Abstract
Coral reefs ecosystems are facing an unprecedented decline due to the action of natural and anthropogenic stressors. The Caribbean Sea is considered to be one of the most impacted areas, as the average estimated scleractinian coral cover in this region decreased from approximately 50% to 10% over the last 30 years. In this study, a ten-year biodiversity survey was used to examine changes in abundance and percentage cover of benthic invertebrates on permanent transects located at four shallow coral reefs around Roatán, Honduras. This study represents the first long-term investigation of the coral ecosystem of Roatán and reports a decrease in scleractinian coral cover from 37.45 [± 5.37]% to 28.95 [± 3.62]% and a concomitant increase in macroalgal (7.02 [± 3.59]% to 13.94 [± 2.69]%) and turf (5.11[ ± 0.84]% to 7.23 [± 1.00]%) cover although no significant differences in the abundance of scleractinian corals, soft corals, or sponges were observed on the transects. While the four reef sites supported more variable benthic communities at the onset of the study, an overall homogenization of the benthic community composition occurred during the study period. Although our study sites were limited to a small region of Roatán’s southern coral reef system, these observations add to results from other Caribbean locations and provide insights into how Mesoamerican coral reefs have changed over the last decade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are among the most biodiverse and productive ecosystems in the world (Hoegh-Guldberg 1999). However, their prevalence has dramatically declined over recent decades (e.g., Pandolfi et al. 2003; Burke and Maidens 2004; Schutte et al. 2010; Burke et al. 2011; Eddy et al. 2021). This decline has been largely attributed to the effects of various stressors, both natural and anthropogenic (Gardner et al. 2003; Carpenter et al. 2008; Vega Thurber et al. 2014). Natural stressors often function as transitional disturbances and are recognized as a component of reef growth and development, including events such as hurricanes that can alleviate thermal stress (Heron et al. 2008) or facilitate propagation (Vroom et al. 2005). However, many of the human-induced stressors impacting coral reefs are considered chronic and do not allow sufficient time or appropriate conditions for coral reef-associated organisms to recover, resulting in the loss of key ecosystem components (Spalding and Brown 2015). Notably, since the 1980’s, increases in sea water temperatures linked to global climate change have triggered mass coral bleaching events, including three widespread occurrences in 1998, 2010 and 2015–2016 that affected all tropical regions within both hemispheres (Hughes et al. 2017).
Within the Caribbean Sea, the Mesoamerican Barrier Reef System (MBRS) extends for ~ 1000 km from Mexico to Guatemala, Belize and Honduras and is the largest coral reef system in the Northern Hemisphere (Chollett et al. 2017). This system provides subsistence for many people within these countries (Gress et al. 2019) but has been heavily impacted by multiple stressors, including overfishing, coral disease outbreaks, hurricanes, coral bleaching, sedimentation, and land-based pollution (Mumby et al. 2007; Smith et al. 2008; Pandolfi 2010; Perry et al. 2013; Jackson et al. 2014; Muñiz-Castillo et al. 2019; Cáceres et al. 2020; França et al. 2020). Over the last 30 years, the average estimated coral cover in this region declined from approximately 50% to 10% (Gardner et al. 2003; Cramer et al. 2020). In particular, the precipitous decline of the three acroporid corals, Acropora cervicornis, A. palmata and A. prolifera, considered the building blocks of Caribbean coral reefs, and the mass mortality of the sea urchin, Diadema antillarum, resulted in a dramatic reduction within three-dimensional reef complexity (Carpenter 1988; Alvarez-Filip et al. 2009; Alvarez-Filip, et al. 2011a, b). These events are thought to have created phase shifts in the composition of the benthic community, moving from hard (scleractinian) coral as the dominant organism to octocorals, sponges, and/or macroalgae, with cascading impacts on the biodiversity and productivity across this region (Carpenter 1988; Hughes 1994; Miller et al. 1999; Aronson et al. 2002; Patterson et al. 2002; Burke and Maidens 2004; Maliao et al. 2008; Wulff 2012; Smith et al. 2016a, b).
Given the alarming rate at which coral ecosystems around the world are declining, coral reef surveys represent a fundamental first step to assess the overall health of the reef ecosystem by measuring benthic cover, species diversity, biomass, and surface complexity. Annual trends in these measures serve as a tool to evaluate reef resistance and resilience to disturbances and to identify areas of particular concern where management strategies need to be implemented. Although the Caribbean region is one of the most extensively studied (Hughes 1994; Burke and Maidens 2004; Jackson et al. 2014), a lack of long-term monitoring in many locations makes it difficult to estimate benthic population and cover trends and develop effective models. In this study, we present results from a decade of surveys of shallow coral reefs off of the southern coast of Roatán, Honduras, providing information on changes that occurred in percentage coverage of benthic invertebrates over a ten-year period. These data represent the first long-term monitoring for this location and provide a baseline for future studies.
Materials and methods
Study sites
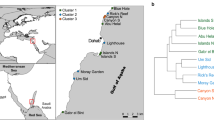
Las Islas de la Bahia (The Bay Islands) within the MBRS lie ~ 65 km off the northern shoreline of Honduras, with Roatán being the largest (approximately 60 km long and 8 km wide) and most populated of the three main islands (Biggs and Olden 2011) (Fig. 1a, b). The islands of Roatán, Guanaja, and Utila are part of the Bonacca Ridge, a mountainous uplift that is thought to have formed along the tectonic plate boundary between the North American and Caribbean plates and separates the region from the Cayman Trough to the North (Sutton 2015). Sea-surface temperatures range from 26 to 30 °C and salinity between 35 and 35.5 PSU (Heyman and Kjerfve 2001; Brenes et al. 2009; Brenes et al. 2017), while processes such as sedimentation, eutrophication, larval dispersion and recruitment are influenced by the cyclonic gyre off the coast of Honduras (Soto et al. 2009). Roatán is part of the Bay Islands Marine National Park, also known as PNMIB (Parque Nacional Marino Islas de la Bahia), a marine protected area (MPA) established in June 2010 by the National Congress of the Republic of Honduras as a management tool to preserve and protect a fundamental portion of the MBRS ecosystem (Carrasco et al. 2013). Four reef sites within the nearshore waters of the southern coast of Roatán were selected for this study: Coco View Wall, Two Tall Two Small, Menagerhea, and Gold Chain (Fig. 1c). Each selected reef was shallow (3–6 m) and represented the diversity of scleractinian corals found within the region (Olson & Radawski, pers. observ.).
Surveys
In 2010, rebar stakes were inserted into the reef while SCUBA diving to generate 30 m permanent transects. Each 30 m transect was divided into two sub-transects of 10 m (from 5 to 15 m and 20 to 30 m), creating four to six 10 m transects per site. In late May to early June from 2010 to 2019, quantitative and qualitative data on the benthic cover and diversity of corals (both hard and soft), sponges, algae, and other benthic invertebrates were retrieved via SCUBA diving by performing point intercept transects (PIT), where the benthic cover at each 10 cm interval along the 10 m length was recorded. Data were not retrieved in 2014 and 2015. Percent coverage along these transects was calculated for hard corals (Scleractinia), soft corals (Octocorallia), macroalgae, turf, crustose coralline algae (CCA), sponges, sand, recently dead coral, rubble and other (including benthic invertebrates that do not belong to the previous categories) as the number of observations within that category divided by 100 observations per transect. On the same transects, 2 m band surveys were performed, with 1 m on each side of the tape measure. The number of hard coral, soft coral, and sponge individuals was recorded within each 10 m by 2 m transect to provide abundance data.
Where possible, all of the organisms within the hard coral, soft coral, macroalgae and sponge categories were identified to the species level (scleractinian taxonomy was mainly based on Veron (2000) with some exceptions to take into account recent reclassification of certain coral species (Wallace et al. 2012)), which allowed for the determination of percent cover of individual species. However, to minimize possible confounding factors associated with the identification of some coral species in situ, the data were grouped and analyzed at the genus level.
Data analysis
Using the vegan package (Oksanen et al. 2018) in R version 3.4.3 (R Core Team 2017), a Bray–Curtis dissimilarity matrix was generated with untransformed data and examined for homogeneity of variance (betadisper). Differences in the benthic community composition between sites and years were assessed using a two-way permutational analysis of variance (PERMANOVA; adonis) with 999 random permutations (Anderson 2006; Anderson et al. 2006). The function metaMDS was used to generate non-metric multidimensional scaling (nMDS) plots to visualize the data in two-dimensional space. A similarity percentage analysis (simper) was implemented to calculate the individual contribution of each benthic category and coral genus toward the differences noted between the first and last years of the study.
To compare the percentage coverage of the investigated categories and hard coral genera across sites and years of the study, linear mixed effects (LME) models were fitted, taking into account spatial autocorrelation. Using the R package lsmeans (Lenth 2016), pairwise comparisons among yearly least square means using Tukey’s HSD tests followed by Bonferroni corrections were employed to identify significant differences in percent cover of the various categories between years. To evaluate associations between hard corals, turf, macroalgae, and CCA, the cor.test function in R was used to run Pearson’s correlations and the R package ggpubr (Kassambara 2018) was used to visualize results.
The wilcox.test of the R package dplyr (Wickham et al. 2018) was used to compare abundances of median values of hard corals, soft corals, and sponges between the start (2010) and end (2019) of the survey period with Mann Whitney U tests. Bonferroni corrections were applied to the results. All data are shown as the mean \(\pm\) standard error (SE) unless otherwise indicated.
Results
During the 2010 to 2019 Roatán surveys, 29 scleractinian coral species, 14 soft coral species (including gorgonians), 6 macroalgal species, and 20 sponge species were identified (Table 1). The benthic community structure of the study area changed across the study period, with significant differences in overall composition between sites (2-way PERMANOVA; Df = 3, F = 24.08, p = 0.001), years (Df = 7, F = 4.21; p = 0.001), and site by year interactions (Df = 21; F = 1.42; p = 0.011). When nMDS plots of the communities at the sites in two-dimensional space were examined over the study period, a gradual homogenization of the benthic assemblages was apparent, with reduced size and spread of the ellipses between the sites in the later years of the study compared to the early years (Fig. 2). To determine which categories were responsible for these changes between 2010 and 2019, simper analyses showed that hard coral cover, followed by sand, macroalgae, and rubble, were most important (Table 2a). However, looking at percent cover of the various categories by site and year suggests that changes in both hard coral and macroalgal cover were largely driven by two of the four sites (Two Tall Two Small, Gold Chain; Fig. 3; Supplementary Table 1). Within the hard coral category, Agaricia and Orbicella were the genera most responsible for the change in benthic cover (Table 2b), with differences again occurring primarily at two of the four sites (Supplementary Table 2).
Mean percent cover of the five most abundant scleractinian genera found on individual transects at the study sites over time. Trend bars are shown in black and different color circles represent the 4 study sites. No data were collected in 2014 or 2015. * denote significance of the trend lines at p < 0.05
LME models were significant by year for the categories hard coral, macroalgae, turf, CCA, sand, rubble, and recently dead coral and by site for macroalgae, soft corals, sponges, and sand (Table 3). All categories except for soft corals, sponges, and sand showed a year by site interaction (Table 3). Although scleractinian coral cover declined significantly across the study period, the hard coral category, with one exception in 2012, remained the most highly represented on the transects (Supplementary Table 1). Conversely, the percent cover of macroalgae and turf significantly increased over time (Fig. 4; Table 3). When LME models for the five most abundant scleractinian coral genera (Acropora, Agaricia, Orbicella, Porites, and Siderastrea) were examined, significant decreases in benthic cover between 2010 and 2019 by Agaricia spp. (Df = 1, F = 3.04, p = 0.0056) were partially offset by significant increases in Siderastrea spp. (Df = 1, F = 7.42, p < 0.0001; Fig. 3; Table 4). However, site was significant for Orbicella, Porites, and Siderastrea and all but Porites showed a year by site interaction (Table 4).
After applying Bonferroni corrections, pairwise comparisons among years using lsmeans followed by Tukey’s post-hoc tests showed significant differences in hard coral cover between 2011 and 2018 (p = 0.0002), macroalgal cover between 2013 and 2016 (p = 0.0016), sand between 2011 and 2013 (p = 0.0015), and multiple years for turf (including 2011–2019: p < 0.0001), rubble (e.g., 2012–2016: p = 0.0009), and dead coral (e.g., 2010–2017: p = 0.0014 and 2017–2019: p < 0.0001; Supplementary Table 3). When examined using the five most abundant scleractinian genera, significant differences in cover between multiple years were only seen for Siderastrea spp. (e.g., 2010 to 2019: p = 0.0003; Supplementary Table 3). While no significant correlation was found between the increase in macroalgal cover and the decrease in hard coral across the study period (R = − 0.03, p = 0.67), there was a significant negative correlation for the increase in turf cover and decrease in hard coral cover (R = − 0.19, p = 0.0093).
Although most corals were visually identified to the species level for abundance data, visual differentiation of species of Agaricia can be difficult (Bongaerts et al. 2013) and no collections were permitted within the marine park to allow for further microscopic or molecular analyses. As a result, all colonies of Agaricia were simply recorded as Agaricia spp. Using Mann–Whitney U tests on abundance data, no significant differences were observed between 2010 and 2019 for any of the categories. Trends suggesting a gradual reduction in the number of individuals within the five most abundant scleractinian genera over time were noted, as was a slight increase in the abundance of soft corals over time (Table 5; Supplementary Fig. 1).
Discussion
Results from this study demonstrated that the composition of the benthic community on shallow, near-shore reefs around Roatán significantly changed from 2010 to 2019. A reduction in scleractinian coral cover and a concomitant increase in macroalgal and turf cover were noted over the study period. This phenomenon, known as a "coral-algal phase shift", has been previously documented on reefs in Belize, Curaçao and Bonaire (Hughes 1994; Nugues and Bak 2008; Jackson et al. 2014; Smith et al. 2016a, b; de Bakker et al. 2017) but has not been previously reported from the Bay Islands. The increased abundance of macroalgae and turf on benthic substrata inhibits growth, fecundity, and survivorship of the scleractinian corals on the reefs (Lewis 1986; Tanner 1995; Hughes et al. 2007), suppresses colonization by coral larval recruits (Kuffner et al. 2006), and increases the prevalence of coral diseases (Nugues et al. 2004). All of these factors work jointly to further decrease the presence of hard coral (Burkepile and Hay 2010).
Although the increased prevalence of both macroalgae and turf algae likely impacted the growth and survival of hard corals, a direct correlation linking the decline in hard coral cover with the increase in macroalgal cover was not found. However, a significant negative correlation was observed between the decrease in hard coral cover and increase in turf cover. This suggests that one of the causes for the shift in benthic composition observed on Roatán’s reefs could be attributed to the ability of algae to outcompete corals for space, but that among the two algal categories, the increased presence of turf algae appears to be more detrimental to coral growth and/or survival. Other benthic categories such as soft corals and sponges remained stable over the study period while significant changes in percent cover of CCA, rubble, sand and dead coral were observed.
Continued development of coastal areas to support the local population as well as to enhance tourism may be one of the leading factors in the benthic transition (Burke and Maidens 2004; Bozec et al. 2008; Burke et al. 2011; Stubler et al. 2015; Pendleton et al. 2016). On the island of Roatán, the amount of tourism has been increasing by approximately an order of magnitude per decade (Doiron and Weissenberger 2014), putting greater stress on the fragile coastal ecosystems such as mangroves and coral reefs that bring tourists to the island (Dorion & Weissenberger 2014; Canty et al. 2018). Correspondingly, the amount of land classified as urban increased over time, from 6.3% in 1985 to 24.6% in 2015 (Helm 2014). The continued development of previously forested land for human use results in greater runoff of sediments (e.g., erosion), nutrients, and pollutants from land-based sources (Carrasco et al. 2013) that can limit the filtration capacity of sponges and other filter-feeding invertebrates (Rogers 1990; Fabricius and Wolanski 2000; Prouty et al. 2008; Bannister et al. 2012; Bell et al. 2015), increase the susceptibility of hard corals and other reef organisms to diseases (Koop et al. 2001; Fabricius 2005; Kaczmarsky and Richardson 2011; D’Angelo and Wiedenmann 2014; Vega Thurber et al. 2014), and create eutrophic conditions that promote the proliferation of algae and algal blooms (Hughes et al. 1999; Anderson et al. 2002; Lapointe 2019). Roatán is also likely impacted by riverine discharges from the mainland Honduran coast, which can easily reach the island via the cyclonic gyre off the Gulf of Honduras and may impact the composition of benthic communities (Harborne et al. 2001; Prouty et al. 2008). To demonstrate the impacts of these activities on near-shore reefs, long term measurements of seawater nutrient concentrations paired with sediment traps are needed.
The changes in benthic cover are likely responsible for the homogenization of species assemblages that occurred from 2010 to 2019. Reef health is influenced by species biodiversity, which confers ecosystem resilience during ecological reorganization (Nyström et al. 2000; Nyström 2006; Camargo et al. 2009). In the absence of any major disturbance (e.g., hurricane, massive disease outbreak) that would rapidly impact the composition the benthic community, significant changes in abundance of benthic categories were not expected over the 10 year study period and were not observed. Instead, the gradual alteration in the presence and abundance of benthic constituents towards a more uniform community may be important for determining optimal management strategies in the future. At present, although biotic homogenization is becoming more common on Caribbean and other reefs (Burman et al. 2012; Mouillot et al. 2014; Graham et al. 2015; Richardson et al. 2018; Estrada-Saldívar et al. 2019), its consequences are not yet fully understood and additional studies are needed to elucidate the long-term repercussions of homogenization on the functional stability of the entire coral reef ecosystem.
Benthic rugosity measurements were not performed in this study, limiting what can be inferred for the architecture of nearshore Roatán reefs. However, the observed decline in branching and structurally complex scleractinian species (e.g., Acropora and branching Porites spp.) across the study period appeared to be partially offset by the increased presence and/or growth of sub-massive coral species (e.g., Siderastrea spp., Porites astreoides). On the reefs of nearby Cayos Cochinos (Honduras), sub-massive scleractinian corals dominate and support high abundance, biomass and richness of fish assemblages compared to locations characterized by fewer sub-massive and more leafy coral species (Cáceres et al. 2020). Similar scenarios have been observed on Caribbean reefs since the 1980’s, where rising seawater temperatures coupled with disease outbreaks led to shifts in the dominance of hard corals from fast growing, branching and structurally complex species to more stress tolerant but less architecturally intricate species (Carpenter 1988; Alvarez-Filip et al. 2009; Alvarez-Filip et al. 2011a, b). Recent studies have also found that some reef-building corals were already declining due to human activities before these additional stressors were documented (Cramer et al. 2020; Cybulski et al. 2020). Coral reefs are one of the most biodiverse ecosystems on the planet but many of the services provided to humans and other species are linked not only to the amount of live coral, but also to the three-dimensional topographic complexity of these habitats (Wilson et al. 2007; Graham 2014; Hoegh-Guldberg et al. 2019). Several studies demonstrated that coral habitats with high structural complexity positively affected reef communities (Messmer et al. 2011; Holbrook et al. 2015), as this element impacts the availability of resources and environmental niches necessary to support diverse and abundant invertebrate and fish communities (Holling 1992; Wilson et al. 2010; Darling et al. 2017). Unfortunately, coral taxa with the highest three-dimensional complexity appear to also be the species that are most susceptible to the wide range of natural and anthropogenic stressors impacting coral reefs (Cramer et al. 2020; Cybulski et al. 2020). Future studies should incorporate benthic rugosity measurements in order to be able to more directly discuss changes in reef architecture.
Species within the genera Acropora and Orbicella represent the primary contributors to building the Caribbean reef framework (Jordan-Dahlgren and Rodriguez-Martinez 2003; Perry et al. 2013; Kuffner and Toth 2016) but the presence of these taxa has dramatically declined over the last three decades. Several recent studies of coral larval recruitment in the Caribbean found that the majority of coral recruits belonged to brooding genera (e.g., Agaricia, Porites) while recruits from broadcast spawning genera (e.g., Acropora, Orbicella) were rarely observed (Arnold et al. 2010; Urvoix et al. 2012; Brandt et al. 2019). This suggests that future reef communities may be dominated by weedier, brooding hard coral species and result in a further reduction of reef framework in the Caribbean. This transition to dominance of non-framework-building corals has been observed for many reefs within the Mesoamerican Reef System (González-Barrios and Álvarez-Filip 2018) but more data from long-term studies are needed to more fully comprehend the implications of these alterations.
The decline in framework-building corals has been largely attributed to severe bleaching events and disease outbreaks (Harvell et al. 2007; Randall and Van Woesik 2017; Van Woesik and Randall 2017), including the current stony coral tissue loss disease outbreak (Alvarez-Filip et al. 2019; Meyer et al. 2019; Muller et al. 2020; Rosales et al. 2020) that has now reached the reefs surrounding Roatán (Precht 2021). Although coral bleaching has been recorded in the Caribbean since 1911 (Rowlands et al. 2008) and severe episodes were documented in years characterized by anomalously high water temperatures (Eakin et al. 2010), the average monthly seawater temperatures remained stable in Roatán during the study period (Supplementary Table 4). This suggests that the changes observed in benthic cover were not directly attributed to the consequences of bleaching.
Information on the incidence and extent of coral diseases in the region are incomplete (Kramer et al. 2000), with relatively few of the over 40 described syndromes (Bruckner 2016) reported from the reefs around Roatán including white band disease, yellow band disease, black band disease (BBD), and white plague type II (Kramer et al. 2000; Riegl et al. 2009; Kroll et al. 2018). Although BBD was noted infrequently affecting various genera of hard corals during this study, we observed a different infection that shared a similar gross appearance. This report, to our knowledge, represents the first documentation of Caribbean ciliate infection in Roatán (Fig. 5) although its presence was observed at other Caribbean locations beginning in 2004 (Cróquer et al. 2006). Visual inspection of the dark band typical of this syndrome on the affected A. palmata coral revealed ciliates with prominent peristomial wings, presumably belonging to the genus Halofolliculina, clustered between the visibly healthy coral tissue and the bare skeleton. Continued increases in sea surface temperatures combined with observations of new diseases on these reefs suggests that further declines in coral cover may be anticipated.
The sites used in this study are located in a marine protected area (MPA) that was established in 2010 to promote sustainable fishing practices, protect migratory species, and control invasive species (Carrasco et al. 2013). Unfortunately, as evidenced from this study, the establishment of an MPA does not guarantee success in conserving reef ecosystems or achieving the stated objectives. It is likely that anthropogenic impacts on shallow reef environments take many years to become apparent, suggesting that early protection and long-term mitigation efforts are needed for a higher probability of successful outcomes. Many of the anthropogenic factors that have been shown to negatively affect coral reefs (e.g., eutrophication, sedimentation, ocean warming, sea level rise, intensity and frequency of storms; e.g., Mumby et al. 2007; Smith et al. 2008; Pandolfi 2010; Perry et al. 2013; Jackson et al. 2014; Muñiz-Castillo et al. 2019; Cáceres et al. 2020; França et al. 2020) cannot be easily mitigated and are outside the reach of MPA management strategies. Until MPAs are staffed and funded at levels that permit effective enforcement and monitoring and governmental agencies work collectively to address the larger issues that threaten reef health, nearshore reefs are likely to face continued gradual declines and phase shifts.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon request.
Code availability
The codes used to analyze the data are available from the corresponding author upon request.
References
Alvarez-Filip L, Dulvy NK, Gill JA, Côte IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc R Soc B Biol Sci 276:3019–3025. https://doi.org/10.1098/rspb.2009.0339
Alvarez-Filip L, Côté IM, Gill JA, Watkinson AR, Dulvy NK (2011) Region-wide temporal and spatial variation in Caribbean reef architecture: is coral cover the whole story? Glob Chang Biol 17(7):2470–2477. https://doi.org/10.1111/j.1365-2486.2010.02385.x
Alvarez-Filip L, Dulvy NK, Côte IM, Watkinson AR, Gill JA (2011) Coral identity underpins reef complexity on Caribbean reefs. Ecol Appl 21(6):2223–2231. https://doi.org/10.2307/41416650
Alvarez-Filip L, Estrada-Saldívar N, Pérez-Cervantes E, Molina-Hernández A, González-Barrios FJ (2019) A rapid spread of the stony coral tissue loss disease outbreak in the Mexican Caribbean. PeerJ. https://doi.org/10.7717/peerj.8069
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62(1):245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication nutrient sources, composition, and consequences. Chem Soc Rev 25(4b):704–726. https://doi.org/10.1039/b709565c
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Arnold SN, Steneck RS, Mumby PJ (2010) Running the gauntlet: Inhibitory effects of algal turfs on the processes of coral recruitment. Mar Ecol Prog Ser 414:91–105. https://doi.org/10.3354/meps08724
Aronson RB, Macintyre IG, Precht WF, Murdoch TJT, Wapnick CM (2002) The expanding scale of species turnover events on coral reefs in belize. Ecol Monogr 72(2):233–249. https://doi.org/10.2307/3100026
Bannister RJ, Battershill CN, de Nys R (2012) Suspended sediment grain size and mineralogy across the continental shelf of the great Barrier reef: impacts on the physiology of a coral reef sponge. Cont Shelf Res 32:86–95. https://doi.org/10.1016/j.csr.2011.10.018
Bell JJ, McGrath E, Biggerstaff A, Bates T, Bennett H, Marlow J, Shaffer M (2015) Sediment impacts on marine sponges. Mar Pollut Bull 94(1–2):5–13. https://doi.org/10.1016/j.marpolbul.2015.03.030
Biggs CR, Olden JD (2011) Multi-scale habitat occupancy of invasive lionfish (Pterois volitans) in coral reef environments of Roatan Honduras. Aquat Invasions 6(3):347–353. https://doi.org/10.3391/ai.2011.6.3.11
Bongaerts P, Frade PR, Ogier JJ, Hay KB, Van Bleijswijk J, Englebert N, Vermeij MJ, Bak RP, Visser PM, Hoegh-Guldberg O (2013) Sharing the slope: depth partitioning of agariciid corals and associated Symbiodinium across shallow and mesophotic habitats (2–60 m) on a Caribbean reef. BMC Evol Biol. https://doi.org/10.1186/1471-2148-13-205
Bozec YM, Acosta-González G, Núñez-Lara E, Arias-González JE (2008) Impacts of coastal development on ecosystem structure and function of Yucatan coral reefs , Mexico. Proceedings of the 11th International Coral Reef Symposium 18(18):691–695
Brandt ME, Olinger LK, Chaves-Fonnegra A, Olson JB, Gochfeld DJ (2019) Coral recruitment is impacted by the presence of a sponge community. Mar Biol 166(4):1–13. https://doi.org/10.1007/s00227-019-3493-5
Brenes CL, Benavides Morera R, Ballestero D (2009) Características hidrográficas del sistema lagunar los micos-quemada en el Caribe Hondureño. Rev Ciencias Mar y Costeras 1:215–229. https://doi.org/10.15359/revmar.1.12
Brenes Rodríguez CL, Benavides Morera R, Loza Álvarez S (2017) Descripción de la distribución espacial de la clorofila a, temperatura y salinidad en la plataforma y el talud continentales del Caribe centroamericano. Revi Ciencias Mar y Costeras 9(1):41. https://doi.org/10.15359/revmar.9-1.3
Bruckner AW (2016) History of coral disease research. In: Woodley CM, Downs CA, Bruckner AW, Porter JW, Galloway SB (eds) Diseases of Coral. Wiley, Hoboken, pp 52–83
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, Washington
Burke L, Maidens J (2004) Reefs at Risk in the Caribbean. World Resources Institute, Washington, http://pdf.wri.org/reefs_caribbean_front.pdf
Burkepile DE, Hay ME (2010) Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS ONE 5(1):e8963. https://doi.org/10.1371/journal.pone.0008963
Burman SG, Aronson RB, Van Woesik R (2012) Biotic homogenization of coral assemblages along the Florida reef tract. Mar Ecol Prog Ser 467:89–96. https://doi.org/10.3354/meps09950
Cáceres I, Ibarra-García EC, Ortiz M, Ayón-Parente M, Rodríguez-Zaragoza FA (2020) Effect of fisheries and benthic habitat on the ecological and functional diversity of fish at the Cayos Cochinos coral reefs (Honduras). Mar Biodivers. https://doi.org/10.1007/s12526-019-01024-z
Camargo C, Maldonado JH, Alvarado E, Moreno-Sánchez R, Mendoza S, Manrique N, Mogollón A, Osorio JD, Grajales A, Sánchez JA (2009) Community involvement in management for maintaining coral reef resilience and biodiversity in southern Caribbean marine protected areas. Biodivers Conserv 18(4):935–956. https://doi.org/10.1007/s10531-008-9555-5
Canty SW, Preziosi RF, Rowntree JK (2018) Dichotomy of mangrove management: a review of research and policy in the Mesoamerican reef region. Ocean & Coastal Manag 157:40–49
Carpenter RC (1988) Mass mortality of a Caribbean sea urchin: Immediate effects on community metabolism and other herbivores. Proc Natl Acad Sci 85(2):511–514. https://doi.org/10.1073/pnas.85.2.511
Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, Devantier L, Graham EJ, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E (2008) Climate change and local impacts. Science 321(5888):560–563. https://doi.org/10.1126/science.1159196
Carrasco J, Secaira E, Lara K (2013) Plan de conservación del Parque Nacional Marino Islas de la Bahía basado en análisis de amenazas, situación y del impacto del cambio climático, y definición de metas y estrategias. ICF y USAID ProParque. 55 pp.
Chollett I, Garavelli L, Holstein D, Cherubin L, Fulton S, Box SJ (2017) A case for redefining the boundaries of the Mesoamerican reef ecoregion. Coral Reefs 36(4):1039–1046. https://doi.org/10.1007/s00338-017-1595-4
Cramer KL, Jackson JBC, Donovan MK, Greenstein BJ, Korpanty CA, Cook GM, Pandolfi JM (2020) Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci Adv 6(17):eaax9395
Cróquer A, Bastidas C, Lipscomp D, Rodríguez-Martínez FE, Jordan-Dahlgren E, Guzman HM (2006) First report of folliculinid ciliates affecting Caribbean scleractinian corals. Coral Reefs 25:187–191. https://doi.org/10.1007/s00338-005-0068-3
Cybulski JD, Husa SM, Duprey NN, Mamo BL, Tsang TPN, Yasuhara M, Xie JY, Qiu J-W, Yokoyama Y, Baker DM (2020) Coral reef diversity losses in China’s Greater Bay area were driven by regional stressors. Sci Adv 6(40):eabb1046
D’Angelo C, Wiedenmann J (2014) Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr Opin Environ Sustain 7(2):82–93. https://doi.org/10.1016/j.cosust.2013.11.029
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36(2):561–575. https://doi.org/10.1007/s00338-017-1539-z
de Bakker DM, van Duyl FC, Bak RPM, Nugues MM, Nieuwland G, Meesters EH (2017) 40 Years of benthic community change on the Caribbean reefs of Curaçao and Bonaire: the rise of slimy cyanobacterial mats. Coral Reefs 36(2):355–367
Doiron S, Weissenberger S (2014) Sustainable dive tourism: social and environmental impacts—The case of Roatan, Honduras. Tour Manag Perspect 10:19–26. https://doi.org/10.1016/j.tmp.2013.12.003
Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L, Baca B, Bartels E, Bastidas C, Bouchon C, Brandt M, Bruckner AW, Bunkley-Williams L, Cameron A, Causey BD, Chiappone M, Christensen TRL, Crabbe MJC, Day O, de la Guardia E, Díaz-Pulido G, DiResta D, Gil- Agudelo DL, Gilliam DS, Ginsburg RN, Gore S, Guzmán HM, Hendee JC, Hernández-Delgado EA, Husain E, Jeffrey CFG, Jones RJ, Jordán-Dahlgren E, Kaufman LS, Kline DI, Kramer PA, Lang JC, Lirman D, Mallela J, Manfrino C, Maréchal JP, Marks K, Mihaly J, Miller WJ, Mueller EM, Muller EM, Toro CAO, Oxenford HA, Ponce- Taylor D, Quinn N, Ritchie KB, Rodríguez S, Ramírez AR, Romano S, Samhouri JF, Sánchez JA, Schmahl GP, Shank B V., Skirving WJ, Steiner SCC, Villamizar E, Walsh SM, Walter C, Weil E, Williams EH, Roberson KW, Yusuf Y (2010) Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5(11). https://doi.org/10.1371/journal.pone.0013969
Eddy TD, Lam VWY, Reygondeau G, Cisneros-Montemayor AM, Greer K, Palomares MLD, Bruno JF, Ota Y, Cheung WWL (2021) Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4:1278–1285. https://doi.org/10.1016/j.oneear.2021.08.016
Estrada-Saldívar N, Jordán-Dalhgren E, Rodríguez-Martínez RE, Perry C, Alvarez-Filip L (2019) Functional consequences of the long-term decline of reef-building corals in the Caribbean: evidence of across-reef functional convergence. R Soc Open Sci. https://doi.org/10.1098/rsos.190298
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50(2):125–146. https://doi.org/10.1016/j.marpolbul.2004.11.028
Fabricius KE, Wolanski E (2000) Rapid smothering of coral reef organisms by muddy marine snow. Estuarine Coastal Shelf Sci 50(1):115–120. https://doi.org/10.1006/ecss.1999.0538
França FM, Benkwitt CE, Peralta G, Robinson JPW, Graham NAJ, Tylianakis JM, Berenguer E, Lees AC, Ferreira J, Louzada J, Barlow J (2020) Climatic and local stressor interactions threaten tropical forests and coral reefs. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2019.0116
Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301(5635):958–960. https://doi.org/10.1126/science.1086050
González-Barrios FJ, Álvarez-Filip L (2018) A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol Indic 95:877–886. https://doi.org/10.1016/j.ecolind.2018.08.038
Graham NAJ (2014) Habitat complexity: coral structural loss leads to fisheries declines. Curr Biol 24(9):R359–R361. https://doi.org/10.1016/j.cub.2014.03.069
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518(7537):94–97. https://doi.org/10.1038/nature14140
Gress E, Voss JD, Eckert RJ, Rowlands G, Andradi-Brown DA (2019) The Mesoamerican Reef. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems. Springer, Cham. https://doi.org/10.1007/978-3-319-92735-0_5
Harborne AR, Afzal DC, Andrews MJ (2001) Honduras: Caribbean coast. Mar Pollut Bull 42(12):1221–1235. https://doi.org/10.1016/S0025-326X(01)00239-9
Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 20(1):172–195
Helm CA (2014) Sattelite-assisted assessment of the effects of human development on coral reefs, Roatan Honduras. Masters Thesis, University of Pennsylvania, pp. 1–36
Heron S, Morgan J, Eakin M, Skirving W (2008) Hurricanes and their effects on coral reefs. pp. 31–36. In: Wilkinson C, Souter D (eds) Status of caribbean coral Reefs after bleaching and hurricanes in 2005. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville, pp 152
Heyman WD, Kjerfve B (2001) The Gulf of Honduras. In: Seelinger U, Kjerfve B (eds) Coastal marine ecosystem of Latin America. Ecological studies (analysis and synthesis), vol 144. Springer, Berlin. https://doi.org/10.1007/978-3-662-04482-7_2
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50(8):839–866. https://doi.org/10.1071/MF99078
Hoegh-Guldberg O, Pendleton L, Kaup A (2019) People and the changing nature of coral reefs. Reg Stud Mar Sci 30:100699. https://doi.org/10.1016/j.rsma.2019.100699
Holbrook SJ, Schmitt RJ, Messmer V, Brooks AJ, Srinivasan M, Munday PL, Jones GP (2015) Reef fishes in biodiversity hotspots are at greatest risk from loss of coral species. PLoS ONE 10(5):1–12. https://doi.org/10.1371/journal.pone.0124054
Holling CS (1992) Cross-scale morphology, geometry, and dynamics of ecosystems. Ecol Monogr 62(4):447–502
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265(5178):1547–1551
Hughes T, Szmant AM, Steneck R, Carpenter R, Miller S (1999) Algal blooms on coral reefs: what are the causes? Limnol Oceanogr 44(6):1583–1586. https://doi.org/10.4319/lo.1999.44.6.1583
Hughes TP, Bellwood DR, Folke CS, McCook LJ, Pandolfi JM (2007) No-take areas, herbivory and coral reef resilience. Trends Ecol Evol 22(1):1–3. https://doi.org/10.1016/j.tree.2006.10.009
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs JPA, Hoogenboom MO, Kennedy EV, Kuo CY, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543(7645):373–377. https://doi.org/10.1038/nature21707
Jackson J, Donovan M, Cramer K, Lam V (2014) Status and trends of Caribbean coral reefs: 1970–2012. Global coral reef monitoring network. IUCN, Gland, pp 1–306
Jordan-Dahlgren E, Rodriguez-Martinez RE (2003) The Atlantic coral reefs of Mexico. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 131–158
Kaczmarsky L, Richardson LL (2011) Do elevated nutrients and organic carbon on Philippine reefs increase the prevalence of coral disease? Coral Reefs 30(1):253–257. https://doi.org/10.1007/s00338-010-0686-2
Kassambara A (2018) Machine learning essentials: practical guide in R. Sthda
Koop K, Booth D, Broadbent A, Brodie J, Bucher D, Capone D, Coll J, Dennison W, Erdmann M, Harrison P, Hoegh-Guldberg O, Hutchings P, Jones GB, Larkum AWD, O’Neil J, Steven A, Tentori E, Ward S, Williamson J, Yellowlees D (2001) ENCORE: The effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar Pollut Bull 42(2):91–120. https://doi.org/10.1016/S0025-326X(00)00181-8
Kramer P, Kramer PR, Arias-Gonzalez E, McField M (2000) Status of coral reefs of northern central America: Mexico, Belize, Guatemala, Honduras, Nicaragua and El Salvador pp. 287–313. In: Wilkinson C (ed) Status of coral reefs of the world: 2000. Global Coral Reef Monitoring Network, p 363
Kroll ML, Rodriguez BA, Edie AC, Phelps KL, Hamilton DE, Randell SM, Lockwood SA (2018) Poriferan abundance is negatively associated with coral health in the Masoamerican Reef. Texas J Sci. https://doi.org/10.32011/txjsci_70_1_Article7
Kuffner IB, Toth LT (2016) A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conserv Biol 30(4):706–715. https://doi.org/10.1111/cobi.12725
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117. https://doi.org/10.3354/meps323107
Lapointe BE (2019) Chasing nutrients and algal blooms in Gulf and Caribbean waters: a personal story. Gulf Caribb Res 30(1):XVI–XXX. https://doi.org/10.18785/GCR.3001.10
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69(1):1–33. https://doi.org/10.18637/jss.v069.i0
Lewis SM (1986) The Role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Soc Am 56(3):183–200
Maliao RJ, Turingan RG, Lin J (2008) Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar Biol 154(5):841–853. https://doi.org/10.1007/s00227-008-0977-0
Messmer V, Jones GP, Munday PL, Holbrook SJ, Schmitt RJ, Brooks AJ (2011) Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92(12):2285–2298. https://doi.org/10.1890/11-0037.1
Meyer JL, Castellanos-Gell J, Aeby GS, Häse CC, Ushijima B, Paul VJ (2019) Microbial community shifts associated with the ongoing stony coral tissue loss disease outbreak on the Florida reef tract. Front Microbiol 10:1–12. https://doi.org/10.3389/fmicb.2019.02244
Miller MW, Hay ME, Miller SL, Malone D, Sotka EE, Szmant AM (1999) Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnol Oceanogr 44(8):1847–1861. https://doi.org/10.4319/lo.1999.44.8.1847
Mouillot D, Villéger S, Parravicini V, Kulbicki M, Arias-González JE, Bender M, Chabanet P, Floeter SR, Friedlander A, Vigliola L, Bellwood DR (2014) Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc Natl Acad Sci U S A 111(38):13757–13762. https://doi.org/10.1073/pnas.1317625111
Muller EM, Sartor C, Alcaraz NI, van Woesik R (2020) Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front Mar Sci. https://doi.org/10.3389/fmars.2020.00163
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of Caribbean coral reefs. Nature 450(7166):98–101. https://doi.org/10.1038/nature06252
Muñiz-Castillo AI, Rivera-Sosa A, Chollett I, Eakin CM, Andrade-Gómez L, McField M, Arias-González JE (2019) Three decades of heat stress exposure in Caribbean coral reefs: a new regional delineation to enhance conservation. Sci Rep 9(1):1–14. https://doi.org/10.1038/s41598-019-47307-0
Nugues MM, Bak RPM (2008) Long-term dynamics of the brown macroalga Lobophora variegata on deep reefs in Curaçao. Coral Reefs 27(2):389–393. https://doi.org/10.1007/s00338-007-0346-3
Nugues MM, Smith GW, Van Hooidonk RJ, Seabra MI, Bak RPM (2004) Algal contact as a trigger for coral disease. Ecol Lett 7(10):919–923. https://doi.org/10.1111/j.1461-0248.2004.00651.x
Nyström M (2006) Redundancy and response diversity of functional groups: Implications for the resilience of coral reefs. Ambio 35(1):30–35. https://doi.org/10.1579/0044-7447-35.1.30
Nyström M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol Evol 15(10):413–417. https://doi.org/10.1016/S0169-5347(00)01948-0
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Harra RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018) Vegan: Community ecology package version 2.5–3 (October 2018) pp. 1–286
Pandolfi JM (2010) Global trajectories of the long-term decline of coral reef ecosystems. Science 955(2003):10–14. https://doi.org/10.1126/science.1085706
Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC (2003) Global trajectories of the long-term decline of coral reef ecosystems. Science 301:955–958
Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW (2002) The etiology of white pox, a lethal disease of the Caribbean elkhorn coral. Acropora Palmata Proc Natl Acad Sci 99(13):8725–8730. https://doi.org/10.1073/pnas.092260099
Pendleton LH, Hoegh-Guldberg O, Langdon C, Comte A (2016) Multiple stressors and ecological complexity require a new approach to coral reef research. Front Mar Sci 3:1–5. https://doi.org/10.3389/fmars.2016.00036
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun 4:1–7. https://doi.org/10.1038/ncomms2409
Precht W (2021) Failure to respond to a coral disease epizootic in Florida: causes and consequences. Rethink Ecol 6:1–47. https://doi.org/10.3897/rethinkingecology.6.56285
Prouty NG, Hughen KA, Carilli J (2008) Geochemical signature of land-based activities in Caribbean coral surface samples. Coral Reefs 27(4):727–742. https://doi.org/10.1007/s00338-008-0413-4
R Core Team (2017) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/
Randall CJ, Van Woesik R (2017) Some coral diseases track climate oscillations in the Caribbean. Sci Rep 7(1):1–8. https://doi.org/10.1038/s41598-017-05763-6
Richardson LE, Graham NAJ, Pratchett MS, Eurich JG, Hoey AS (2018) Mass coral bleaching causes biotic homogenization of reef fish assemblages. Glob Chang Biol 24(7):3117–3129. https://doi.org/10.1111/gcb.14119
Riegl B, Purkis SJ, Keck J, Rowlands GP (2009) Monitored and modeled coral population dynamics and the refuge concept. Mar Pollut Bull 58(1):24–38. https://doi.org/10.1016/j.marpolbul.2008.10.019
Rogers CS (1990) Responses of coral reefs and reef organisms to sedimentation. Mar Ecol Prog Ser 62:185–202. https://doi.org/10.1109/acssc.2005.1599849
Rosales SM, Clark AS, Huebner LK, Ruzicka RR, Muller EM (2020) Rhodobacterales and Rhizobiales are associated with stony coral tissue loss disease and its suspected sources of transmission. Front Microbiol 11(April):1–20. https://doi.org/10.3389/fmicb.2020.00681
Rowlands GP, Purkis SJ, Riegl BM (2008) The 2005 coralbleaching event Roatan (Honduras): use of pseudoinvariant features (PIFs) in satellite assessments. J Spat Sci 53(1):99–112. https://doi.org/10.1080/14498596.2008.9635139
Schutte VGW, Selig ER, Bruno JF (2010) Regional spatio-temporal trends in Caribbean coral reef benthic communities. Mar Ecol Prog Ser 402:115–122. https://doi.org/10.3354/meps08438
Smith TB, Nemeth RS, Blondeau J, Calnan JM, Kadison E, Herzlieb S (2008) Assessing coral reef health across onshore to offshore stress gradients in the US Virgin Islands. Mar Pollut Bull 56(12):1983–1991. https://doi.org/10.1016/j.marpolbul.2008.08.015
Smith JE, Brainard R, Carter A, Dugas S, Edwards C, Harris J, Lewis L, Obura D, Rohwer F, Sala E, Vroom PS, Sandin S (2016a) Re-evaluating the health of coral reef communities : baselines and evidence for human impacts across the central Pacific. Proc R Soc B Biol Sci 283:20151985. https://doi.org/10.1098/rspb.2015.1985
Smith T, Kadison E, Ennis R, Gyory J, Brandt M, Wright V, Nemeth R, Henderson L (2016b) The United States Virgin Island Territorial Coral Reef Monitoring Program: Annual Report. pp 273. https://doi:https://doi.org/10.13140/RG.2.1.3993.5125
Soto I, Andréfouët S, Hu C, Muller-Karger FE, Wall CC, Sheng J, Hatcher BG (2009) Physical connectivity in the Mesoamerican barrier reef system inferred from 9 years of ocean color observations. Coral Reefs 28(2):415–425. https://doi.org/10.1007/s00338-009-0465-0
Spalding MD, Brown BE (2015) Warm-water coral reefs and climate change. Science 350(6262):769–771. https://doi.org/10.1126/science.aad0349
Stubler AD, Duckworth AR, Peterson BJ (2015) The effects of coastal development on sponge abundance, diversity, and community composition on Jamaican coral reefs. Mar Pollut Bull 96(1–2):261–270. https://doi.org/10.1016/j.marpolbul.2015.05.014
Sutton DS (2015) Structural and geophysical interpretation of Roatán Island, Honduras, western Caribbean. Dissertation, University of Louisiana at Lafayette
Tanner JE (1995) Competition between scleractinian corals and macroalgae: An experimental investigation of coral growth, survival and reproduction. J Exp Mar Biol Ecol 190:151–168. https://doi.org/10.1016/S0196-0644(88)80272-5
Urvoix LEA, Fauvelot C, Bouchon C (2012) Monitoring of coral larval recruitment on artificial settlement plates at three different depths using genetic identification of recruits ( Guadeloupe Island). Proceedings of the 65th Gulf and Caribbean Fisheries Institute (65):114–120
Van Woesik R, Randall CJ (2017) Coral disease hotspots in the Caribbean. Ecosphere 8(5):1–10. https://doi.org/10.1002/ecs2.1814
Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, Mcminds R, Zaneveld JR (2014) Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob Chang Biol 20(2):544–554. https://doi.org/10.1111/gcb.12450
Veron JEN (2000) Corals of the world, vol 1. Australian Institute of Marine Science, Townsville, pp 204–205
Vroom PS, Walters LJ, Beach KS, Coyer JA, Smith J, Abgrall M-J, Byron D (2005) Hurricane-induced propagation and rapid growth of the weedy brown alga Dictyota in the Florida Keys.pdf. Florida Sci 68(3):161–174
Wallace J, Karim F, Wilkinson S (2012) Assessing the potential underestimation of sediment and nutrient loads to the Great Barrier Reef lagoon during floods. Mar Pollut Bull 65(4–9):194–202. https://doi.org/10.1016/j.marpolbul.2011.10.019
Wickham H, François R, Henry L, Müller K (2018) Dplyr: a grammar of data manipulation. R package version 0.7.6. https://CRAN.Rproject.org/package=dplyr
Wilson SK, Graham NAJ, Polunin NV (2007) Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar Biol 151(3):1069–1076. https://doi.org/10.1007/s00227-006-0538-3
Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakacaka A, Polunin NVC (2010) Habitat degradation and fishing effects on the size structure of coral reef fish communities. Ecol Appl 20(2):442–451. https://doi.org/10.1890/08-2205.1
Wulff J (2012) Ecological interactions and the distribution, abundance, and diversity of sponges. Adv Mar Biol 61:273–344. https://doi.org/10.1016/B978-0-12-387787-1.00003-9
Acknowledgements
This study was supported by the James “Doc” Radawski Marine Science Internship, generously provided by CoCo View Resort. We thank Julia Stevens, Emily Parker, Corey McCormick, Alex Thompson, Lindsay Short, Ryan Jones, Aimee O’Keefe, Alexis Schutz, Sam Wade, Josh Campbell, Abbie Ward, Aislyn Galford, and Alyssa Bentley for assistance with data collection. We are eternally grateful to CoCo View Resort, the Bay Island Conservation Association (BICA), and the Roatán Marine Park for access to the dive sites and logistical support.
Funding
This study was supported by the James “Doc” Radawski Marine Science Internship provided by CoCo View Resort in Roatán, Honduras.
Author information
Authors and Affiliations
Contributions
AG and MM: collected the data for the last two years of the study, analyzed the data and wrote the manuscript. JDR and JBO: conceived, coordinated and designed the research. All authors, with the exception of JDR: (deceased), read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Consent to participate
All the authors approved their participation in this publication.
Consent for publication
Approval for inclusion of James Doc Radawski as an author has been granted and all living authors are supportive of publication.
Sampling and field study
No sampling was conducted in this observational field study so no permits were required but authors have gratefully acknowledged logistical support from the CoCo View Dive Resort, the Bay Islands Conservation Association, and the Roatán Marine Park.
Additional information
Communicated by Paolo G Albano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Coastal and marine biodiversity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giorgi, A., Monti, M., Radawski, J.D. et al. Long term benthic survey demonstrates a shift in the composition of benthic reef communities at shallow sites in Roatán, Honduras. Biodivers Conserv 31, 1689–1708 (2022). https://doi.org/10.1007/s10531-022-02421-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02421-w