22 m; Raceway 2: 1.83 × 0.51 × 0.22 m).
Temperature treatments
All corals recovered at 24 °C for 12 d in their randomly assigned raceways, at which point tank temperatures were gradually increased to 28 °C over 5 d. This temperature was selected to mimic a plausible summertime temperature that would promote healthy coral growth. Following a 27-d acclimation at 28 °C, 82 fragments were subjected to one of two treatments for 89 d (March 21, 2019–June 17, 2019). The first treatment involved an oscillating temperature regime, where corals (referred herein as the “Variable” group, N = 39) experienced two 3-h exposures to 31 °C d−1 prefaced by a 3-h increase from 28 °C and followed by a 3-h decrease to 28 °C (Fig. 1b). The laboratory control fragments (N = 43) remained at 28 °C for 89 d (Fig. 1b).
Heat-stress assays
There were two heat-stress assays conducted during this experiment to assess the effect of pre-acclimation on coral thermotolerance in high temperature stress. The first heat-stress assay was conducted on a subset of corals (referred to herein as the “Field control” group, N = 46) with the temperature ramp-up to 32 ºC starting on March 15, 2019, 37 d after collection from the in situ nursery, to evaluate genotypic differences in thermotolerance prior to stress-hardening. Corals were randomly distributed across ten glass aquaria (0.58 × 0.58 × 0.27 m), and the temperature was increased by + 0.5 °C d−1 for 8 d until 32 °C was reached. This temperature was chosen to reflect a plausible summertime condition that would lead to acute thermal stress on the order of days. This temperature was maintained for 20 d, concluding on April 10, 2019 (Fig. 1c). The second heat-stress assay was conducted using the fragments from the laboratory control and variable temperature groups after their 89-d treatment period. Corals were randomly distributed across six glass aquaria and subjected to the same heat-stress assay described above for 15 d, concluding on July 9, 2019 (Fig. 1d).
Laboratory tank conditions
The raceways and glass aquaria (collectively called “tanks”) used during this experiment had similar environmental parameters for holding A. cervicornis fragments for an extended period and are described in detail below.
Each tank featured one or two circulation pumps (depending on volume of tank) set to an output flow rate of 53 gallons per hour (Nanostream 6040, Tunze). Seawater from Biscayne Bay was filtered to 25 µm and supplied to each tank via continuous fresh seawater drip at 150 mL min−1 (calibrated weekly). Two or three 135-W LED arrays (Hydra 52 HD, Aqua Illumination) illuminated each tank, depending on the length of the tank. Across a 24-h diel cycle, lights were off from 19:00 to 6:00, followed by a 3-h gradual increase from darkness to midday target levels of approximately 250 µmol photons m−2 s−1, which were sustained for 7 h (9:00–16:00). At 16:00, light was gradually decreased over 3 h to complete darkness by 19:00 (Fig. 1b). Photosynthetically active radiation (PAR) was continually monitored throughout the experiment using a spherical quantic sensor (MQ-200, Apogee). Coral fragments were rotated around the tanks daily to balance light irradiance exposure. Temperature was (1) maintained with a 300-W aquarium heater (TH-300, Finnex) and a titanium chiller coil (Hotspot Energy), (2) measured using a high-accuracy RTD sensor (TTD25C, ProSense), and (3) controlled and logged using custom software written in LabVIEW (National Instruments) following Enochs et al. (2018). All coral fragments were fed via a broadcast feeding method, with each tank dosed with 5.5 µg/mL of Reef-Roids (Polyplab), once per evening during both the temperature treatments and the heat-stress assays. Given the high concentration of food administered, the tank flow was maintained during feeding.
Mean number of days until coral fragments exhibited signs of tissue loss or bleaching (receiving a color score of D1) during the heat-stress assay after being subjected to one of two treatments (laboratory control or variable) or after removal from the field, separated by original reef site where corals were sourced (North, Mid, or South reef). Green = field control, blue = laboratory control, red = variable temperature treatment. Within the area of each bar, the proportion of fragments within each treatment that experienced tissue loss versus bleaching are encoded as either dark shading (tissue loss) or light shading (bleaching). Error bars are standard deviation
Coral response variables
Coral health was monitored throughout the experiment for the three treatment groups (field controls, laboratory controls, and variable temperature fragments) via four response variables: (1) coral tissue color (visual scale and/or photographic color score quantification), (2) number of days until either tissue sloughing or bleaching to a color score of D1 (both described in detail below), (3) coral appearance, and (4) photosynthetic efficiency of the in hospite dinoflagellate endosymbionts (pulse amplitude-modulated [PAM] fluorometry). Each is described in detail below.
Coral tissue color
Coloration of the coral fragments was visually determined daily during both the field control and laboratory control/variable 32 °C heat-stress assays using the D1–D6 scale on the Coral Color Reference Card (Siebeck et al. 2006). For the heat-stress assay involving the laboratory controls and variable temperature treatment group, an additional, more quantitative assessment of coral tissue coloration was conducted using photographs that were taken daily using an underwater camera housing setup to standardize the distance of image capture (Canon Powershot G1X) with the reference card and Kodak Gray Scale attached (Fig. S1). Each coral fragment's coloration was converted into a numeric value using a method adapted from Winters et al. (2009). Briefly, photos were imported into ImageJ software (Schneider et al. 2012) and white balance was standardized to the M (8) value on the Kodak Gray Scale. Next, four points of a 3-pixel-radius were randomly selected on each coral (omitting shadows and apical tips) to calculate red–green–blue (RGB) intensity values on the scale of 0 (black) to 255 (white). As previous research has shown that the R-intensity value correlates most closely with chlorophyll a concentrations of in hospite dinoflagellate endosymbionts (Winters et al. 2009), R-intensities of 4 points were acquired and averaged for each coral fragment. The ImageJ scripts for this analysis are provided in the Supplemental Materials.
Coral thermal stress responses
During each day of the 32 °C heat-stress assays, when a fragment was assigned a color score of D1 on the color reference card, indicating complete bleaching, the coral was removed from the heat-stress assay and relocated to separate glass aquaria for recovery. These recovery tanks were set at an ambient temperature of 28 °C with the same lighting, water flow, and feeding regime parameters as described above for the other tanks. The number of days until bleaching was recorded as a metric for assessing tolerance in thermal stress. In addition to coral bleaching, symptoms of rapid tissue sloughing and necrosis were observed at such a high rate that the occurrence of this response was also recorded. Corals that displayed signs of rapid tissue loss (RTL), where epithelial tissue quickly and completely dissociated from the coral skeleton, were removed from the heat-stress assay. The number of days until tissue loss was also quantified and used as a secondary metric for gauging thermal stress. Note that the number of days until removal was calculated with day zero equating to the first day that the temperature of the heat-stress assay reached 32 °C.
Coral appearance
On the day that a fragment was removed from the heat-stress assay due to either reaching a color score of D1 on the color reference card or due to signs of RTL, the type of response, or coral appearance (bleaching vs. RTL), was recorded.
Photosynthetic efficiency
PAM fluorometry was used to measure the maximum, dark-adapted yield of photosystem II (Fv/Fm) of the dinoflagellate endosymbionts in hospite twice during the 89-d laboratory control/variable temperature treatment and every 3–4 d during the 32 °C heat-stress assays (sensu Warner et al. 1996; Ralph et al. 2015). Corals were dark-acclimated for 45 min prior to use of Imaging-PAM MAXI Version (Walz, Germany). During each PAM session, one area of interest was selected per coral fragment (avoiding their apical tips).
Statistical analyses
All analyses were performed in R Programming Language version 4.0.2 (RStudio Team 2015). The numbers of days until coral fragments bleached to a score of D1 or exhibited RTL in the 32 °C heat-stress assay among the three treatments and six genotypes were assessed using a two-way ANOVA: Genotype + Treatment + Genotype*Treatment. To assess the effect of original reef site on the number of days until visible stress in the heat-stress assay, genotypes A and B from each donor reef (North, Mid, and South) were pooled and assessed using the following two-way ANOVA: Reef + Treatment + Reef*Treatment. For both ANOVAs, post hoc Tukey’s HSD tests were used to determine pairwise significant differences (α = 0.05). Normality and heteroscedasticity of the residuals were analyzed using Shapiro–Wilk and Levene’s tests, respectively. A Pearson’s Chi-squared test was used to assess the effect of treatment on likelihood of coral bleaching or RTL during the heat-stress assay. A Kruskal–Wallis test was used to assess the difference in mean maximum, dark-adapted yield of photosystem II (Fv/Fm) between the laboratory control and variable temperature treatment at a single time point, which was after 89 d of exposure to either the stable or variable temperature regimes. The R markdown file containing the code for this analysis can be found at https://github.com/ademerlis/acerstresshardening2022.
Results
Precision of tank temperature regimes
For the 89-d temperature treatment, mean temperatures were calculated at 15-min intervals and found to be maintained within a standard deviation of 1.45 °C for the variable treatment and within 0.96 °C for the laboratory controls (Fig. 1b). Temperatures during the heat-stress assays for the field controls (N = 10 aquaria) and laboratory controls/variable temperature treatment (N = 6 aquaria) were 31.96 ± 0.10 °C (mean ± SD [for this and all following error terms]) and 31.98 ± 0.09 °C over the 20- and 15-d periods, respectively (Fig. 1c–d).
Coral coloration
Corals were deemed visually healthy after their acclimation and treatment periods based on their color scores assigned using the coral reference card. Mean visual color scores of the corals entering their respective heat-stress assays for the field controls, laboratory controls, and the variable group were 4.8 ± 0.5, 4.1 ± 0.3, and 3.9 ± 0.5, respectively (Supplemental Table 2). Additionally, mean R-intensity values, used as a proxy for chlorophyll a concentration, for the laboratory controls and variable temperature-treated corals were at similar levels entering the heat-stress assay (Fig. 3c).
a Mean photochemical efficiency for field controls during the heat-stress assay, measured as Fv/Fm. Error bars are standard deviation. b Mean photochemical efficiency for laboratory controls and variable temperature-treated corals during the heat-stress assay, measured as Fv/Fm. Error bars are standard deviation. Color indicates treatment group; blue = laboratory control, and red = variable temperature treatment. Asterisk above one time point indicates a significant difference in mean Fv/Fm between laboratory control and variable temperature treatment groups (Kruskal–Wallis test, H(1) = 59.937, p < 0.001). c Mean R-intensity values for laboratory controls and variable temperature-treated corals during the heat-stress assay. Y-axis is inverted for ease of cross-panel comparison (as a higher R-intensity value correlates with lower chlorophyll a concentration)
Number of days until bleaching or RTL
Corals initially treated with the 89-d variable temperature regime maintained a color score greater than D1 or maintained tissue integrity significantly longer in the 32 °C heat-stress assay (mean number of days = 12.4 ± 2.3) in comparison to both the laboratory (6.4 ± 2.0 d) and field control groups (9.7 ± 2.8d; Fig. 2a, Tables 1 and 2). There was a significant effect of original reef site as well (Table 3), with North reef enduring thermal stress longer than Mid and South reefs (Fig. 2b; Table 4). While the field control group specifically had a significant difference between North Reef versus Mid and South Reefs, this was not seen in the variable temperature treatment (Fig. 2b; Supplemental Table 3).When comparing genotypes individually, no significant differences were observed within the variable temperature treatment, however, there were significant differences among genotypes within the laboratory control group and the field control group (Fig. S2; Supplemental Table 3).
Prior exposure to a variable temperature treatment also influenced the manifestation of prolonged high-temperature stress; namely, there was a significant difference in the proportion of fragments that bleached versus those that experienced RTL and necrosis (Table 5). Additionally, there was a significant effect of original reef on likelihood of succumbing to bleaching versus RTL during the heat-stress assay, with the North reef corals most likely to perish via tissue loss (Table 6).
Photosynthetic efficiency
During the 89-d treatment, the photosynthetic efficiency of the laboratory control corals displayed a steep decline while the variable group maintained efficiency closer to their initial values (Fig. 3b). At the start of the heat-stress assay, when temperatures reached 32 ºC, the average Fv/Fm of the variable group was significantly higher (0.528 ± 0.25) than the laboratory control fragments (0.361 ± 0.036; H(1) = 59.937, p < 0.001).
Discussion
The significant increase in number of days before bleaching or RTL in the 32 °C heat-stress assay following exposure to a variable temperature treatment indicates that this treatment enhanced coral thermotolerance. Additionally, the higher frequency of bleaching of the variable temperature-treated corals compared to field and laboratory controls (which were more likely to manifest RTL) suggests that the enhanced thermotolerance was also coupled with an enhanced stress response, where epithelial integrity was maintained in thermally stressful conditions for the treated group. The higher incidence of bleaching rather than tissue loss following the variable temperature regime suggests that the coral host innate immune system may also be implicated in the heat-stress response. RTL, in which degraded tissues peel away from the skeleton, may be caused by host cell autolysis, allelopathic toxins, or pathogenic infection (Bornemann 2001; Luna et al. 2007; Calfo 2009; Bartlett 2013) and has only ever been described in diseased corals (Luna et al. 2007). It is possible, then, that the RTL witnessed is a result of a high temperature-driven immune-compromised state in which endogenous or exogenous microbes capitalized on necrotic tissues, and that periodic pre-exposure to higher temperatures prevented the variable corals from reaching this point. This differential response is further supported by in situ research during the 2014 mass bleaching event in South Florida that demonstrated a significant negative correlation between bleaching and tissue loss disease in nursery-propagated A. cervicornis colonies (Merselis et al. 2018). Future molecular analyses are necessary to determine whether the differential thermal stress response observed can be attributed to a high temperature-induced weakening of the coral immune system.
Additionally, the significantly reduced photosynthetic efficiency of the laboratory control group at the start of the heat-stress assay indicates that the health of the dinoflagellate endosymbionts, and thus homeostasis of the coral-algal symbiosis, was already at risk. It is possible that the 28 °C static temperature regime in which the laboratory controls were kept was unsatisfactory for the A. cervicornis holobiont during that time of year (April–June), while the oscillating temperatures from 28 to 31 °C were closer to the temperatures that these fragments would experience in the in situ nursery. Furthermore, while all coral colonies used in this experiment had been propagated and maintained in an in situ coral nursery for over a year, there was a significant difference in number of days until bleaching or RTL in the heat-stress assay based on their native reef. Genotypes from the North reef had significantly higher rates of RTL across all treatment groups compared to those of the Mid and South reefs. The differing results of these three distinct reefs in the heat-stress assay may be due to local acclimatization driven by different environmental conditions, as was observed in previous research (Drury et al. 2017). It may also be driven by genet identity, as supported by prior studies using nursery-propagated A. cervicornis (Drury et al. 2017; Ladd et al. 2017; Drury and Lirman 2021). Future research should incorporate a larger subset of genotypes to test these hypotheses.
Importantly, North, Mid, and South reefs did not significantly differ in number of days until RTL or bleaching in the heat-stress assay following the variable temperature treatment. This points to another potential benefit of the variable temperature treatment to confer thermotolerance of A. cervicornis regardless of genotype. In the field control group, the North genotypes survived the longest in the 32 °C heat-stress assay, which may be explained by more similar in situ temperatures shared between their native reef to the in situ nursery. A tidal flow channel separates North reef and the Key Biscayne nursery from the Mid and South reefs, and the MMM temperatures are more similar on either side of the channel (listed from north to south: North Reef = 29.71 °C, in situ coral nursery = 29.92 ºC, Mid Reef = 30.00 °C, South Reef = 30.01 °C; Kaufman et al. 2021). This indicates another potential driver of baseline thermal response, reef origin site, that was no longer shown following the variable temperature treatment.
There are several important next steps for determining the applicability of this technique. First, applying this variable temperature treatment to a subset of nursery-propagated A. cervicornis genotypes and observing their survivorship and physiological response during thermal stress in situ is necessary. Second, understanding the recovery of variable temperature-treated coral fragments after heat stress, as well as upon exposure to subsequent thermal stress events, will address the duration of this observed thermotolerance. Third, analysis of factors contributing to the two different physiologically compromised states is important, and an experiment should be conducted with replication sufficient to examine bleaching and RTL responses independently. Finally, utilization of molecular approaches to investigate changes in gene expression following the variable temperature stress-hardening, namely in heat stress response and innate immunity pathways, will provide insight into the contrasting physiological responses, RTL versus bleaching, of A. cervicornis to acute warming. Nonetheless, the results of this experiment illuminate an avenue whereby we can apply conferred benefits from a variable temperature regime as a restoration technique in Florida.
References
National Academies of Sciences, Engineering, and Medicine (2019) A research review of interventions to increase the persistence and resilience of coral reefs. The National Academies Press, Washington. https://doi.org/10.17226/25279
Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W (2016) Climate change disables coral bleaching protection on the great barrier reef. Sci 352:338–342
Anthony KRN (2016) Coral reefs under climate change and ocean acidification: challenges and opportunities for management and policy. Annu Rev Environ Resour 41:59–81
Bartlett TC (2013) Small scale experimental systems for coral research: considerations, planning, and recommendations. NOAA Technical Memorandum NOS NCCOS 165 and CRCP 18. p 68
Baums IB, Baker AC, Davies SW, Grottoli AG, Kenkel CD, Kitchen SA, Kuffner IB, LaJeunesse TC, Matz MV, Miller MW, Parkinson JE, Shantz AA (2019) Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecol Appl 29:1–23
Bay RA, Palumbi SR (2015) Rapid acclimation ability mediated by transcriptome changes in reef-building corals. Genome Biol Evol 7:1602–1612
Bellantuono AJ, Hoegh-Guldberg O, Rodriguez-Lanetty M (2012a) Resistance to thermal stress in corals without changes in symbiont composition. Proc R Soc B 279:1100–1107
Bellantuono AJ, Granados-Cifuentes C, Miller DJ, Hoegh-Guldberg O, Rodriguez-Lanetty M (2012b) Coral thermal tolerance: tuning gene expression to resist thermal stress. PLoS ONE 7:e50685
Borneman EH (2001) Aquarium corals: selection, husbandry, and natural history. T.F.H. Publications, Inc., Neptune City, p 432
Boström-Einarsson L, Babcock RC, Bayraktarov E, Ceccarelli D, Cook N, Ferse SCA, Hancock B, Harrison P, Hein M, Shaver E, Smith A, Suggett D, Stewart-Sinclair PJ, Vardi T, McLeod IM (2020) Coral restoration: a systematic review of current methods, successes, failures and future directions. PLoS One 15:e0226631. https://doi.org/10.1371/journal.pone.0226631
Bruckner AW (2002) Potential application of the U.S. endangered species act as a conservation strategy. In: Proceedings of the Caribbean Acropora workshop 18–22 April 2002, Miami, FL. NOAA technical memorandum NMFS-OPR-24. NOAA, Silver Spring, MD, p 199
Calfo A (2009) Book of coral propagation: reef gardening for aquarists. Volume 1. 2nd Edition. Reading Trees Publications. Monroeville, PA. p 416
Carilli J, Donner SD, Hartmann AC (2012) Historical temperature variability affects coral response to heat stress. PLoS ONE 7:e34418. https://doi.org/10.1371/journal.pone.0034418
Casey KS, Brandon TB, Cornillon P, Evans R (2010) The past, present, and future of the AVHRR pathfinder SST program. Oceanography from space. Springer, Dodrecht, pp 273–287
Dilworth J, Caruso C, Kahkejian VA, Baker AC, Drury C (2021) Host genotype and stable differences in algal symbiont communities explain patterns of thermal stress response of Montipora capitata following thermal pre-exposure and across multiple bleaching events. Coral Reefs 40(1):151–163
Drury C, Lirman D (2021) Genotype by environment interactions in coral bleaching. Proc R Soc B. https://doi.org/10.1098/rspb.2021.0177
Drury C, Dale KE, Panlilio JM, Miller SV, Lirman D, Larson EA, Bartels E, Crawford DL, Oleksiak MF (2016) Genomic variation among populations of threatened coral: Acropora cervicornis. BMC Genom 17(1):1–14
Drury C, Manzello D, Lirman D (2017) Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral Acropora cervicornis. PLoS One 12:e0174000
Enochs IC, Manzello DP, Jones PJ, Aguilar C, Cohen K, Valentino L, Schopmeyer S, Kolodziej G, Jankulak M, Lirman D (2018) The influence of diel carbonate chemistry fluctuations on the calcification rate of Acropora cervicornis under present day and future acidification conditions. J Exp Mar Biol Ecol 506:135–143
Fisch J, Drury C, Towle EK, Winter RN, Miller MW (2019) Physiological and reproductive repercussions of consecutive summer bleaching events of the threatened Caribbean coral Orbicella faveolata. Coral Reefs 38:863–876
Gintert BE, Manzello DP, Enochs IC, Kolodziej G, Carlton R, Gleason ACR, Gracias N (2018) Marked annual coral bleaching resilience of an inshore patch reef in the Florida keys: a nugget of hope, aberrance, or last man standing? Coral Reefs 37:533–547
Goergen EA, Gilliam DS (2018) Outplanting technique, host genotype, and site affect the initial success of outplanted Acropora cervicornis. PeerJ 6:e4433. https://doi.org/10.7717/peerj.4433
Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One 7:e33353. https://doi.org/10.1371/journal.pone.0033353
Hein MY, Beeden R, Birtles A, Gardiner NM, Le Berre T, Levy J, Marshall N, Scott CM, Terry L, Willis BL (2020) Coral restoration effectiveness: multiregional snapshots of the long-term responses of coral assemblages to restoration. Diversity 12:153
Hughes TP, Kerry JT, Connolly SR, Baird AH, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Jacobson M, Liu G, Pratchett MS, Skirving W, Torda G (2019) Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat Clim Chang 9:40–43
Johnson ME, Lustic C, Bartels E, Baums IB, Gilliam DS, Larson EA, Lirman D, Miller MW, Nedimyer K, Schopmeyer S (2011) Caribbean Acropora restoration guide: best practices for propagation and population enhancement. The Nature Conservancy, Arlington
Kaufman ML, Watkins E, van Hooidonk R, Baker AC, Lirman D (2021) Thermal history influences lesion recovery of the threatened Caribbean staghorn coral Acropora cervicornis under heat stress. Coral Reefs 40:289–293
Kenkel CD, Matz MV (2016) Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nat Ecol Evol 1:1–6
Kitchen SA, Ratan A, Bedoya-Reina OC, Burhans R, Fogarty ND, Miller W, Baums IB (2019) Genomic variants among threatened Acropora corals. G3 Genes Genom Genet 9(5):1633–1646
Ladd MC, Shantz AA, Bartels E, Burkepile DE (2017) Thermal stress reveals a genotype-specific tradeoff between growth and tissue loss in restored Acropora cervicornis. Mar Ecol Prog Ser 572:129–139
Liew YJ, Howells, EJ, Wang X, Michell CT, Burt JA, Idaghdour Y, Aranda M (2020) Intergenerational epigenetic inheritance in reef-building corals. Nat Clim Change 10(3):254–259. https://doi.org/10.1038/s41558-019-0687-2
Lirman D, Schopmeyer S (2016) Ecological solutions to reef degradation: optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ 4:e2597. https://doi.org/10.7717/peerj.2597
Lirman D, Thyberg T, Herlan J, Hill C, Young-Lahiff C, Schopmeyer S, Huntington B, Santos R, Drury C (2010) Propagation of the threatened staghorn coral Acropora cervicornis: Methods to minimize the impacts of fragment collection and maximize production. Coral Reefs 29:729–735
Luna GM, Biavasco F, Danovaro R (2007) Bacteria associated with the rapid tissue necrosis of stony corals. Environ Microbiol 9:1851–1857
Manzello DP (2015) Rapid recent warming of coral reefs in the Florida keys. Sci Rep. https://doi.org/10.1038/srep16762
Merselis DG, Lirman D, Rodriguez-Lanetty M (2018) Symbiotic immuno-suppression: is disease susceptibility the price of bleaching resistance? PeerJ 4:1–18
Middlebrook R, Hoegh-Guldberg O, Leggat W (2008) The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J Exp Biol 211:1050–1056
Morikawa MK, Palumbi SR (2019) Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc Natl Acad Sci U S A 116:10586–10591
National Marine Fisheries Service (2006) Endangered and threatened species: final listing determinations for Elkhorn coral and Staghorn coral. Fed Regist 71:26852–26861
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Sci 344:895–898
Putnam HM, Gates RD (2015) Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J Exp Biol 218:2365–2372
Ralph PJ, Hill R, Doblin MA, Davy SK (2015) Theory and application of pulse amplitude modulated chlorophyll fluorometry in coral health assessment. Dis Coral. https://doi.org/10.1002/9781118828502.ch38
Rivest EB, Comeau S, Cornwall CE (2017) The role of natural variability in shaping the response of coral reef organisms to climate change. Curr Clim Change Rep 3:271–281
RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc. http://www.rstudio.com/
Safaie A, Silbiger NJ, McClanahan TR, Pawlak G, Barshis DJ, Hench JL, Rogers JS, Williams GJ, Davis KA (2018) High frequency temperature variability reduces the risk of coral bleaching. Nat Commun. https://doi.org/10.1038/s41467-018-04074-2
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to imagej: 25 yr of image analysis. Nat Methods 9:671–675
Schoepf V, Jung MU, McCulloch MT, White N, Stat M, Thomas L (2020) Thermally variable, macrotidal reef habitats promote rapid recovery from mass coral bleaching. Front Mar Sci 7:245. https://doi.org/10.3389/fmars.2020.00245
Schopmeyer SA, Lirman D, Bartels E, Gilliam DS, Goergen EA, Griffin SP, Johnson ME, Lustic C, Maxwell K, Walter CS (2017) Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 36:1047–1057
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25:453–460
Sully S, Burkepile DE, Donovan MK, Hodgson G, van Woesik R (2019) A global analysis of coral bleaching over the past two decades. Nat Commun 10:1264. https://doi.org/10.1038/41467-019-09238-2
Torda G, Donelson JM, Aranda M, Barshis DJ, Bay L, Berumen ML, Bourne DG, Cantin N, Foret S, Matz M (2017) Rapid adaptive responses to climate change in corals. Nat Clim Change 7:627
van Hooidonk R, Maynard J, Tamelander J, Gove J, Ahmadia G, Raymundo L, Williams G, Heron SF, Planes S (2016) Local-scale projections of coral reef futures and implications of the Paris agreement. Sci Rep. https://doi.org/10.1038/srep39666
van Oppen MJH, Oliver JK, Putnam HM, Gates RD (2015) Building coral reef resilience through assisted evolution. Proc Natl Acad Sci U S A 112:2307–2313
van Woesik R, Banister RB, Bartels E, Gilliam DS, Goergen EA, Lustic C, Maxwell K, Moura A, Muller EM, Schopmeyer S, Winters RS, Lirman D (2021) Differential survival of nursery-reared Acropora cervicornis outplants along the Florida reef tract. Restor Ecol 29:1–10
Wall CB, Ricci CA, Wen AD, Ledbetter BE, Klinger DE, Mydlarz LD, Gates RD, Putnam HM (2021) Shifting baselines: physiological legacies contribute to the response of reef corals to frequent heatwaves. Funct Ecol 35:1366–1378
Ware M, Garfield EN, Nedimyer K, Levy J, Kaufman L, Precht W, Scott Winters R, Miller SL (2020) Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida keys national marine sanctuary. PLoS One 15:e0231817. https://doi.org/10.1371/journal.pone.0231817
Warner ME, Fitt WK, Schmidt GW (1996) The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ 19:291–299
Winters G, Holzman R, Blekhman A, Beer S, Loya Y (2009) Photographic assessment of coral chlorophyll contents: implications for ecophysiological studies and coral monitoring. J Exp Mar Biol Ecol 380:25–35
Young CN, Schopmeyer S, Lirman D (2012) A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and western Atlantic. Bull Mar Sci 88:1075–1098
Acknowledgements
We would like to thank L. Chomiak, L. Shea, L. Dutra, C. Aguilar, J. Morris, J. Unsworth, N. Soderberg, R. van Hooidonk, B. Young, M. D’Alessandro, C. Dennison, and A. Palacio for their contributions to this project and manuscript. This project was funded by NOAA’s Coral Reef Conservation Program. Corals were collected under Florida’s Fish and Wildlife Commission Permit SAL-19-1794-SCRP issued to Diego Lirman. We would like to thank the reviewers and the editor of Coral Reefs for their constructive feedback.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Mark Vermeij
Supplementary Information
Below is the link to the electronic supplementary material.
338_2022_2232_MOESM1_ESM.eps
(a) Image of setup with Coral Color Reference Card (Siebeck et al. 2006) and Kodak Gray Scale bar used for initial color score categorization (D1–D6) and white balance standardization in ImageJ, respectively. This image was taken prior to the start of the 8 d temperature increase to 32 ºC for the heat-stress assay. (b) Observed signs of paling (first and third fragments) and rapid tissue loss (the central fragment) during the heat-stress assay. (EPS 88084 kb)
338_2022_2232_MOESM2_ESM.eps
Mean number of days until coral fragments demonstrated tissue sloughing (dark shading) or bleached to a color score of D1 (light shading) for each genotype during the heat-stress assay after being subjected to one of two treatments (laboratory control or variable) or directly after removal from the field (field control). Error bars are standard deviation. (EPS 1102 kb)
338_2022_2232_MOESM3_ESM.xlsx
Supplementary file3: Supplemental Table 1: Recorded temperatures during both the temperature treatment and heat-stress assays, as well as their respective calculated means and standard deviations. (XLSX 4574 kb)
338_2022_2232_MOESM4_ESM.xlsx
Supplementary file4: Supplemental Table 2: Metadata for each individual coral fragment during both the temperature treatment and heat-stress assays. Color scores (D1-D6), R-intensities, maximum dark-adapted yield of photosystem II (Fv/Fm), and number of days in heat-stress assay before either reaching a color score of D1 (fully bleached) or exhibiting signs of rapid tissue loss. (XLSX 208 kb)
338_2022_2232_MOESM5_ESM.xlsx
Supplementary file5: Supplemental Table 3: Results of post hoc Tukey's HSD test of multiple comparison of means for 2 analyses of variances: 1) the effect of genotype and treatment on the number of days corals persisted in the heat-stress assay, and 2) the effect of original reef site and treatment on the number of days corals persisted in the heat-stress assay, both with a 95% family-wise confidence interval. (XLSX 23 kb)
Rights and permissions
About this article
Cite this article
DeMerlis, A., Kirkland, A., Kaufman, M.L. et al. Pre-exposure to a variable temperature treatment improves the response of Acropora cervicornis to acute thermal stress. Coral Reefs 41, 435–445 (2022). https://doi.org/10.1007/s00338-022-02232-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02232-z