Abstract
Net calcification rates for coral reef and other calcifiers have been shown to decline as ocean acidification (OA) occurs. However, the role of calcium carbonate dissolution in lowering net calcification rates is unclear. The objective of this study was to distinguish OA effects on calcification and dissolution rates in dominant calcifying macroalgae of the Florida Reef Tract, including two rhodophytes (Neogoniolithon strictum, Jania adhaerens) and two chlorophytes (Halimeda scabra, Udotea luna). Two experiments were conducted: (1) to assess the difference in gross (45Ca uptake) versus net (total alkalinity anomaly) calcification rates in the light/dark and (2) to determine dark dissolution (45CaCO3), using pH levels predicted for the year 2100 and ambient pH. At low pH in the light, all species maintained gross calcification rates and most sustained net calcification rates relative to controls. Net calcification rates in the dark were ~84% lower than in the light. In contrast to the light, all species had lower net calcification rates in the dark at low pH with chlorophytes exhibiting net dissolution. These data are supported by the relationship (R2 = 0.82) between increasing total alkalinity and loss of 45Ca from pre-labelled 45CaCO3 thalli at low pH in the dark. Dark dissolution of 45CaCO3-labelled thalli was ~18% higher in chlorophytes than rhodophytes at ambient pH, and ~ twofold higher at low pH. Only Udotea, which exhibited dissolution in the light, also had lower daily calcification rates integrated over 24 h. Thus, if tropical macroalgae can maintain high calcification rates in the light, lower net calcification rates in the dark from dissolution may not compromise daily calcification rates. However, if organismal dissolution in the dark is additive to sedimentary carbonate losses, reef dissolution may be amplified under OA and contribute to erosion of the Florida Reef Tract and other reefs that exhibit net dissolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ocean acidification (OA), a consequence of anthropogenic CO2 absorption by the ocean, leads to a rise in hydrogen ion concentration [H+] that negatively affect calcifying marine organisms (Orr et al. 2005). Global oceanic pH is projected to decline 0.3–0.4 units from a current 8.1 pH by 2100, causing a concurrent shift in dissolved inorganic carbon (DIC) species. Ocean pH decline by the year 2100 leads to an increase in pCO2 and bicarbonate ion (HCO3−) concentration (+200% and +30%, respectively), and a decrease in carbonate ion (CO32−) concentration (−60%), resulting in a lower (−60%) calcium carbonate (CaCO3) saturation state (Ω) of seawater (Fabry et al. 2008; IPCC 2013). While the specific mechanisms are only recently being articulated (Marubini et al. 2008; Hurd et al. 2011; Jokiel 2011; McCulloch et al. 2012; Hendriks et al. 2015), higher pCO2, greater [H+], and reduced Ω in seawater have been shown to coincide with lower calcification rates of reef corals and macroalgae (reviewed in, Nelson 2009; Pandolfi et al. 2011; Koch et al. 2013; Hofmann and Bischof 2014; Hoegh-Guldberg et al. 2017). Calcified tropical reef macroalgae are fundamental to coral reef ecosystem function, including the enhancement of reef sediment production, accretion, and stability, as well as facilitating recruitment of coral larvae (Goreau 1963; Winman and McKendree 1975; Littler and Littler 1984; Davies and Marshall 1985; Heyward and Negri 1999; Chisholm 2000; Rees et al. 2007). Thus, there is uncertainty in how OA will affect the future role of calcified algae on reefs, necessitating a more complete understanding of the mechanisms leading to lower calcification rates at elevated pCO2 and lower pH observed in some groups of macroalgae.
Currently, a number of studies show negative effects of elevated pCO2 and lower pH on macroalgal calcification (Gao et al. 1993; Anthony et al. 2008; Price et al. 2011; Diaz-Pulido et al. 2014; Kato et al. 2014; Meyer et al. 2016); however, there is also evidence for continued calcification under these conditions for many species, including both rhodophytes and chlorophytes dominant on coral reefs (Vogel et al. 2015a, 2015b; Peach et al. 2016, 2017a; Dutra et al. 2016; Comeau et al. 2017, 2018; Cornwall et al. 2017). Some rhodophytes may be more structurally vulnerable to lower pH due to the relatively high solubility of their high-magnesium calcite skeletal CaCO3 polymorph at low pH, and the close proximity of calcification sites to bulk seawater (Adey 1998; Morse et al. 2006; Jokiel et al. 2008; Smith et al. 2012). However, some species appear to be able to resist OA conditions due to strong organismal control of pH and carbonate chemistry at the thalli surface and calcification site (Cornwall et al. 2017; Comeau et al. 2018; McNicholl et al. 2019). Chlorophytes build their skeletons of the metastable aragonite CaCO3 polymorph within semi-isolated calcification spaces (e.g. fused utricles or sheaths) that may minimize exposure to bulk seawater (Borowitzka and Larkum 1987; Peach et al. 2017a, 2017b). Nevertheless, some chlorophytes have less biological control of their thalli pH (McNicholl et al. 2019) and CaCO3 crystal isolation may be weak in some species (Price et al. 2011; Peach et al. 2017b). In addition to morphology and the CaCO3 polymorph affecting macroalgal calcification responses to lower pH, photophysiology may also be instrumental to sustain calcification rates (Hurd et al. 2011; Cornwall et al. 2012; Zweng et al. 2018; Comeau et al. 2017; McNicholl et al. 2019).
Photosynthesis is known to stimulate calcification (Borowitzka and Larkum 1976b, 1987; Gao et al. 1993), buffer the effects of OA through the formation of a high pH micro-boundary at the thalli surface, and provide energy for ATP-driven H+ pumps (De Beer and Larkum 2001; Cornwall et al. 2013; Hofmann et al. 2016; McNicholl et al. 2019). Thus, the effects of OA on calcification and dissolution of calcifying autotrophs, such as reef macroalgae, are likely to have distinct responses in the light compared to the dark. Net calcification in the dark occurs at slower rates, potentially due to reduced ability to precipitate CaCO3 without photosynthesis or dissolution overcoming biomineralization or recrystallization processes (Borowitzka and Larkum 1976a, 1976b, 1987; Chisholm 2000; Comeau et al. 2012). Enhanced dissolution at low bulk seawater pH may in part be explained by a lower capacity of the thalli surface to buffer H+ and a build-up of respiratory CO2 and H+, thereby reducing pH in the calcifying space (Borowitzka 1976b; Comeau et al. 2012). Consequently, calcifying autotrophs may be more vulnerable to night-time dissolution with higher external pCO2 and [H+] in seawater (Martin and Gattuso 2009; Comeau et al. 2012; Kamenos et al. 2013; Vogel et al. 2015a, 2015b; Chou et al. 2020).
Ocean acidification research on coral reefs, including macroalgae, has largely focused on calcification (reviewed in, Fabry et al. 2008; Pandolfi et al. 2011; Hofmann and Bischof 2014); however, there is growing interest in dissolution effects on low net calcification rates found near CO2 seeps (Rodolfo-Metalpa et al. 2011; Vogel et al. 2015a, 2015b) and in elevated pCO2 experiments (Pickett and Andersson 2015; Chou et al. 2020). Recent field studies (Andersson and Gledhill 2013; Eyre et al. 2014) and mesocosm experiments (Andersson et al. 2009; Anthony et al. 2013; Dove et al. 2013) highlight the importance of carbonate sediment dissolution, particularly at low porewater pH (Cyronak et al. 2013), in driving net reef dissolution. While sediment dissolution is now well recognized, there have been fewer studies examining individual reef organisms’ potential contribution to dissolution (Rodolfo-Metalpa et al. 2011; Vogel et al. 2015a, 2015b). In coral reef macroalgae, as well as other calcifiers, the role of carbonate dissolution in reducing net calcification is an important mechanistic question that remains. The question is whether reduced net calcification rates under OA are a result of an organism’s diminished capacity to precipitate CaCO3, or if dissolution is enhanced (Jokiel 2011; Roleda et al. 2012; Cyronak et al. 2016).
The effects of OA on calcification have primarily been determined by buoyant weight, total alkalinity anomaly, imaging, or staining, that quantify net calcification rates without the ability to distinguish between gross versus net calcification and identify the role of dissolution (Jokiel et al. 1978; Chisholm and Gattuso 1991; Holcomb et al. 2013; Schoepf et al. 2017). As net calcification is defined by gross calcification minus gross dissolution (Smith and Key 1975), understanding both of these processes is critical to elucidate the mechanisms influencing low net calcification rates under OA conditions. The fact that they can occur simultaneously complicate the disentanglement of calcification and dissolution processes. For example, respiratory CO2 can disrupt CaCO3 precipitation by lowering pH at the calcification site in the dark and lead to dissolution of crystals (Borowitzka and Larkum 1976b). Under OA conditions, positive net CaCO3 production will be dependent on an organism’s ability to sustain gross calcification to counter dissolution (Findlay et al. 2009, 2011). Negative net calcification rates, or net dissolution, observed in OA studies (Anthony et al. 2008; Jokiel et al. 2008; Martin and Gattuso 2009; Diaz-Pulido et al. 2012; Vogel et al. 2015a, 2015b) may be misinterpreted as a reduced capacity to calcify, while the principal effect may be enhanced dissolution. In contrast to methods that only measure net calcification, gross calcification can be estimated using 45Ca radioisotopes or measuring Ca2+ uptake (Borowitzka and Larkum 1976a; Böhm 1978; Tambutté et al. 1995; Findlay et al. 2011; Gazeau et al. 2015). While limitations of these techniques also exist, such as overestimation with isotopic exchange (Borowitzka and Larkum 1976a), these constraints have been partially resolved by employing a lower total activity and increasing rinse times to ensure measurements are only based on radioisotopes incorporated into the skeleton (Böhm 1978; Tambutté et al. 1995). Using techniques that measure net and gross calcification in tandem may provide the best opportunity to separate the effects of OA on calcification versus dissolution (Gómez-Batista et al. 2020) to gain a more robust mechanistic understanding of OA effects on net calcification (Rodolfo-Metalpa et al. 2011; Cohen and Fine 2012; Cohen et al. 2017). Understanding these mechanisms could also assist in explaining some of the current discrepancies in the literature that show positive, negative, and neutral responses of net calcification to OA in reef macroalgae, even within the same species (Ries et al. 2009; Sinutok et al. 2011; Vogel et al. 2015b; Peach et al. 2016, 2017a; Cornwall et al. 2017; Comeau et al. 2018).
The objective of this study was to distinguish the effects of elevated pCO2 and lower pH predicted for the year 2100 (IPCC 2013) on gross and net calcification in the light and dark, as well as dark dissolution, in dominant calcifying macroalgae from the Florida Reef Tract. Further, the role of irradiance in raising the calcification to dissolution ratio at low pH was examined. We hypothesized gross calcification rates would increase at lower pH and greater pCO2 in the light to accommodate higher dissolution rates and sustain net calcification rates. We further predicted that species with lower net calcification rates under OA, particularly in the dark, would exhibit higher dissolution rates, rather than lower gross calcification rates. All species are likely to have reduced gross and net calcification rates in the dark under OA conditions, as calcification becomes more reliant on light-triggered processes (Comeau et al. 2012; Vogel et al. 2015a; Hofmann et al. 2016; McNicholl et al. 2019; Chou et al. 2020).
Materials and methods
Macroalgal collection and maintenance
Four dominant Florida Reef Tract calcifying macroalgal species (Neogoniolithon strictum, Jania adhaerens, Halimeda scabra, and Udotea luna; hereafter referred to by genus) were collected (August–September 2018) at ~3 m depth via scuba from a patch reef off Looe Key Reef (24.62055° N, 81.37078° W). Irradiance measured at midday just above the benthos was ~800 µmol photons m−2 s−1 (4π spherical PAR quantum sensor; LI-193, LI-COR Inc.) Samples were transported in aerated coolers within 4 h to Florida Atlantic University’s (FAU) marine laboratory in Boca Raton, FL. Species were identified and placed in a flow-through seawater system (coastal Atlantic seawater) under full-spectrum LED lights set for ~500 µmol photons m−2 s−1 (Build My LED©). Irradiance was set to ~500 µmol photons m−2 s−1 for maintaining macroalgae before the experiments and during the experiment to correspond to a saturating light level previously determined for these species (Zweng et al. 2018), and achievable with the full-spectrum lights employed. The incoming seawater had a salinity of 35.5 and 29 °C temperature. After 24 h, samples were shipped to the International Atomic Energy Agency’s (IAEA) Environment Laboratories in the Principality of Monaco and acclimated for 2 weeks to laboratory conditions before experiments commenced. Macroalgae were maintained in two open-circuit 40 L aquaria with natural UV-sterilized filtered (1 µm) seawater renewed 50% h−1 (salinity: 38; T°: 29 °C) under full-spectrum LED lights (Kessil, A360W E-Series Tuna Sun) set to 550 µmol photons m−2 s−1 on a 12:12 h photoperiod.
Experimental design and analytical techniques
Two experiments were conducted: (1) a calcification experiment to assess net and gross calcification rates in the light and dark at low and ambient pH and (2) a dissolution experiment to determine dark dissolution (loss of 45CaCO3) and net calcification (TAA) rates at low and ambient pH for each species. Treatment and control pH values, and resulting carbonate parameters, achieved in each experiment (average ± SD) are presented in Table 1. The targeted pH was a 0.3–0.4 pH reduction forecasted for the year 2100 (7.7) from present-day values (~8.1).
Net and gross calcification experiment
Experiment setup
Net and gross calcification rates were determined by conducting short-term incubations on individual algal thalli in a closed system (200 or 500 mL polyethylene beakers). The length of incubation was established by the change in seawater total alkalinity (TA) necessary to estimate net calcification rate using the total alkalinity anomaly (TAA) method, while limiting metabolic-induced changes in seawater pH (i.e. photosynthesis or respiration) based on OA experimental protocols (Langdon et al. 2010). Incubation periods were adjusted for time and volume of seawater (200 mL Udotea; 500 mL all other species) so the δTA was 3–10 times the control (seawater only containers; δTA -5.0 ± 9.1 µmol kg−1), but did not exceed 10% of overall δTA (Langdon et al. 2010). The TAA technique assumes a 2:1 molar ratio of TA change (− / +) and CaCO3 precipitation/dissolution, respectively (Smith and Key 1975; Chisholm and Gattuso 1991).
To determine pH effects on calcification rates during the day, incubations were conducted for 4 h in the light (~550 µmol photons m−2 s−1) between 0800 and 1500. Calcification rates in the dark were determined for 13 h from 1800 to 0700. Ambient and low pH experiments (n = 3–4) were conducted simultaneously for all species with one individual per beaker and blank controls with only seawater. Seawater pH was determined with calibrated pH-loggers (Metrohm 826; pHNBS) and corrected to total pHT scale based on a standard TRIS buffer (Andrew Dickson Lab, Scripps Institute of Oceanography; Batch # T33).
Experimental seawater was prepared in batches for each experiment by filtering (0.45 µm), and adjusting to treatment pH (ambient or bubbled with pure CO2) and temperature (29 °C). Macroalgae were randomly selected and conditioned to non-labelled batch seawater under experimental conditions (as above) for 30 min. To achieve a total experimental activity of 50 Bq mL−1 45Ca, 11 µL of 45CaCl2 source activity (13,895 kBq mL−1; PerkinElmer) was added to 3 L of the experimental seawater devoid of macroalgae, or 150 kBq of total activity. Homogenized subsamples (1 mL) were collected before each experiment to assess the initial 45Ca activity, total alkalinity (TA; 150 mL), pHT, salinity, dissolved O2, and temperature. Experimental beakers were weighed (Balance Sartorius BP 3100 S; 0.01 g) before and after being filled with the 45Ca-labelled seawater (200–500 mL) to determine initial seawater volume based on mass and density. Macroalgal samples were then transferred to assigned beakers with labelled seawater and sealed with clear plastic wrap (Azpack™ Wrap film) in contact with seawater surface to minimize headspace and water–air gas exchange. All beakers were then placed in a water bath to keep temperature constant (29 ± 0.2 °C) during the incubation. Seawater was continuously mixed in each beaker via a stir-bar (30 mm; 180 rpm) below the algae separated by a perforated plastic disc. Two stir plates (Thermo Scientific Variomag) operating six stirrers each were used to ensure similar flow rates between treatments and replicates.
At the end of each incubation, alga was removed from the beaker and prepared for 45Ca analysis. Seawater pHT, dissolved O2 and temperature were immediately measured in the beakers, and subsamples taken for TA (150 mL). A subsample (1 mL) was also obtained from each beaker for final seawater 45Ca determination. Seawater inorganic nitrogen (NH4+, NO3−, NO2−) concentration (1 mL) was determined at the beginning and end of the first experimental runs (Spectroquant method; Merck Spectroquant Pharo 100) to ensure nutrients were low and not influencing TA measurements (Gazeau et al. 2015).
Net calcification determination
Replicate seawater samples (n = 3) for TA were collected at the beginning and end of each incubation, poisoned with mercuric chloride (0.02%), sealed in sample tubes (Falcon 50 mL), and stored in the dark at 4 °C for subsequent analysis. Total alkalinity was measured by open-cell titration (Metrohm Titrando 888) with 0.1 N HCl and verified using an alkalinity reference (CRM Batch #174; Andrew Dickson Lab, Scripps Institute of Oceanography). TA measurements were taken in triplicate unless the initial two measurements were within ± 5 µmol kg−1 of each other. Net calcification rate was calculated from δTA as follows:
where the net calcification rate (Gnet) is µmol CaCO3 g dwt−1 h−1, ρw is the seawater density (kg L−1), δTA is the difference between TAfinal minus TAinitial (µmol kg−1), v is the volume of seawater (L), Wa is the algal dry weight (g) and t is the incubation time in hours.
Photosynthesis and respiration rates
Photosynthesis (O2 production) and respiration (O2 consumption) rates were calculated from dissolved O2 concentration (mg L−1) measurements (YSI ProODO Optical Dissolved Oxygen Meter) before and after each incubation in the light and dark, respectively, and normalized to dry weight of the alga. Gross primary production was calculated by adding net O2 production to the absolute value of respiration. Change in dissolved O2 from control, seawater-only beakers was subtracted from photosynthesis and respiration rates to account for microbial activity and gas exchange.
Gross calcification determination
Gross calcification rates were determined using 45Ca as a tracer based on the protocol developed for corals by Tambutté et al. (1995) and Cohen et al. (2017) and modified by Gómez-Batista et al. (2020). This technique assumes a 1:1 molar ratio of the rate of 45Ca2+ uptake from seawater to the rate of CaCO3 precipitation. At the end of each incubation period, macroalgal samples were rinsed with fresh seawater for 30 min in a flow-through aquaria (20 L) to allow dilution of potential isotopic exchange (Tambutté et al. 1995) and/or release of 45Ca within inter-utricle spaces (i.e. Halimeda) (Borowitzka and Larkum 1976a; Böhm 1978). Following the rinse period, samples were dipped in de-ionized water to remove salt, gently blotted dry, and moved to a drying oven (60 °C). Once dried (4–10 d), algal samples were manually crushed and completely dissolved in a 1:1 perchloric (70%) and nitric (70%) acid solution (0.6–1.5 mL). From each dissolved algal sample, 2–4 aliquots (0.2 mL) were separately transferred to scintillation vials and then each filled with 10 mL scintillation liquid Ultima Gold™ XR, homogenized, and kept in the dark for 24 h before radio analyses. On average, the difference of activity between aliquots was 7.5%. If the difference was > 15%, two additional aliquots were counted. For the determination of specific activity in the radiolabelled seawater, a 1 mL subsample was collected from each beaker at the end of the experiment, transferred to a 20 mL glass scintillation vial, mixed in proportion 1:10 (v:v) with scintillation liquid, and kept in the dark for 24 h before radio analyses.
The radioactivity of 45Ca was counted using a Tri-Carb 2900 Liquid Scintillation Counter for a total of 275 samples. Counting time was adjusted to achieve < 5% counting error. Average 45Ca activity of aliquots was used to calculate total 45Ca activity in the algal skeleton based on known standards and corrected for counting efficiency, radioactive decay, and quenching effect. Gross calcification (Ggross) rates (µmol CaCO3 g dwt−1 h−1) were calculated from algal skeletal activity and seawater activity using the following formula:
where 45Ca activitysp is equal to the total 45Ca activity in counts per minute (CPM) based on average of replicate aliquots (n = 2–4) of dissolved algal sample, 11.44 is the concentration of Ca2+ in 1 mL of seawater (µmol), δ45Ca activitysw is the total activity of 45Ca in 1 mL of seawater (CPM) calculated from average initial and final seawater activity, Wa is the algal dry weight (g), and t is the incubation time in hours.
Calculating dissolution rates from calcification estimates
To calculate the dissolution rate from the net and gross calcification experiments, the calcification data using the 45Ca uptake and TAA techniques would have to be quantitatively combined. However, this was not possible due to net calcification rates being higher in some cases than gross calcification, particularly in the light. This issue has been found in experiments on corals, bivalves, and macroalgae using the TAA and 45Ca and changes in seawater [Ca2+] (Tambutté et al. 1995; Rodolfo-Metalpa et al. 2011; Gazeau et al. 2015; Cohen and Fine 2012; Cohen et al. 2017; this study). A detailed discussion of factors potentially causing higher net than gross calcification rates is presented in the supplemental section (Supplemental Notes on Methodology). While calculating dissolution from net and gross calcification rates were constrained by methodology, comparisons of calcification rates using the TAA and 45Ca uptake techniques across treatments revealed similar trends (Fig. S1), and identified the importance of dissolution in calcifying macroalgae. Our approach of labelling of 45CaCO3 in the thalli and examining dissolution directly (method described below) allowed us to quantify the dissolution response to OA in the dark.
Dissolution experiment
As an estimate of dissolution, 45Ca loss from pre-labelled thalli was determined in the dark. To allow 45Ca incorporation into the algal CaCO3 framework, macroalgae were grown in two 20-L tanks of radiolabelled seawater for 72 h (12 h photoperiod). Activity was added to the experimental seawater before the addition of macroalgae. To achieve an experimental activity of 35 Bq mL−1 45Ca, 11 µL of 45CaCl2 source activity (61,870 kBq mL−1; PerkinElmer) was added to 20 L of experimental seawater, or ~700 kBq of total activity. At the end of the 72 h 45Ca exposure, all thalli samples were rinsed in flow-through seawater for 30 min to control for potential isotopic exchange (Tambutté et al. 1995). The dissolution experiment was conducted similarly to the calcification experiment, except incubations were in unlabelled seawater. Algal samples were randomly assigned to beakers (n = 3) with unlabelled seawater at ambient or low pH treatments (Table 1). The dissolution experiments were conducted in the dark for 13 h at night (between 1800 and 0700). Initial and final pHT, O2, temperature, and TA (150 mL) for each beaker were measured. Seawater samples (2 mL; n = 2) were obtained from each beaker at the end of the incubation and total 45Ca activity released from the thalli measured as an estimate of 45CaCO3 dissolution. Replicate seawater samples (n = 3) for TA were collected at the beginning and end of each dark incubation, poisoned, and kept in the dark at 4 °C until subsequent analysis, as described above.
Statistical analysis
To compare calcification rates across light/dark and ambient/low pH, we used a 2-way ANOVA (SigmaPlot v13.0, Systat Software Inc.). A post hoc Tukey’s test was employed to discern significance within ANOVA factors. A t test was used to compare gross and net calcification rates within treatment pH in the light and dark. The assumptions of normality and homogeneity of variances were tested using the Shapiro–Wilk and Levene tests, respectively. Significance levels are p < 0.05 unless otherwise stated.
Results
Net calcification rates under low and ambient pH
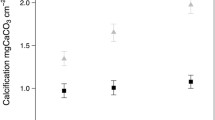
Net calcification rates in the light were similar at ambient and low pH for most of the calcifying macroalgae based on the TAA technique (Fig. 1). Neogoniolithon and Halimeda had less than a 5% difference in net calcification rate between pH treatments in the light. Udotea was the only species with a significantly lower net calcification rate (−44%) in the light at low pH compared to controls. Net calcification rates for all species (Fig. 1) were significantly lower in the dark compared to the light, regardless of low (−78 to −108%) or ambient pH (−78 to −94%) treatment. Neogoniolithon and Jania maintained positive net calcification rates in the dark at both ambient and low pH, although net calcification rates were significantly lower (~50%) at low compared to ambient pH (Fig. 1a, b). Halimeda and Udotea exhibited net dissolution in the dark at low pH, while at ambient pH in the dark, net calcification rates remained positive (Fig. 1c, d). All species had significantly (p < 0.05; Neogoniolithon, p = 0.06) lower net calcification rates in the dark under low pH compared to ambient controls. Udotea was the only species with significantly lower daily net calcification rates (−52%) integrating light and dark calcification rates over 24 h, a consequence of the combined effect of lower net calcification in the light and dark at low pH.
Net calcification rates (TAA) of a Neogoniolithon, b Jania, c Halimeda, d Udotea at ambient and low pH in the light for 4 h (between 0800 and 1500 h) or 13 h in the dark (1800 to 0700 h). Daily net calcification rates were calculated by combining light and dark calcification rates normalized to 24 h for each pH treatment. Asterisk indicates a significant difference (p < 0.05) between pH treatments within light and dark experiments. Means ± standard error
Gross calcification rates under low and ambient pH
Similar to results for net calcification, gross calcification rates were highest in the light with no significant effect of low pH on gross calcification rates in the light (Fig. 2). Gross calcification rates in the dark were lower than in the light for both ambient (−41 to −83%) and low (−46 to −91%) pH treatments for all species. In the dark, the rhodophytes sustained gross calcification rates at low pH (Fig. 2a, b), while the chlorophytes had significantly lower gross calcification rates (−50%) at low compared to ambient pH (Fig. 2c, d). In contrast to net calcification, gross calcification remained positive for chlorophytes at low pH in the dark (Fig. 2c, d). The relatively high gross calcification rates in the light at ambient and low pH resulted in similar daily gross calcification rates for all species (Fig. 2).
Gross calcification rates (45Ca uptake) of a Neogoniolithon, b Jania, c Halimeda, d Udotea at ambient and low pH in the light for 4 h (between 0800 and 1500 h) or 13 h in the dark (1800–0700 h). Daily gross calcification rates were calculated by combining light and dark calcification rates normalized to 24 h for each pH treatment. Asterisk indicates a significant difference (p < 0.05) between pH treatments within light and dark experiments. Means ± standard error
Dissolution and net calcification in the dark
The activity of 45Ca in seawater from pre-labelled 45CaCO3 thalli was highest at low compared to ambient pH in the dark (Table 2). Udotea had relatively high seawater 45Ca activity in both ambient and low pH, suggesting high dark dissolution rates regardless of pH. This apparent high dissolution rate for Udotea is supported by the negative net calcification rates (Fig. 1d, Table 2) and high TA increase under low pH (Table 2) in the dark. At low pH in the dark, the δTA was positive and net calcification rates negative for each species, indicating dissolution. The dissolution of 45CaCO3 explained 82% of the variance of seawater δTA under low pH conditions (Fig. 3a), while there was no significant relationship at ambient pH (Fig. 3b). The change in 45CaCO3 dissolution from ambient to low pH was lower in the rhodophytes (7–21%) compared to the chlorophytes (29–32%), consistent with dark net calcification results (Fig. 1, Table 2).
Photosynthesis and respiration
The O2 flux in the light (photosynthesis) and dark (respiration) was not significantly different between ambient and low pH treatments for any species in the two experiments (Table S1).
Discussion
The present study demonstrates that dissolution under elevated pCO2 and lower pH predicted for the year 2100 (IPCC 2013) can explain lower net calcification rates of calcified macroalgae, particularly in the dark, even when the ability to precipitate new CaCO3 in the light or on a daily basis was not constrained. This effect may explain the diminished net calcification rates frequently measured in tropical reef macroalgae at lower pH (Nelson 2009; Pandolfi et al. 2011; Koch et al. 2013; Hofmann and Bischof 2014; Hoegh-Guldberg et al. 2017). The major effects of acidification on reef macroalgae dissolution occur in darkness when calcification rates are ~5 to 10 times lower than during the day. The conclusion that lower net calcification rates under OA conditions in the dark are associated with dissolution was supported by the close relationship (R2 = 0.82) between increasing TA and loss of 45Ca from pre-labelled 45CaCO3 thalli only in the low pH treatment. Although calcification experiments on reef organisms examining OA effects infrequently examine light and dark conditions separately, those that have been conducted typically find lower net calcification rates at low pH in the dark (Martin and Gattuso 2009; Kamenos et al. 2013; Vogel et al. 2015a, 2015b; Chou et al. 2020). At the Milne Bay Province, Papua New Guinea (PNG) cold CO2 seep site, Halimeda opuntia and H. digitate had higher net calcification rates at low pH (7.7) seeps during the day compared to controls (8.1). At night, however, a significant decline (−167%) in net calcification was observed for H. opuntia, and minimal calcification at both seep and control sites for H. digitate (Vogel et al. 2015b). Venn et al. (2019) found in some species of coral (e.g. Stylophora pistillata) calcification rates were more sensitive to low pH in darkness. They also observed no net calcification at pH 7.4 and dissolution at pH 7.2 for the coral species Acropora hyacinthus in the dark. Similarly, a major dark effect was found at pH 7.7 in Acropora millepora with reduced (−155%) net calcification rates at seep sites in PNG (Vogel et al. 2015a). Although it is presumptive to compare across reef organisms with a diversity of calcification mechanisms, our results on macroalgae contribute to the growing awareness that dissolution occurs in many calcifying reef organisms (Andersson et al. 2009; Cyronak et al. 2013; Silbiger and Donahue 2015), and may be enhanced at low pH in the dark for autotrophic calcifiers (Martin and Gattuso 2009; Vogel et al. 2015a, 2015b; Chou et al. 2020).
Since dark dissolution appears to be an important mechanism influencing the effect of OA on net calcification rate in reef calcifiers, the ability to calcify in the dark or maintain net positive calcification may be an indicator of OA resistance. This supposition is supported by the divergent responses of the rhodophyte and chlorophyte macroalgal species to OA conditions examined herein. Dark dissolution of 45CaCO3 labelled thalli was on average 18% higher in chlorophytes compared to rhodophytes even at ambient pH. The chlorophytes also had approximately twofold higher 45CaCO3 dissolution rates relative to the rhodophytes at low seawater pH compared to ambient. In the dark, dissolution or lack of CaCO3 re-precipitation of labelled thalli 45CaCO3 was greater (124%) in chlorophytes than rhodophytes at low pH. The observed resistance to dark dissolution in rhodophytes is in contrast to what was expected based on the higher potential solubility of the rhodophyte’s high-Mg calcite polymorph, compared to the lower solubility of aragonite found in chlorophytes (Borowitzka and Larkum 1987; Ries et al. 2009; Ries 2011a; Wizemann et al. 2015). Thus, polymorph chemistry may not be a good indicator of macroalgal species’ susceptibility to OA, particularly in species with the ability to modify or control polymorph chemistry to enhance resistance to OA (Ries 2009, 2011b; Egilsdottir et al. 2013; Kamenos et al. 2016; Nash et al. 2016). Instead, processes such as H+ control, morphology, and mechanisms of calcification are likely more important in constraining OA impacts on thalli CaCO3 dissolution (Jokiel 2011; Price et al. 2011; Ries 2011b; Hofmann et al. 2016; McNicholl et al. 2019). It is well documented that rhodophytes have strong biotic control over internal calcification–dissolution processes (Cornwall et al. 2017; Comeau et al. 2018), as many continuously shed and reconstitute epithallial cells to remove epiphytes (Wegeberg and Pueschel 2002; Pueschel et al. 2005) and decalcify and recalcify in specific regions of the thalli to form reproductive structures (Adey et al. 2013). Further, rhodophytes typically form highly structured calcite crystals associated with the production of organic matrices (Nash et al. 2019) that may contribute to active biotic control of dissolution, as found in corals that show less dissolution with tissue present, even in undersaturated seawater (Rodolfo-Metalpa et al. 2011). Calcification in rhodophytes also occurs within cell walls that may semi-isolate CaCO3 from night-time dissolution, or perhaps more readily allow the efflux of respiratory CO2 to seawater. Based on stable isotope δ13C signatures of tropical macroalgal thalli, Lee and Carpenter (2001) propose both Halimeda and Udotea calcification is dependent on metabolic effects with a relatively large component of DIC calcification arising from respiratory CO2 in the light, albeit more research is needed in this regard. This light-dependent calcification process may increase the exposure of aragonite crystals to respiratory CO2 at night when respiratory DIC is not being sequestered. This would correspond to negative net calcification and greater dissolution at night for this group with greater CO2 exposure from external seawater that may be additive to respiratory CO2, as no difference in respiration rates in low pH treatments was found in the present study or others (Semesi et al. 2009; Kamenos et al. 2013; Comeau et al. 2017). At night, CO2 released into the semi-enclosed inter-utricular calcifying space (IUS) of Halimeda spp. partially dissolves some CaCO3 to be recrystallized for different skeletal structures in the utricle or cellular-IUS interface (Borowitzka and Larkum 1976b; Wizemann et al. 2014). The IUS may become further undersaturated causing more dissolution (Wizemann et al. 2015), but aragonite crystal deformation is not obvious in all species at lower pH (Peach et al. 2017a). In contrast, the rhodophyte Neogoniolithon showed δ13C carbonate isotopic fractionation that was positively correlated to δ18O, more in-line with carbonate-secreting organisms (Lee and Carpenter 2001), thus may be less dependent on bulk seawater chemistry, and respiratory CO2 exposure at night. Comparisons between rhodophytes and chlorophytes in our study show that the ability to precipitate new CaCO3 in the dark at low pH may affect the extent to which low pH exposure impacts net dissolution, although this link is not well understood and requires further mechanistic studies and an examination of more species.
While enhanced dark dissolution appears to be common in calcifying autotrophs in response to OA conditions (Martin and Gattuso 2009; Kamenos et al. 2013; Vogel et al. 2015a, 2015b; Venn et al. 2019; Chou et al. 2020; this study), some species may be able to offset these negative impacts with light-enhanced calcification (LEC) during the day, resulting in no net effect on daily calcification rates (Kamenos et al. 2013; Vogel et al. 2015b). In some scenarios, dissolution may explain compromised structural integrity (Ragazzola et al. 2012), or altered crystal content in macroalgae, even when net calcification remains unaffected at low pH (Hofmann et al. 2012, 2014). Differences in the daily net calcification rates were primarily driven by light calcification rates that were ~5 to 10 times higher than dark calcification rates in the macroalgae examined; a similar response was shown by others (Gao et al. 1993; Chisholm 2000). This LEC effect may be attributed to enhanced biotic calcification (biomineralization) and/or abiotic precipitation or cementation due to carbonate oversaturation. The present study illustrates the relatively close relationship between gross and net calcification rates, and the importance of light-driven calcification in overcoming low pH conditions predicted for the year 2100. LEC has been observed across macroalgal calcifiers (Borowitzka and Larkum 1987) and some zooxanthellae coral (Jokiel 2011; Venn et al. 2019). We found all four macroalgal calcifiers maintained gross calcification in the light at low pH similar to ambient pH controls. These data highlight the importance of irradiance for some autotrophic calcifiers to sustain their maximum calcification rates to overcome enhanced dissolution.
Dissolution in the dark appears to be the major driver of OA effects on net calcification rates in tropical macroalgae; however, our results also indicate that dissolution under low pH can depress net calcification rates in the light in some species. However, if tropical macroalgae can maintain high calcification rates in the light, lower net calcification rates in the dark from dissolution may not compromise daily calcification rates. Effects of lower pH in the light on net calcification rates driven by enhanced dissolution may be more prevalent in chlorophytes than rhodophytes; however, more species need to be examined to validate this supposition. Dissolution at the organismal scale may be amplified under OA conditions when considering the whole reef added to sedimentary carbonate losses with implications for carbonate platform growth, stability, and formation in the long term. This would be crucial for the Florida Reef Tract where some reefs are already eroding at −1.1 ± 0.4 kg CaCO3 m−2 year−1 (Muehllehner et al. 2016), and across reefs globally showing enhanced dissolution of sediments and organismal skeletons under global change (Andersson et al. 2009; Silbiger and Donahue 2015; Eyre et al. 2018).
References
Adey WH (1998) Review-Coral Reefs: Algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J Phycol 34:393–406
Adey WH, Halfar J, Williams B (2013) The coralline genus Clathromorphum Foslie emend Adey Biological, physiological, and ecological factors controlling carbonate production in an Arctic-Subarctic climate archive. Smithson Inst Sch Press 40:1–39
Andersson AJ, Gledhill D (2013) Ocean acidification and coral reefs: Effects on breakdown, dissolution, and net ecosystem calcification. Annu Rev Mar Sci 5:321–348
Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, Tan A (2009) Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: mesocosm-scale experimental evidence. Biogeosci 6:1811–1823
Anthony KRN, Diaz-Pulido G, Verlinden N, Tilbrook B, Andersson AJ (2013) Benthic buffers and boosters of ocean acidification on coral reefs. Biogeosci 10:4897–4909
Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Nat Acad Sci 105:17442–17446
Böhm L (1978) Application of the 45Ca tracer method for determination of calcification rates in calcareous algae: effect of calcium exchange and differential saturation of algal calcium pools. Mar Biol 47:9–14
Borowitzka MA, Larkum AWD (1976a) Calcification in the green alga Halimeda II The exchange of Ca2+ and the occurrence of age gradients in calcification and photosynthesis. J Exp Bot 27:864–878
Borowitzka MA, Larkum AWD (1976b) Calcification in the green alga Halimeda III The sources of inorganic carbon for photosynthesis and calcification and a model of the mechanism of calcification. J Exp Bot 27:879–893
Borowitzka MA, Larkum AWD (1987) Calcification in algae: mechanisms and the role of metabolism. Crit Rev Plant Sci 6:1–45
Chisholm JRM (2000) Calcification by crustose coralline algae on the northern Great Barrier Reef, Australia. Limnol Oceanogr 45:1476–1484
Chisholm JRM, Gattuso J-P (1991) Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol Oceanogr 36:1232–1239
Chou W-C, Liu P-J, Chen Y-H, Huang W-J (2020) Contrasting changes in diel variations of net community calcification support that carbonate dissolution can be more sensitive to ocean acidification than coral calcification. Front Mar Sci 7:3
Cohen S, Fine M (2012) Measuring gross and net calcification of a reef coral under ocean acidification conditions: methodological considerations. Biogeosci 9:8241–8272
Cohen S, Krueger T, Fine M (2017) Measuring coral calcification under ocean acidification: methodological considerations for the 45Ca-uptake and total alkalinity anomaly technique. PeerJ 5:e3749
Comeau S, Carpenter RC, Edmunds PJ (2012) Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc R Soc B Biol Sci 280:1753
Comeau S, Carpenter RC, Edmunds PJ (2017) Effects of pCO2 on photosynthesis and respiration of tropical scleractinian corals and calcified algae. ICES J Mar Sci 74:1092–1102
Comeau S, Cornwall CE, DeCarlo TM, Krieger E, McCulloch MT (2018) Similar controls on calcification under ocean acidification across unrelated coral reef taxa. Glob Change Biol 24:4857–4868
Cornwall CE, Comeau S, McCulloch MT (2017) Coralline algae elevate pH at the site of calcification under ocean acidification. Glob Change Biol 23:4245–4256
Cornwall CE, Hepburn CD, Pilditch CA, Hurd CL (2013) Concentration boundary layers around complex assemblages of macroalgae: Implications for the effects of ocean acidification on understory coralline algae. Limnol Oceanogr 58:121–130
Cornwall CE, Hepburn CD, Pritchard D, Currie KI, McGraw CM, Hunter KA, Hurd CL (2012) Carbon-use strategies in macroalgae: Differential responses to lowered pH and implications for ocean acidification: response of macroalgae to lowered pH. J Phycol 48:137–144
Cyronak T, Santos IR, Eyre BD (2013) Permeable coral reef sediment dissolution driven by elevated pCO2 and pore water advection. Geophys Res Lett 40:4876–4881
Cyronak T, Schulz KG, Jokiel PL (2016) The omega myth: what really drives lower calcification rates in an acidifying ocean. ICES J Mar Sci 73:558–562
Davies PJ, Marshall JF (1985) Halimeda bioherms - low energy reefs, northern Great Barrier Reef. Proc 5th Int Coral Reef Symp 5:1–7
De Beer D, Larkum AWD (2001) Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors. Plant Cell Environ 24:1209–1217
Diaz-Pulido G, Anthony KRN, Kline DI, Dove S, Hoegh-Guldberg O (2012) Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae: warming, high CO2 and corallines. J Phycol 48:32–39
Diaz-Pulido G, Nash MC, Anthony KRN, Bender D, Opdyke BN, Reyes-Nivia C, Troitzsch U (2014) Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs. Nat Commun 5:3310
Dove SG, Kline DI, Pantos O, Angly FE, Tyson GW, Hoegh-Guldberg O (2013) Future reef decalcification under a business-as-usual CO2 emission scenario. Proc Nat Acad Sci 110:15342–15347
Dutra E, Koch M, Peach K, Manfrino C (2016) Tropical crustose coralline algal individual and community responses to elevated pCO2 under high and low irradiance. ICES J Mar Sci 73:803–813
Egilsdottir H, Noisette F, Noël LM-LJ, Olafsson J, Martin S (2013) Effects of pCO2 on physiology and skeletal mineralogy in a tidal pool coralline alga Corallina elongata. Mar Biol 160:2103–2112
Eyre BD, Andersson AJ, Cyronak T (2014) Benthic coral reef calcium carbonate dissolution in an acidifying ocean. Nat Clim Change 4:969–976
Eyre BD, Cyronak T, Drupp P, De Carlo EH, Sachs JP, Andersson AJ (2018) Coral reefs will transition to net dissolving before end of century. Science 359:908–911
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Findlay HS, Wood HL, Kendall MA, Spicer JI, Twitchett RJ, Widdicombe S (2009) Calcification, a physiological process to be considered in the context of the whole organism. Biogeosci Discuss 6:2267–2284
Findlay HS, Wood HL, Kendall MA, Spicer JI, Twitchett RJ, Widdicombe S (2011) Comparing the impact of high CO2 on calcium carbonate structures in different marine organisms. Mar Biol Res 7:565–575
Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M (1993) Calcification in the articulated coralline alga Corallina pilulifera, with special reference to the effect of elevated CO2 concentration. Mar Biol 117:129–132
Gazeau F, Urbini L, Cox T, Alliouane S, Gattuso J (2015) Comparison of the alkalinity and calcium anomaly techniques to estimate rates of net calcification. Mar Ecol Prog Ser 527:1–12
Gómez-Batista M, Metian M, Oberhänsli F, Pouil S, Swarzenski PW, Tambutté E, Gattuso JP, Hernández CMA, Gazeau F (2020) Intercomparison of four methods to estimate coral calcification under various environmental conditions. Biogeosci 17:887–899
Goreau TF (1963) Calcium carbonate deposition by coralline algae and corals in relation to their roles as reef-builders. Ann NY Acad Sci 109(1):127–167
Hendriks IE, Duarte CM, Olsen YS, Steckbauer A, Ramajo L, Moore TS, Trotter JA, McCulloch M (2015) Biological mechanisms supporting adaptation to ocean acidification in coastal ecosystems. Estuar Coast Shelf Sci 152:A1–A8
Heyward AJ, Negri AP (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279
Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S (2017) Coral reef ecosystems under climate change and ocean acidification. Front Mar Sci 4:158
Hofmann LC, Bischof K (2014) Ocean acidification effects on calcifying macroalgae. Aquat Biol 22:261–279
Hofmann LC, Koch M, de Beer D (2016) Biotic control of surface pH and evidence of light-induced H+ pumping and Ca2+-H+ exchange in a tropical crustose coralline alga. PLoS ONE 11:e0159057
Hofmann LC, Yildiz G, Hanelt D, Bischof K (2012) Physiological responses of the calcifying rhodophyte, Corallina officinalis (L.), to future CO2 levels. Mar Biol 159:783–792
Hofmann LC, Heiden J, Bischof K, Teichberg M (2014) Nutrient availability affects the response of the calcifying chlorophyte Halimeda opuntia (L.) J.V. Lamouroux to low pH Planta 239:231–242
Holcomb M, Cohen AL, McCorkle DC (2013) An evaluation of staining techniques for marking daily growth in scleractinian corals. J Exp Mar Biol Ecol 440:126–131
Hurd CL, Cornwall CE, Currie K, Hepburn CD, McGraw CM, Hunter KA, Boyd PW (2011) Metabolically induced pH fluctuations by some coastal calcifiers exceed projected 22nd century ocean acidification: a mechanism for differential susceptibility? Glob Change Biol 17:3254–3262
IPCC (2013) Climate change 2013: The physical science basis. In: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jokiel PL (2011) Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull Mar Sci 87:639–657
Jokiel PL, Maragos JE, Franzisket L (1978) Coral growth: buoyant weight technique. In: Stoddart DR, Johannes RE (eds) Coral reefs: research methods. UNESCO Monographs on Oceanographic Methodology, Paris, pp 529–542
Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT (2008) Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27:473–483
Kamenos NA, Perna G, Gambi MC, Micheli F, Kroeker KJ (2016) Coralline algae in a naturally acidified ecosystem persist by maintaining control of skeletal mineralogy and size. Proc R Soc B Biol Sci 283:20161159
Kamenos NA, Burdett HL, Aloisio E, Findlay HS, Martin S, Longbone C, Dunn J, Widdicombe S, Calosi P (2013) Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Glob Change Biol 19:3621–3628
Kato A, Hikami M, Kumagai NH, Suzuki A, Nojiri Y, Sakai K (2014) Negative effects of ocean acidification on two crustose coralline species using genetically homogeneous samples. Mar Environ Res 94:1–6
Koch M, Bowes G, Ross C, Zhang X-H (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Change Biol 19:103–132
Langdon C, Gattuso J, Andersson Al (2010) Part 3: Measurements of calcification and dissolution of benthic organisms and communities. In: Riebesell U, Fabry VJ, Hansson L GJ-P (eds) Guide to best practices for ocean acidification research and data reporting. European Commission, Office CDMA 03/115, B-1049, Brussels, pp 213–232
Lee D, Carpenter SJ (2001) Isotopic disequilibrium in marine calcareous algae. Chem Geo 172:307–329
Littler MM, Littler DS (1984) Relationships between macroalgal functional form groups and substrata stability in a subtropical rocky intertidal system. J Exp Mar Bio Ecol 74:13–34
Martin S, Gattuso J-P (2009) Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob Change Biol 15:2089–2100
Marubini F, Ferrier-Pagès C, Furla P, Allemand D (2008) Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism. Coral Reefs 27:491–499
McCulloch M, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change 2:623–627
McNicholl C, Koch MS, Hofmann LC (2019) Photosynthesis and light-triggered proton pumps increase boundary layer pH in tropical reef macroalgae: A proposed mechanism to sustain calcification under ocean acidification. J Exp Mar Bio Ecol 521:151208
Meyer FW, Schubert N, Diele K, Teichberg M, Wild C, Enríquez S (2016) Effect of inorganic and organic carbon enrichments (DIC and DOC) on the photosynthesis and calcification rates of two calcifying green algae from a Caribbean reef lagoon. PLoS ONE 11:e0160268
Morse JW, Andersson AJ, Mackenzie FT (2006) Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and “ocean acidification”: role of high Mg-calcites. Geochim Cosmochim Acta 70:5814–5830
Muehllehner N, Langdon C, Venti A, Kadko D (2016) Dynamics of carbonate chemistry, production, and calcification of the Florida Reef Tract (2009–2010): evidence for seasonal dissolution: seasonal dissolution on the FRT. Global Biogeochem Cycles 30:661–688
Nash MC, Martin S, Gattuso J-P (2016) Mineralogical response of the Mediterranean crustose coralline alga Lithophyllum cabiochae to near-future ocean acidification and warming. Biogeosci 13:5937–5945
Nash MC, Diaz-Pulido G, Harvey AS, Adey W (2019) Coralline algal calcification: a morphological and process-based understanding. PLoS ONE 14:e0221396
Nelson WA (2009) Calcified macroalgae - critical to coastal ecosystems and vulnerable to change: a review. Mar Freshwater Res 60:787
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner G-K, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig M-F, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422
Peach K, Koch M, Blackwelder P (2016) Effects of elevated pCO2 and irradiance on growth, photosynthesis and calcification in Halimeda discoidea. Mar Ecol Prog Ser 544:143–158
Peach KE, Koch MS, Blackwelder PL, Manfrino C (2017a) Calcification and photophysiology responses to elevated pCO2 in six Halimeda species from contrasting irradiance environments on Little Cayman Island reefs. J Exp Mar Biol Ecol 486:114–126
Peach KE, Koch MS, Blackwelder PL, Guerrero-Given D, Kamasawa N (2017b) Primary utricle structure of six Halimeda species and potential relevance for ocean acidification tolerance. Bot Mar 60:1–11
Pickett M, Andersson AJ (2015) Dissolution rates of biogenic carbonates in natural seawater at different pCO2 conditions: a laboratory study. Aquat Geochem 21:459–485
Pierrot D, Lewis E, Wallace DWR (2006) MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Oak Ridge: U.S. Department of Energy
Price N, Hamilton S, Tootell J, Smith J (2011) Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Mar Ecol Prog Ser 440:67–78
Pueschel CM, Judson BL, Wegeberg S (2005) Decalcification during epithallial cell turnover in Jania adhaerens (Corallinales, Rhodophyta). Phycologia 44:156–162
Ragazzola F, Foster LC, Form A, Anderson PSL, Hansteen TH, Fietzke J (2012) Ocean acidification weakens the structural integrity of coralline algae. Glob Change Biol 18:2804–2812
Ries JB (2009) Effects of secular variation in seawater Mg/Ca ratio (calcite-aragonite seas) on CaCO3 sediment production by the calcareous algae Halimeda, Penicillus and Udotea - evidence from recent experiments and the geological record. Terra Nova 21:323–339
Ries JB (2011a) Skeletal mineralogy in a high-CO2 world. J Exp Mar Biol Ecol 403:54–64
Ries JB (2011b) A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim Cosmochim Acta 75:4053–4064
Ries JB, Cohen AL, McCorkle DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rees SA, Opdyke BN, Wilson PA, Henstock TJ (2007) Significance of Halimeda bioherms to the global carbonate budget based on a geological sediment budget for the Northern Great Barrier Reef. Coral Reefs 26:177–188
Rodolfo-Metalpa R, Houlbrèque F, Tambutté E, Boisson F, Baggini C, Patti FP, Jeffree R, Fine M, Foggo A, Gattuso J-P, Hall-Spencer JM (2011) Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat Clim Change 1:308–312
Roleda MY, Boyd PW, Hurd CL (2012) Before ocean acidification: Calcifier chemistry lessons. J Phycol 48:840–843
Schoepf V, Hu X, Holcomb M, Cai W-J, Li Q, Wang Y, Xu H, Warner ME, Melman TF, Hoadley KD, Pettay DT, Matsui Y, Baumann JH, Grottoli AG (2017) Coral calcification under environmental change: a direct comparison of the alkalinity anomaly and buoyant weight techniques. Coral Reefs 36:13–25
Semesi IS, Kangwe J, Björk M (2009) Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp. (Rhodophyta). Estuar Coast Shelf Sci 84:337–341
Silbiger NJ, Donahue MJ (2015) Secondary calcification and dissolution respond differently to future ocean conditions. Biogeosci 12:567–578
Sinutok S, Hill R, Doblin MA, Wuhrer R, Ralph PJ (2011) Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol Oceanogr 56:1200–1212
Smith SV, Key GS (1975) Carbon dioxide and metabolism in marine environments. Limnol Oceanogr 20:493–495
Smith AM, Sutherland JE, Kregting L, Farr TJ, Winter DJ (2012) Phylomineralogy of the Coralline red algae: Correlation of skeletal mineralogy with molecular phylogeny. Phytochemistry 81:97–108
Tambutté E, Allemand D, Bourge I, Gattuso J-P, Jaubert J (1995) An improved 45Ca protocol for investigating physiological mechanisms in coral calcification. Mar Biol 122:453–459
Venn AA, Tambutté E, Caminiti-Segonds N, Techer N, Allemand D, Tambutté S (2019) Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci Rep 9:2201
Vogel N, Meyer F, Wild C, Uthicke S (2015a) Decreased light availability can amplify negative impacts of ocean acidification on calcifying coral reef organisms. Mar Ecol Prog Ser 521:49–61
Vogel N, Fabricius KE, Strahl J, Noonan SHC, Wild C, Uthicke S (2015b) Calcareous green alga Halimeda tolerates ocean acidification conditions at tropical carbon dioxide seeps: Halimeda growing at CO2 seeps. Limnol Oceanogr 60:263–275
Wegeberg S, Pueschel CM (2002) Epithallial and initial cell fine structure in species of Lithothamnion and Phymatolithon (Corallinales, Rhodophyta). Phycologia 41:228–244
Winman SK, McKendree WG (1975) Distribution of Halimeda plants and sediments on and around a patch reef near Old Rhodes Key, Florida. J Sediment Petrol 45:415–421
Wizemann A, Meyer FW, Westphal H (2014) A new model for the calcification of the green macro-alga Halimeda opuntia (Lamouroux). Coral Reefs 33:951–964
Wizemann A, Meyer FW, Hofmann LC, Wild C, Westphal H (2015) Ocean acidification alters the calcareous microstructure of the green macro-alga Halimeda opuntia. Coral Reefs 34:941–954
Zweng RC, Koch MS, Bowes G (2018) The role of irradiance and C-use strategies in tropical macroalgae photosynthetic response to ocean acidification. Sci Rep 8:9479
Acknowledgements
This research was funded by the National Science Foundation Ocean Acidification Program-CRI-OA Grant #1416376. The IAEA is grateful for the support provided to the Environment Laboratories by the Government of the Principality of Monaco. The authors thank Dr. Steeve Comeau and Dr. Frederic Gazeau for their feedback on the initial results of this study. We also thank Chris Johnson, Kimberly McFarlane, and the undergraduate students that assisted in field collections and in the FAU laboratory. We appreciate Dr. Andre Wizemann and an anonymous reviewer and Coral Reefs’ editors for their time and comments that significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McNicholl, C., Koch, M.S., Swarzenski, P.W. et al. Ocean acidification effects on calcification and dissolution in tropical reef macroalgae. Coral Reefs 39, 1635–1647 (2020). https://doi.org/10.1007/s00338-020-01991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-020-01991-x