Abstract
Coral reefs around the world are changing rapidly, with overfishing of herbivorous fishes and increased sediment inputs being two of the major local-scale stressors. We therefore assessed the effects of sediment loads and overfishing on the nutritional quality and yield to grazing fishes of algal turfs, within the epilithic algal matrix, on a coral reef at Lizard Island, Australia. Low, ambient and high sediment loads were maintained on turf-covered coral tiles, with and without grazer exclusion cages, for 1 month. Subsequently, algal turfs were removed and analysed for organic carbon and nitrogen content. Under grazer exclusion, sediment additions decreased algal turf biomass by approximately 63%, while algal turf biomass was the highest on tiles with sediments removed. In the presence of grazing fishes, algal turfs in all treatments were cropped by grazers to similar low biomass levels. Nitrogen content of algal turfs followed a similar trend. Effectively, added sediments decreased the potential yield of algal turf biomass and nitrogen to grazing fishes by an average of 2000 and 3300%, respectively. Sediments profoundly affect algal turf yield to grazing herbivorous fishes and, therefore, the productivity of algal turf-based food chains, potentially diminishing reef-based fisheries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, coral reefs are often regarded as impacted, degraded systems with a decreasing capacity to sustain the humans who depend on them (Jackson et al. 2014; Hughes et al. 2017a). Climate change is a major driver of reef declines, yet there are currently multiple interacting stressors acting on coral reefs that can erode their resilience and limit their ability to regenerate after disturbance (Graham et al. 2013; Ban et al. 2014). Of these stressors, overfishing of herbivorous fishes and increased sediment inputs appear to be two of the most prevalent, especially at local scales (Jackson et al. 2001; Bellwood et al. 2004; Maina et al. 2013; Brodie and Pearson 2016). Sediments naturally occur on all coral reefs, yet increased sediment inputs can arise from storms, dredging, coastal development and river discharge, especially following altered land use practices (McCulloch et al. 2003; Maina et al. 2013; Pollock et al. 2014; Brodie and Pearson 2016). Fortunately, overfishing and sediment inputs can be managed at local scales to improve the resilience of coral reef ecosystems (McCook et al. 2010; Brodie and Pearson 2016). However, a thorough understanding of the effects of both stressors is essential if we are to prioritise reforms when managing sediment inputs and artisanal fishing.
On coral reefs, the epilithic algal matrix (EAM) is a critical interface where both sediments and herbivory can interact (Goatley et al. 2016; Adam et al. 2018). On many coral reefs, the EAM is the most widespread benthic covering (Goatley and Bellwood 2011; Smith et al. 2016) and, as such, it represents a key habitat where benthic interactions occur, including the settlement of benthic organisms such as scleractinian corals (Birrell et al. 2005). It also provides a vast reservoir of nutritional resources which underpin the productivity of shallow-water coral reef ecosystems (Hatcher 1988; Klumpp and McKinnon 1992; Wilson et al. 2003; Kramer et al. 2013; Bellwood et al. 2018). A wide variety of EAM-based resources are exploited by fishes on coral reefs, including cyanobacteria (Clements et al. 2017), cryptofauna (Kramer et al. 2013) and detritus (Crossman et al. 2001; Tebbett et al. 2017a). In addition, the algal turfs themselves represent a highly productive and nutritious resource that underpins the growth of many herbivorous reef fishes (Hatcher 1988; Russ 2003; Kelly et al. 2017). Indeed, experimental evidence suggests that algal turfs may completely regrow in just 4 d following fish grazing (Bonaldo and Bellwood 2011). Fishes that rely on algal turfs as a nutritional resource often make up the bulk of the catch from artisanal finfish fisheries in developing countries throughout the tropics (Craig et al. 1997; Comeros-Raynal et al. 2012; Condy et al. 2015). Thus, factors that affect the productivity or quality of algal turfs may have bottom-up effects on the food chain, culminating in lower fisheries yields for people (Bellwood et al. 2018). Furthermore, herbivorous coral reef fishes are generally nitrogen limited (Fong and Paul 2011), highlighting the link between algal nitrogen content and aquatic food chains. However, our understanding of the effects of sediments within the EAM on algal turf nutritional content is limited.
To date, overfishing of herbivores and nutrient enrichment have received the greatest focus in studies examining algal growth and productivity on reefs (reviewed in Burkepile and Hay 2006). There are two problems: (a) nutrient enrichment often does not have a major effect, and (b) most studies focus on large, foliose macroalgae rather than turf algae (e.g. Burkepile and Hay 2006, 2009; Smith et al. 2010; Rasher et al. 2012). While several studies have identified macroalgal phase shifts as a threat to coral reefs (Hughes 1994; Cheal et al. 2010; Nash et al. 2013), such phase shifts, while visually apparent, may not be the predominant degraded stable state on coral reefs (Bruno et al. 2009). Low-complexity coral reef systems dominated by long sediment-laden algal turfs (LSATs sensu Goatley et al. 2016) are increasingly viewed as the major alternative degraded state on coral reefs (Bellwood and Fulton 2008; Jouffray et al. 2014; Goatley et al. 2016; Smith et al. 2016; Brown et al. 2017). Within these turfs, sediment appears to be a major determinant of benthic processes (Birrell et al. 2005; Gowan et al. 2014; Ricardo et al. 2017; Tebbett et al. 2017b), potentially shaping primary productivity and associated food chains (Crossman et al. 2001; Purcell and Bellwood 2001; Wilson et al. 2003).
Unfortunately our understanding of the interaction between algal turfs, overfishing and sediments is still in its infancy (but see Goatley and Bellwood 2013; Clausing et al. 2014; Muthukrishnan and Fong 2014; Fong et al. 2018), while the bottom-up effects for herbivory and the reef food chains that underpin fisheries and human food supply have only recently begun to be considered (Bellwood et al. 2018). To reconcile this knowledge gap, we present experimental work undertaken on the Great Barrier Reef (GBR) in 1993/1994, before recent mass coral bleaching events (Hughes et al. 2017b) and cyclones (Khan et al. 2017). In this work, we examine the impacts of herbivorous fishes and sediments on algal turfs and reef processes, in a relatively intact coral reef ecosystem, while considering the potential impacts of these factors on fish populations and fisheries yields to humans.
Materials and methods
Study site and design
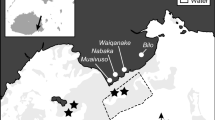
Experiments were conducted on the reef between Palfrey and South Island (14°42.0′S, 145°26.7′E), near to Lizard Island on the Great Barrier Reef (GBR) (Fig. 1a). Two subtidal sites (30 × 10 m; denoted as ‘A’ and ‘B’) were situated on the reef flat, just behind the reef crest, approximately 20–40 cm below zero tide datum (Fig. 1a). The reef surface was covered by an EAM of predominantly small, branched and non-geniculate turfing algae. Turf algae were grazed conspicuously during the day by site-attached damselfishes Pomacentrus chrysurus and P. wardi, and a diverse community of roving surgeonfishes, parrotfishes and rabbitfishes (Electronic supplementary material [ESM]; Table S11).
Square tiles, 12 × 12 × 1.5 cm, were cut from coral heads (Porites sp.) to provide replicate, natural coral surfaces for experiments. In early June 1993, 60 tiles (10 blocks of 6 tiles) were attached to the substratum at both sites (120 tiles in total), using stainless steel screws inserted through a hole in the centre of the tiles (Fig. 1b). Tiles in each block were aligned in a row, parallel to the reef crest edge, and spaced 10 cm apart, except for the middle two tiles which were 15 cm apart, creating two ‘subsets’ of three tiles. Blocks were situated haphazardly at least 2 m apart on substrata that were: (a) approximately horizontal, (b) free of corals, (c) not adjacent to sand pits, (d) dominated by epilithic algae and (e) not adjacent to damselfish territories.
Experiment 1: loss and accumulation of sediments within EAMs
An experiment during October 1993 at site B gauged sediment accrual and loss on algal turf-covered tiles. We added or removed sediments on tiles and then measured sediment loads at successive time intervals to determine the rates at which sediments would naturally accumulate on, or wash off, tiles. Tiles were firstly attached to the reef for 4 months to establish an EAM following Russ (1987) and Scott and Russ (1987).
Tiles (all uncaged) were randomly assigned to one of the three sediment treatments: addition (n = 24), removal (n = 24) or ambient (n = 12). Upon commencing the experiment, sediments on six ambient tiles were collected on SCUBA using an electronic vacuum sampler (Purcell 1996), to estimate the initial sediment loads. The remaining six ambient-treatment tiles were left untreated and sampled at the end of the experiment. For the addition treatment, 30 g of sediments were then sprinkled onto each ‘addition’ treatment tile within a fitted shield to prevent dispersion. A 30 g addition results in a sediment load of about 2080 g m−2 (dry weight), a realistic high level in comparison with the ambient sediment load on the surrounding reef substrata (Purcell 1997; see preliminary sediment samples in ESM) and is within the natural reported range for sediments on GBR reefs (23–8400 g m−2) (Purcell 2000; Goatley et al. 2016; Tebbett et al. 2017d). The sediment allocations were formulated to the grain size composition of the preliminary sediment samples using washed and dried coral reef sediments from Lizard Island (ESM; Table S1). These sediments are generally coarser, contain higher carbonate content and differ in terms of hardness, solubility and ability to adsorb nutrients compared to terrestrially derived sediments (Gordon et al. 2016). As such, the sediments used herein offer a conservative estimate of the impacts of sediment on the EAM as many of the qualities of siliceous terrestrial sediments are likely to cause even greater effects (Flores et al. 2012; Storlazzi et al. 2015; Tebbett et al. 2017c). Sediments < 63 μm were omitted, as these contributed little (by weight) to natural sediment samples from this and neighbouring reefs (Purcell 2000) and would probably be lost (via re-suspension) during the allocation onto tiles. For the removal treatment, sediments were hosed off the tiles using the exhalant water current (30 cm s−1) from the pump of the vacuum sampler at a distance of 5 mm above each tile for 60 s.
Sediments on addition and removal treatment tiles from each of the six blocks were collected using the vacuum sampler after 1 h (representing the ‘settled load’), and 1, 3 and 5 d. In the laboratory, each sample (sediment and water) was placed for 3 h into a 50-cm-high column, with collection funnel at the bottom. Supernatant water was then drained, and the settled sediments washed into vials. Sediments were cleared of organic material using bleach (see Purcell 1996), oven-dried at 70 °C, and weighed to the nearest 0.1 mg. Organic material was removed so that only the effects of inorganic sediments were examined herein. Sediment load data were analysed using generalised least squares models due to heteroscedasticity. Time since addition or removal was treated as a fixed categorical factor with a variance structure allowing different variances for each level. Analyses were performed in the statistical software R (R Core Team 2014) using the nlme package (Pinheiro et al. 2014).
Experiment 2: effects of overfishing and sediment on the EAM
This experiment was carried out from 11 February to 9 March 1994 at sites A and B, using the full array of conditioned tiles (n = 120). Following previous manipulations in Experiment 1 and the Assessment of Caging Artefacts Experiment (see ESM for full details of the caging experiment), all tiles were left uncaged for at least 10 weeks to develop a natural EAM. Caging and sediment load treatments were assigned to tile blocks within each site using a two-step randomisation. The treatment loads were: removal = sediment hosed off daily; ambient = sediment load not altered; and added = 13 g of sediment added every 2 d to maintain approximately 30 g per tile. The treatments were based on the results of Experiment 1 [see ESM (Extra details for Experiment 2) for a comparison of naturally occurring sediments at both sites]. On average, the sediment addition and sediment removal treatments resulted in sediment loads 8.5-fold higher and 9.7-fold lower, respectively, than sediment loads on ambient tiles (ESM; Fig. S4a). The grazer exclusion cages (71 × 27 × 10 cm) were covered with galvanised wire mesh (12 × 12 mm), including a 5-cm bottom fringe. Cages were fastened to steel mounts bolted on the reef, with the mesh on the fringe contoured to the substratum (Fig. 1b). A full evaluation of cage effects using cage controls is provided in the ESM.
On day one, sediments on removal treatment tiles were hosed off for 60 s. On subsequent mornings, a 30-s interval was used, which appeared sufficient to remove sediments accumulated over the 24-h period. For sediment addition treatment tiles, sediments were formulated following the same methods in Experiment 1. On day one, 30 g of sediments were sprinkled onto each addition treatment tile, within a fitted shield, to initiate the treatment load. To maintain this load, 13 g of the sediments were applied every second day. Each cage was removed briefly to allow for sediment manipulations and was brushed to remove fouling. All manipulations were carried out between 8:00 and 11:00 am.
Sediment treatments were maintained for 4 weeks. Sediment loads were not manipulated on the morning of collection, when tiles were unbolted from the reef, taking care not to disturb the accumulated sediments or turf algae. While underwater, the sides and bottom of each tile were brushed to remove all sediments retaining only those on the upper surface. Each tile was then sealed in a plastic bag, chilled on ice and then refrigerated at 5 °C until processed.
Within 2 d of removal, each tile and bag was rinsed into containers to collect loose sediments. The remaining sediments were then vacuumed from the EAMs, with tiles submersed in filtered seawater; as noted by Purcell (1996), this method does not remove turf algae. Sediments were collected on a 63-μm mesh screen and added to the loose sediments. After 1-h settling in containers, water was decanted and each sediment sample rinsed into a vial. These were then cleared of organic material using bleach, dried at 70 °C and weighed to the nearest 0.1 mg as above; these values represent the sediment loads at the end of the experiment.
Following sediment collection, each tile was rinsed with freshwater and the turf algae scraped and collected. The upper surface of tiles was scraped to a depth of approximately 1 mm to remove epilithic turf algae and upper endolithic algae, while using templates to exclude turf algae at the edges (within 5 mm) and next to the central hole (4 square cm). Algal turf samples, each from 117 cm2 of tile surface, were immediately frozen in vials, freeze-dried, weighed to the nearest 1 mg and ground with a mortar and pestle. The organic carbon content of algal turf samples was analysed in triplicate using a LECO™ auto-analyser and acid pre-treatment following Purcell (1997), providing a measure of the final biomass (in g organic carbon) of the algal turfs on tiles. For nitrogen analyses, aliquots (0.1–0.4 g) of ground turf algae were treated with a modified Kjedahl digestion (Anderson and Ingram 1989) which oxidises the samples in catalysed hydrogen peroxide and sulphuric acid. Nitrogen content was then determined colorimetrically using the salicylate–hypochlorite method of Baethgen and Alley (1989). The average precision of duplicate trials was 3%. Algal turfs herein include the fleshy turf algae, coralline algae and attached microbes and cyanobacteria that remained following vacuuming and rinsing. Most particulate material (detritus), invertebrates, microbes and microalgae would have been lost during processing but represent important additional nutritional resources for fishes (Wilson et al. 2003; Clements et al. 2017).
The difference in both algal turf biomass and nitrogen between caged and uncaged treatments provides a conservative estimate of the yield to grazing fishes over the 1-month exposure period. Yields of algal turfs to herbivores might be underestimated due to algal turf biomass loss due to breakage (Russ 2003), the beneficial effects of herbivore grazing on algal turf productivity (Russ 1987), decreased photosynthesis from self-shading of algae turfs at high biomass (Carpenter 1985; Williams and Carpenter 1990) or cage effects reducing algal turf biomass by about 25% probably through partial shading by the mesh (see ESM).
The final algal turf biomass (g C) and nitrogen yield (total mg N) of EAMs on tiles were compared among sediment treatments and between caging treatments using generalised linear mixed effects models (GLMMs) with a Gamma distribution and log link. Initially, in all cases, a full model was formulated with sediment treatment and caging level fitted as interacting fixed effects. Site was fitted as a random factor, and tile block was fitted as a random factor nested within site to account for the lack of independence between treatments. The most parsimonious model was selected based on AICc scores (ESM; Table S8). Analyses were performed in the statistical software R (R Core Team 2014) using the lme4 (Bates et al. 2014) and AICcmodavg (Mazerolle 2015) packages.
Results
Experiment 1: loss and accumulation of sediments within EAMs
About one-third of the added sediment was lost from the addition treatment tiles within the first hour, leaving the ‘settled load’. Approximately 46% of this settled load was lost over the following 3 d (Fig. 2a). All sediment loads on subsequent days were significantly lower than the settled load (ESM; Table S9). Despite a high initial loss of unbound sediments, a substantial proportion from a single addition is held within EAMs over a long period (Fig. 2a). This analysis indicated that a sediment addition of 13 g every 2 d was needed to maintain a high treatment load of 30 g per tile in Experiment 2.
Sediment loads on ‘removal’ tiles after 1 h were approximately 90% lower than those on ambient tiles at the beginning of the experiment. Analysis of data from the ‘removal’ treatment (Fig. 2b) indicated that sediments accrued rapidly on tiles over the first 24 h and reached ambient loads within approximately 3 d. All loads for periods exceeding 24 h were significantly higher than the load of sediment after 1 h (ESM; Table S9). We concluded that sediments should be hosed off tiles daily to maintain low treatments in Experiment 2.
Experiment 2: effects of sediment loading on EAMs
Increased sediment loads negatively affected the turf algae (Fig. 3a). In caged treatments, sediment addition tiles had on average 60% less algal turf biomass than tiles with sediments removed or at ambient levels. However, EAMs in the open appear to have been subjected to variable grazing pressure by herbivorous fishes because, despite the effects of sediment on algal turf biomass, algal turfs were cropped to similar low biomass levels across all three sediment loads. This resulted in a significant interaction between caging and sediment treatments (ESM; Table S10).
Experiment 2. The final a algal turf biomass [organic carbon (OC)] and b nitrogen [organic nitrogen (ON)] on caged and uncaged tiles under different sediment treatments. Note the potential yield of algal turf biomass (green) and nitrogen (orange) to fishes above (black) caged treatments. The yield represents the difference in the final algal turf biomass and nitrogen mass between caged and uncaged treatments. Dots above figures represent sediment. See ESM Fig. S4 for full-figure details
Sediment load and grazing pressure also affect the total amount of nitrogen within algal turfs (Fig. 3b; ESM Fig. S4). Patterns of total nitrogen per tile among the three sediment treatments were similar to those shown for algal turf biomass (Fig. 3; ESM Fig. S4). Like algal turf biomass, nitrogen on caged sediment addition tiles was on average just 44% of that on tiles with sediments removed or at ambient levels. However, there were minimal differences in nitrogen between open tiles, again leading to a significant interaction between caging and sediment treatments (ESM; Table S10).
Final algal turf biomass in caged treatments was 2.9-fold and 2.6-fold higher than on uncaged tiles, with sediments removed or at ambient loads, respectively (Fig. 3a). Similarly, the final nitrogen on caged treatments was 2.6-fold and threefold higher than on uncaged tiles with sediments removed or at ambient loads, respectively (Fig. 3b). This suggests that fishes remove at least 2–3 times the standing biomass and nitrogen from algal turfs every month. However, high sediment loads appeared to have a substantial effect on the productivity of these algal turfs and their potential yield to fishes. The potential yield of algal turf biomass and nitrogen from high sediment treatments was on average 2000 and 3300% lower, respectively, than that recorded from sediment removed or ambient sediment treatments (Fig. 3). Total algal productivity removed by fishes is therefore 0.44, 0.34 and 0.02 g C m−2 d−1 and 0.03, 0.04 and 0.001 g N m−2 d−1 for removed, ambient and high sediment treatments, respectively.
Discussion
The highly productive nature of coral reef ecosystems is dependent on the algal turfs within the EAM (Carpenter 1985; Hatcher 1988; Klumpp and McKinnon 1992). These turfs support key trophic pathways within these systems (Russ 2003; Kelly et al. 2017; Bellwood et al. 2018) and can contribute disproportionately to algal production compared to foliose macroalgae (e.g. turfs can be more than 15-fold more productive than macroalgae; Kelly et al. 2017). Productivity of algal turfs can vary widely depending on physical and biological factors such as light intensity and nutrient availability (Carpenter 1985; Klumpp and McKinnon 1992; Russ and McCook 1999), and our results make it clear that sediment loads are also a critical factor. Indeed, we revealed that potential algal turf yield to herbivorous fishes was reduced by 94% under high sediment loads and slightly enhanced (by 22%) under low sediment loads. We infer that this suppression of algal turf yield by sediments reflects a natural process, since sediments were manipulated on established EAMs on coral–carbonate surfaces at levels (31–3042 g m−2) well within the natural reported range on GBR reefs (23–8400 g m−2) (Purcell 2000; Goatley et al. 2016; Tebbett et al. 2017d). Our results, therefore, show that increased sediment loads can reduce algal turf productivity, potentially leading to significant bottom-up effects on algal-based food chains by limiting the food supply to herbivorous fishes. The absence of a detectable effect of sediment loading on EAMs in the open (uncaged) treatments highlights that such effects can be masked by grazing, probably by herbivorous fishes (ESM; Table S11). Reduced grazing pressure by large herbivorous fishes through caging led to marked increases in algal turf biomass when sediment was removed or at ambient levels. This supports previous studies in highlighting the strong grazing pressure exerted on algal communities by intact (i.e. not heavily fished) fish populations (Burkepile and Hay 2006; Rasher et al. 2012; Goatley and Bellwood 2013; Clausing et al. 2014; Kelly et al. 2017).
Evidently, the nature of interactions among sediments, algal turfs and herbivorous fishes appears to be complex and dependent on both bottom-up and top-down control. Sediment loading clearly had a marked effect on both algal turf biomass and nutrient availability (nitrogen) within the EAM, with the potential for bottom-up effects on herbivorous fish communities. This supports the results of Clausing et al. (2014) who also found negative effects of sediments on algal turfs, with algal turf growth reduced by around 50% at sediment depths of 2 mm, and near to zero at depths of 4 mm. By contrast, sediment-mediated top-down control of algal turfs by herbivorous fishes is often reported, i.e. sediments trapped in algal turfs suppressing herbivory and facilitating the growth and expansion of turfing algae (Goatley and Bellwood 2013; Goatley et al. 2016, Tebbett et al. 2017b; Fong et al. 2018). Indeed, Goatley and Bellwood (2013) found that in high-energy locations, a 1-week pulse of sediment resulted in 60% longer algal turfs, while Muthukrishnan and Fong (2014) found sediment pulses every 2 weeks for 24 weeks led to an approximate 10% increase in algal turf cover. Although these studies used different metrics, the relationship among algal turf length, cover and biomass, as well as between sediment mass and depth, might be more complex than previously thought (e.g. Purcell and Bellwood 2001; Gordon et al. 2016). Furthermore, these relationships might differ across spatial scales, as the species composition of algal turfs can be highly variable (Harris et al. 2015). Irrespective of the complexity, it is clear that both sediments and herbivory play key roles in mediating the nature of algal turfs.
From the present study, it is evident that sediment-laden algal turfs represent a relatively non-productive algal turf state where algal turf biomass is relatively inaccessible to grazing fishes. This supports the apparent division in the state of coral reef EAMs, or turfs, between resilient and degraded reef systems. In relatively ‘healthy’ reef systems, the EAM is typified by short productive algal turfs (SPATs) (sensu Goatley et al. 2016), which are relatively free of sediments and underpin high-turnover herbivorous, particulate or detrital trophic pathways (Hatcher 1988; Klumpp and McKinnon 1992; Russ 2003; Wilson et al. 2003). An alternate state can arise from overfishing of herbivorous fishes or increased sedimentation, culminating in the expansion of long sediment-laden algal turfs (LSATs) (Steneck 1997; Goatley et al. 2016), which are unproductive, and can further impede other reef processes such as coral settlement (Birrell et al. 2005).
What is especially disconcerting is that, once trapped in the EAM, sediment loads can be particularly stable. The results of Experiment 1 demonstrated that despite a rapid loss of added sediment, sediment loss from the EAM slowed and, after 5 d, sediment loads still remained approximately five times higher than ambient loads (Fig. 2). This supports recent evidence suggesting that EAM sediment loads are temporally stable over extended periods (Gordon et al. 2016). Once sediments are trapped, they can lead to chronic, long-term bottom-up effects on the ecosystem. This may trigger a positive feedback, where more sediments result in fewer herbivores (or vice versa) ratcheting down the productivity of high-turnover shallow-water coral reefs. Unfortunately for coral reefs, feedbacks of this nature could be common since overfishing and increased sediment inputs (e.g. from coastal development, dredging or river discharge) are widespread and often occur simultaneously (Wilkinson 2008; Seemann et al. 2014; Hamilton et al. 2017).
Our field experiments demonstrate that the amount of sediment within EAMs can have a profound effect on algal turf and nitrogen yields, potentially effecting algal turf-based food chains on coral reefs. Under conditions of increased EAM sediment loads, maintaining herbivorous fish communities may be an important factor in facilitating recovery of coral populations and in the maintenance of reef productivity. Unfortunately, these herbivorous fishes are often overfished. This represents a complex problem in that the decreased productivity of algal turf-based food chains could lead to less productive ecosystems for humans to harvest, culminating in lower fisheries yields and increasing fishing pressure. We found that reducing grazing and/or increasing sediments resulted in a > 2000% reduction in algal turf yield from the EAM. As the reefs biggest single primary producer, these impacts are likely to have serious implications for both reefs and the people who depend on them.
References
Adam TC, Duran A, Fuchs CE, Roycroft MV, Rojas MC, Ruttenberg BI, Burkepile DE (2018) Comparative analysis of foraging behavior and bite mechanics reveals complex functional diversity among Caribbean parrotfishes. Mar Ecol Prog Ser 597:207–220
Anderson JM, Ingram JS (1989) Tropical soil biology and fertility: a handbook of methods. CAB International, Aberystwyth
Baethgen WE, Alley MM (1989) A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjedahl digests. Commun Soil Sci Plant Anal 20:961–969
Ban SS, Graham NAJ, Connolly SR (2014) Evidence for multiple stressor interactions and effects on coral reefs. Glob Chang Biol 20:681–697
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7
Bellwood DR, Fulton CJ (2008) Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea levels and climate change? Limnol Oceanogr 53:2695–2701
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833
Bellwood DR, Tebbett SB, Bellwood O, Mihalitsis M, Morais RA, Streit RP, Fulton CJ (2018) The role of the reef flat in coral reef trophodynamics: past, present, and future. Ecol Evol 8:4108–4119
Birrell CL, McCook LJ, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. Mar Pollut Bull 51:408–414
Bonaldo RM, Bellwood DR (2011) Spatial variation in the effects of grazing on epilithic algal turfs on the Great Barrier Reef, Australia. Coral Reefs 30:381–390
Brodie JE, Pearson RG (2016) Ecosystem health of the Great Barrier Reef: time for effective management action based on evidence. Estuar Coast Shelf Sci 183:438–451
Brown KT, Bender-Champ D, Bryant DEP, Dove S, Hoegh-Guldberg O (2017) Human activities influence benthic community structure and the composition of the coral-algal interactions in the central Maldives. J Exp Mar Bio Ecol 497:33–40
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VGW (2009) Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–1484
Burkepile DE, Hay ME (2006) Herbivore vs. nutrient control of marine primary producers: context-dependent effects. Ecology 87:3128–3139
Burkepile DE, Hay ME (2009) Nutrient versus herbivore control of macroalgal community development and coral growth on a Caribbean reef. Mar Ecol Prog Ser 389:71–84
Carpenter RC (1985) Relationships between primary production and irradiance in coral reef algal communities. Limnol Oceanogr 30:784–793
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H (2010) Coral-macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–1015
Clausing RJ, Annunziata C, Baker G, Lee C, Bittick SJ, Fong P (2014) Effects of sediment depth on algal turf height are mediated by interactions with fish herbivory on a fringing reef. Mar Ecol Prog Ser 517:121–129
Clements KD, German DP, Piché J, Tribollet AD, Choat JH (2017) Integrating ecological roles and trophic resources on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc 120:729–751
Comeros-Raynal MT, Choat JH, Polidoro BA, Clements KD, Abesamis R, Craig MT, Lazuardi ME, McIlwain J, Muljadi A, Myers RF, Nañola CL Jr, Pardede S, Rocha LA, Russell B, Sanciangco JC, Stockwell B, Harwell H, Carpenter KE (2012) The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: the parrotfishes and surgeonfishes. PLoS One 7:e39825
Condy M, Cinner JE, McClanahan TR, Bellwood DR (2015) Projections of the impacts of gear-modification on the recovery of fish catches and ecosystem function in an impoverished fishery. Aquat Conserv Mar Freshw Ecosyst 410:396–410
Craig PC, Choat JH, Axe LM, Saucerman S (1997) Population biology and harvest of a coral reef surgeonfish (Acanthurus lineatus) in American Samoa. Fish Bull 95:680–693
Crossman DJ, Choat JH, Clements KD, Hardy T, McConochie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Flores F, Hoogenboom MO, Smith LD, Cooper TF, Abrego D, Negri AP (2012) Chronic exposure of corals to fine sediments: lethal and sub-lethal impacts. PLoS One 7:e37795
Fong CR, Bittick SJ, Fong P (2018) Simultaneous synergist, antagonist and additive interactions between multiple local stressors all degrade algal turf communities on coral reefs. J Ecol 106:1390–1400
Fong P, Paul VJ (2011) Coral reef algae. In: Dubinsky Z, Stambler N (eds) Coral Reefs: an ecosystem in transition. Springer, Dordrecht, pp 241–272
Goatley CHR, Bellwood DR (2011) The roles of dimensionality, canopies and complexity in ecosystem monitoring. PLoS One 6:e27307
Goatley CHR, Bellwood DR (2013) Ecological consequences of sediment on high-energy coral reefs. PLoS One 8:e77737
Goatley CHR, Bonaldo RM, Fox RJ, Bellwood DR (2016) Sediments and herbivory as sensitive indicators of coral reef degradation. Ecol Soc 21:29
Gordon SE, Goatley CHR, Bellwood DR (2016) Composition and temporal stability of benthic sediments on inner-shelf coral reefs. Mar Pollut Bull 111:178–183
Gowan JC, Tootell JS, Carpenter RC (2014) The effects of water flow and sedimentation on interactions between massive Porites and algal turf. Coral Reefs 33:651–663
Graham NAJ, Bellwood DR, Cinner JE, Hughes TP, Norstrom AV, Nyström M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548
Hamilton RJ, Almany GR, Brown C, Pita J, Peterson NA, Choat JH (2017) Logging degrades nursery habitat for an iconic coral reef fish. Biol Conserv 210:273–280
Harris JL, Lewis LS, Smith JE (2015) Quantifying scales of spatial variability in algal turf assemblages on coral reefs. Mar Ecol Prog Ser 532:41–57
Hatcher BG (1988) Coral reef primary productivity: a beggar’s banquet. Trends Ecol Evol 3:106–111
Hughes TP (1994) Catastrophes, phase shifts and large-scale degradation of a Caribbean coral reef. Science 265:1547–1551
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough JM, Morrison TH, Palumbi SR, Van Nes EH, Scheffer M (2017a) Coral reefs in the Anthropocene. Nature 546:82–90
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TCL, Butler I, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin M, Figueira W, Gilmour J, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-Y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm H, McWilliam M, Pandolfi JM, Pears R, Pratchett MS, Schoepf V, Simpson T, Skirving W, Sommer B, Torda G, Wachenfeld D, Willis BL, Wilson SK (2017b) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jackson JBC, Donovan MK, Cramer KL, Lam V (2014) Status and trends of Caribbean coral reefs: 1970–2012. Global Coral Reef Monitoring Network, Washington
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Jouffray J-B, Nyström M, Norstrom AV, Williams ID, Wedding LM, Kittinger JN, Williams GJ (2014) Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos Trans R Soc B 370:20130268
Kelly ELA, Eynaud Y, Williams ID, Sparks RT, Dailer ML, Sandin SA, Smith JE (2017) A budget of algal production and consumption by herbivorous fish in an herbivore fisheries management area, Maui. Hawaii. Ecosphere 8:e01899
Khan JA, Goatley CHR, Brandl SJ, Tebbett SB, Bellwood DR (2017) Shelter use by large reef fishes: long-term occupancy and the impacts of disturbance. Coral Reefs 36:1123–1132
Klumpp DW, McKinnon AD (1992) Community structure, biomass and productivity of epilithic algal communities on the Great Barrier Reef: dynamics at different spatial scales. Mar Ecol Prog Ser 86:77–89
Kramer MJ, Bellwood O, Bellwood DR (2013) The trophic importance of algal turfs for coral reef fishes: the crustacean link. Coral Reefs 32:575–583
Maina J, de Moel H, Zinke J, Madin J, McClanahan T, Vermaat JE (2013) Human deforestation outweighs future climate change impacts of sedimentation on coral reefs. Nat Commun 4:1986
Mazerolle MJ (2015) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.0-3
McCook LJ, Ayling T, Cappo M, Choat JH, Evans RD, De Freitas DM, Heupel M, Hughes TP, Jones GP, Mapstone B, Marsh H, Mills M, Molloy FJ, Pitcher CR, Pressey RL, Russ GR, Sutton S, Sweatman H, Tobin R, Wachenfeld DR, Williamson DH (2010) Adaptive management of the Great Barrier Reef: a globally significant demonstration of the benefits of networks of marine reserves. Proc Natl Acad Sci 107:18278–18285
McCulloch M, Fallon S, Wyndham T, Hendy E, Lough J, Barnes D (2003) Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421:727–730
Muthukrishnan R, Fong P (2014) Multiple anthropogenic stressors exert complex, interactive effects on a coral reef community. Coral Reefs 33:911–921
Nash KL, Graham NAJ, Bellwood DR (2013) Fish foraging patterns, vulnerability to fishing, and implications for the management of ecosystem function across scales. Ecol Appl 23:1632–1644
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2014) nlme:linear and nonlinear mixed effects models. R package version 3.1-118
Pollock FJ, Lamb JB, Field SN, Heron SF, Schaffelke B, Shedrawi G, Bourne DG, Willis BL (2014) Sediment and turbidity associated with offshore dredging increase coral disease pevalence on nearby reefs. PLoS One 9:e102498
Purcell SW (1996) A direct method for assessing sediment load in epilithic algal communities. Coral Reefs 15:211–213
Purcell SW (1997) The role of sediments in epilithic algal communities on coral reefs. Ph.D. thesis. James Cook University, Townsville
Purcell SW (2000) Association of epilithic algae with sediment distribution on a windward reef in the northern Great Barrier Reef, Australia. Bull Mar Sci 66:199–214
Purcell SW, Bellwood DR (2001) Spatial patterns of epilithic algal and detrital resources on a windward coral reef. Coral Reefs 20:117–125
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasher DB, Engel S, Bonito V, Fraser GJ, Montoya JP, Hay ME (2012) Effects of herbivory, nutrients, and reef protection on algal proliferation and coral growth on a tropical reef. Oecologia 169:187–198
Ricardo GF, Jones RJ, Nordborg M, Negri AP (2017) Settlement patterns of the coral Acropora millepora on sediment-laden surfaces. Sci Total Environ 609:277–288
Russ GR (1987) Is rate of removal of algae by grazers reduced inside territories of tropical damselfishes? J Exp Mar Bio Ecol 110:1–17
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67
Russ GR, McCook LJ (1999) Potential effects of a cyclone on benthic algal production and yield to grazers on coral reefs across the central Great Barrier Reef. J Exp Mar Bio Ecol 235:237–254
Scott FJ, Russ GR (1987) Effects of grazing on species composition of the epilithic algal community on coral reefs of the central Great Barrier Reef. Mar Ecol Prog Ser 39:293–304
Seemann J, González CT, Carballo-Bolaños R, Berry K, Heiss GA, Struck U, Leinfelder RR (2014) Assessing the ecological effects of human impacts on coral reefs in Bocas del Toro, Panama. Environ Monit Assess 186:1747–1763
Smith JE, Brainard R, Carter A, Grillo S, Edwards C, Harris J, Lewis L, Obura D, Rohwer F, Sala E, Vroom PS, Sandin S (2016) Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc R Soc B 283:20151985
Smith JE, Hunter CL, Smith CM (2010) The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 163:497–507
Steneck RS (1997) Crustose corallines, other algal functional groups, herbivores and sediments: complex interactions along reef productivity gradients. In: Proceedings of the 8th international coral reef symposium 1:695–700
Storlazzi CD, Norris BK, Rosenberger KJ (2015) The influence of grain size, grain color, and suspended-sediment concentration on light attenuation: why fine-grained terrestrial sediment is bad for coral reef ecosystems. Coral Reefs 34:967–975
Tebbett SB, Goatley CHR, Bellwood DR (2017a) Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus on coral reefs. Coral Reefs 36:803–813
Tebbett SB, Goatley CHR, Bellwood DR (2017b) The effects of algal turf sediments and organic loads on feeding by coral reef surgeonfishes. PLoS One 12:e0169479
Tebbett SB, Goatley CHR, Bellwood DR (2017c) Fine sediments suppress detritivory on coral reefs. Mar Pollut Bull 114:934–940
Tebbett SB, Goatley CHR, Bellwood DR (2017d) Algal turf sediments and sediment production by parrotfishes across the continental shelf of the northern Great Barrier Reef. PLoS One 12:e0170854
Wilkinson C (2008) Status of coral reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville
Williams S, Carpenter R (1990) Photosynthesis/photon flux density relationships among components of coral reef algal turfs. J Phycol 26:36–40
Wilson SK, Bellwood DR, Choat JH, Furnas MJ (2003) Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr Mar Biol Annu Rev 41:279–309
Acknowledgements
We thank M. Emslie, J. Colquhoun, D. Hocking, Z. Rosser, K. Bonair, D. Zeller, A. Miles, J. Semmens, M. Fogg, K. Buckley, G. Smith and Lizard Island Research Station staff for field and laboratory support; three anonymous reviewers for helpful comments. Financial support was provided by a James Cook University Merit Research Grant (SWP), an Australian Museum Postgraduate Grant (SWP) and the Australian Research Council (DRB, Grant Number: CE140100020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Topic Editor Dr. Mark R. Patterson
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tebbett, S.B., Bellwood, D.R. & Purcell, S.W. Sediment addition drives declines in algal turf yield to herbivorous coral reef fishes: implications for reefs and reef fisheries. Coral Reefs 37, 929–937 (2018). https://doi.org/10.1007/s00338-018-1718-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-018-1718-6