Abstract
The trophic behavior of some deep-sea Newfoundland cold-water corals was explored using fatty acid (FA) and lipid profiles. No significant effect of geographic location and/or depth was identified in lipid or FA composition. However, differences were detected between and within taxon groups in hexa- or octocoral subclasses. Phospholipids constituted the main lipid class in all groups except black-thorny corals which had less structural lipids likely due to their morphology (stiff axes) and slower growth rates. Within each subclass, major differences in the identity of dominant FAs were detected at the order level, whereas differences between species and taxon groups of the same order were mainly driven by a variation in proportions of the dominant FA. Soft corals and gorgonians (Order Alcyonacea) were close in composition and are likely relying on phytodetritus resulting from algae, macrophytes and/or foraminifera, while sea pens (Order Pennatulacea) seem to consume more diatoms and/or herbivorous zooplankton with the exception of Pennatula sp. In the hexacoral subclass, black-thorny corals (Stauropathes arctica) differed significantly from the stony-cup corals (Flabellum alabastrum); S. arctica was seemingly more carnivorous (zooplankton markers) than F. alabastrum, which appears omnivorous (phyto- and zooplankton markers). Our results suggest that deep-sea corals are not as opportunistic as expected but have some selective feeding associated with taxonomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cold-water corals (CWCs) are ecosystem-engineer species supporting high biodiversity and providing habitat and food sources for several other types of organisms. CWCs constitute nurseries for many fish species and promote taxonomic richness and diversity by offering a multitude of microhabitats for associated fauna (epifauna, crypto-fauna, parasitic, and surrounding organisms) with variation according to coral age, shape, and species (Buhl-Mortensen and Mortensen 2005; Wareham and Edinger 2007; Baillon et al. 2014; Buhl-Mortensen et al. 2016). In the Northwest Atlantic, CWCs are threatened by bottom-contact fishing, especially trawling (Wareham and Edinger 2007; Edinger et al. 2007; Gilkinson and Edinger 2009; Buhl-Mortensen et al. 2016), but may encounter other threats such as hydrocarbon drilling or ocean acidification (Roberts et al. 2006). Northwest Atlantic coral faunas consist of a variety of slow-growing and long-lived species (Sherwood and Edinger 2009; Neves et al. 2015; Neves 2016) in several functional groups, including gorgonian sea fans, soft corals, sea pens, solitary stony-cup corals, and black-thorny corals (Wareham and Edinger 2007; Baker et al. 2012). Slow growth rates might jeopardize potential recovery after a perturbation (Sherwood and Edinger 2009; Lacharité and Metaxas 2013; Huvenne et al. 2016). In addition, there is a lack of knowledge on coral recruitment in deep waters; identification of the factors driving the distribution of coral species on the seafloor such as food availability and trophic behavior is essential to predict their presence and work toward better conservation measures and management.
Food availability is one of the factors that dictate CWC species distribution and colony shape (Mortensen and Buhl-Mortensen 2004, 2005; Dullo et al. 2008; Baillon et al. 2015; Buhl-Mortensen et al. 2015a, b, 2016). For deep-sea CWCs, food availability depends on the deposition of particles and plankton migration through the water column, current regimes, internal waves propagated along water mass boundaries, and the thickness of benthic boundary layer (Roberts et al. 2006; Zedel and Fowler 2009; Buhl-Mortensen et al. 2016). CWCs have two feeding mechanisms: (1) mucus entrapment of the prey and digestion outside the coral polyp and (2) capture and digestion of prey directly by the polyp (Buhl-Mortensen et al. 2016). CWCs may also directly absorb dissolved materials from seawater (Mueller et al. 2014). Feeding mechanisms may vary according to species and food availability but also by other factors inherent to the colony such as coral polyp spacing, polyp diameter, or polyp distance from the seafloor (Sherwood et al. 2008; Baillon et al. 2015; Buhl-Mortensen et al. 2016). Many CWC species are cosmopolitan, and most species can apparently feed on various food sources including detrital organic matter, bacteria, or zooplankton (Mortensen et al. 2001; Roberts et al. 2006; Naumann et al. 2011; Mueller et al. 2014), but little is known about their trophic ecology (Roberts et al. 2006). The purpose of this paper is to explore the trophic ecology of CWCs in Northwest Atlantic waters using lipid composition and fatty acid analysis.
Lipid composition and fatty acid (FA) profiles change with the ecology, nutrition, and health of organisms and are powerful tools when used as trophic markers (e.g., Dalsgaard et al. 2003). Some primary producers synthesize specific FAs such as 22:6ω3 (docosahexaenoic acid, DHA: dinoflagellates) or 20:5ω3 (eicosapentaenoic acid, EPA: diatoms) that can be assimilated and identified in primary consumers (Graeve et al. 1994, 1997; Dalsgaard et al. 2003). Moreover, some ratios (DHA/EPA, 18:1ω9/18:1ω7) are also indicators of degree of carnivory (Graeve et al. 1997; Dalsgaard et al. 2003). Understanding the feeding process in deep-sea ecosystem-engineering organisms is essential to anticipate potential changes in these ecosystems due to modifications of current circulation, primary production cycles, or ocean acidification. The loss of ecosystem-engineer species may have irreversible effects (Jordán and Scheuring 2004).
On the Northwest Atlantic coast (Newfoundland and Labrador, NL), Sherwood et al. (2008) studied the diet of 11 CWC species using carbon and nitrogen stable isotopes and found some differences in carnivory among species/taxon group. In addition, bulk lipid analysis on CWC species revealed variation in lipid composition among higher taxonomic groups (Hamoutene et al. 2008). However, stable isotope signatures and lipid composition of CWCs did not vary with depth or latitude (Hamoutene et al. 2008; Sherwood et al. 2008). From these studies, the authors hypothesized that the strong interspecific variation in feeding habits was due to differences in habitats and colony morphologies but mostly associated with the need to reduce interspecific competition in food-limited, deep-sea environments (Iken et al. 2001; Sherwood et al. 2008). However, these studies did not always explore taxon differences in conjunction with species differences and environmental factors. We evaluate the potential diet of a variety of coral species from one region of the Northwest Atlantic using FA and lipid class profiles to better understand the effect of location, depth, taxon group, and species on the trophic behavior of CWCs. Through this exploration, we comment on the potential effect of species, clades and morphological differences on trophic choices and/or contrasting opportunist feeding behaviors.
Materials and methods
Coral collection
This study took advantage of routine multispecies research surveys conducted by Fisheries and Oceans Canada (DFO) along the continental shelf and slope of NL (eastern Canada; Fig. 1). Surveys follow a stratified transect sampling design using a Campelen 1800 trawl towed for 15 min or ~ 1.4 km of seafloor (Wareham and Edinger 2007). In this study, we used samples collected during one complete trip of the Fall 2007 surveys in the Flemish Pass region (Fig. 1), between the Grand Bank and the Flemish Cap, at depths ranging from 770 to 1370 m (depths are in 50 m ranges in Table 1). Temperature was recorded at depth during the trawl using a CTD attached to the safety line of the net and was stable, ranging from 3.5 to 3.9 °C. Eighteen of the 37 transects contained corals as bycatch: seven in North Atlantic Fisheries Organization (NAFO) Division 3L, nine in 3M, and two in 3N (Fig. 1). The species sampled were identified to the lowest taxonomic level and classified in taxon groups as described in Table 1. After collection, samples were individually bagged with identification tag and frozen in liquid nitrogen. Within the gorgonian group, individuals isolated from the Paramuricea genus were considered separately (P. placomus, Paramuricea sp. [B3a and b]) according to preliminary genetic analysis on 28S; distribution of Paramuricea genus in the North Atlantic includes cryptic species (Radice et al. 2016).
Lipid and fatty acid analyses
Lipid extraction was performed as described previously (Parrish 1999). Samples were homogenized in a 2:1 mixture of ice-cold chloroform/methanol and homogenized with a Polytron PCU-2-110 homogenizer (Brinkmann Instruments, Rexdale, Ontario, Canada). Chloroform-extracted water was added to bring the ratio of chloroform/methanol/water to 8:4:3. Samples were sonicated for 4–10 min in an ice bath and centrifuged at 5000 rpm for 2 min. The bottom, organic layer was removed using the double pipetting technique of placing a long, lipid-cleaned Pasteur pipette inside a short one, to remove the organic layer without disturbing the top, aqueous layer. Chloroform was then added back to the extraction test tube, and the entire procedure was repeated three times. All organic layers were pooled into lipid-cleaned vials and concentrated using a flash evaporator (Buchler Instruments, Fort Lee, NJ).
Lipid class composition was determined using an Iatroscan Mark VI TLC–FID, silica-coated Chromarods, and a three-step development method (Parrish 1987). The lipid extracts were applied to the Chromarods and focused on a narrow band using 100% acetone. The first development system was hexane/diethyl ether/formic acid (99.95:1:0.05). The rods were developed for 25 min, removed from the system for 5 min, and replaced for 20 min. The second development was for 40 min in hexane/diethyl ether/formic acid (79:20:1). The final development system had two steps; the first was 100% acetone for two 15 min time periods, followed by two 10 min periods in chloroform/methanol/chloroform-extracted water (5:4:1). Before placing them in each solvent system, the rods were dried in a constant humidity chamber. After each development system, the rods were scanned in the Iatroscan and the data collected using Peak Simple software (v3.67, SRI Inc). The Chromarods were calibrated using standards from Sigma Chemicals (St. Louis, MO). Lipid classes (corresponding standards are indicated in brackets) identified were: hydrocarbons [nanodecane]; steryl esters/wax esters [cholesteryl stearate]; ethyl esters, methyl esters, ethyl ketones, and methyl ketones [3-hexadecanone]; glyceryl ethers and triacylglycerols [tripalmitin]; free FAs [palmitic acid]; alcohols [1-hexadecanol]; sterols [cholesterol]; acetone mobile polar lipids (AMPL) [1-mono-palmyoyl-rac-glycerol]; and phospholipids [dipalmitoylphosphatidylcoline]. For all samples, lipid extracts were transesterified using methylene chloride and Hilditch reagent for 1 h at 100 °C. The fatty acid methyl esters (FAME) were analyzed on a HP 6890 gas chromatography flame ionization detector equipped with a 7683 auto-sampler. The GC column was a ZB wax + (Phenomenex, USA). The column length was 30 mm with an internal diameter of 0.32 mm. The column temperature began at 65 °C and was held at this temperature for 0.5 min. Temperature then ramped to 195 °C at a rate of 40 °C min−1, was held for 15 min, and then ramped to a final temperature of 220 °C at a rate of 2 °C min−1. This final temperature was held for 0.75 min. The carrier gas was hydrogen flowing at a rate of 2 mL min−1. The injector temperature started at 150 °C and ramped to a final temperature of 250 °C at a rate of 120 °C min−1. The detector temperature stayed constant at 260 °C. Peaks were identified using retention times from standards purchased from Supelco, 37 component FAME mix (product number 47885-U), bacterial acid methyl ester mix (product number 47080-U), PUFA 1 (product number 47033), and PUFA 3 (product number 47085-U). Chromatograms were integrated using the Varian Galaxie Chromatography Data System, version 1.9.3.2.y.
The analytical method used in this study for FA analysis is standard and did not assess tetracosapolyenoic acid (TPA) proportions as the analyses predate a series of articles (Imbs and Dautova 2008; Imbs et al. 2010, 2016; Baptista et al. 2012) on the importance of TPAs (24:5ω6 and 24:6ω3). The total time of GC analysis was too short; the retention time of TPA methyl esters is known to be 75–90 min on a polyethylene glycol column of 30 m length at 210–220 °C. TPAs are known to be present in octocoral species and absent from hexacoral species (Imbs et al. 2010); this dictates our decision to run FA statistical analysis on each subclass separately. This limitation is further discussed in the “Discussion” section.
Some FAs were combined into classes: “bacterial biomarker” consists of i15:0, ai15:0, 15:0, 15:1, i16:0, ai16:0, i17:0, ai17:0, 17:0, 17:1, and 16:1ω6 (we did not include 18:1ω6 here); “bacterial (ω7)” includes the latter set (“bacterial biomarker”) plus 18:1ω7 and 16:1ω7. “SAFA” is the sum of saturated FA and PUFA and MUFA are the sum of poly- and mono- (respectively) unsaturated fatty acids, while EPA (20:5ω3) and DHA (22:6ω3) are the FA biomarkers of diatoms and dinoflagellates, respectively. The “ω3” and “ω6” classes correspond to the sum of omega-3 FA and omega-6 FA from a sample. Two trophic ratios were used: DHA/EPA and 18:1ω9/18:1ω7 to evaluate the level of carnivory (Graeve et al. 1997; Dalsgaard et al. 2003).
Lipid and fatty acid analyses were run consecutively on 19 coral samples, and an extra analysis of FA was performed on an additional set of 54 coral samples for a total of 75 coral samples.
Statistical analyses
Proportions of lipid classes and FA are expressed in the text as averaged percentages ± standard deviation. Kruskal–Wallis ANOVAs on ranks followed by pairwise comparisons when applicable were performed in Sigmaplot 13.0 to compare total lipid percentages (n = 19) and DHA/EPA and 18:1ω9/18:1ω7 ratios (n = 75) between taxon groups.
Lipid classes and fatty acid composition (only FAs representing more than 1% of the total FA composition of at least one individual were selected) were analyzed using a multivariate approach in Primer 7.0 and PERMANOVA add-on (Anderson et al. 2008; Clarke and Gorley 2015). Analyses were run with a full comparison (all individuals in the hexacoral and octocoral subclasses) of lipid class composition. In contrast, statistical analyses on FA compositions were completed separately for octocoral and hexacoral subclasses. Resemblance matrices between individuals were built using Bray–Curtis distances without data transformation. First, principal coordinates analysis (PCO) was run on the resemblance matrix among individuals for FA analyses. No PCO analysis was completed on lipid classes data due to a smaller data set (n = 19 vs n = 75 for FA). Second, on each matrix, we ran a PERMANOVA (9999 permutations of residuals under a reduced model, sums of squares type I) to test the effect of depth (depth was used as covariate), geographic location (NAFO divisions, fixed factor), taxon groups as described in Table 1 (fixed), and species nested in the taxon group (fixed) and their interactions. The model was adjusted to pool interactions or factors when p values exceeded 0.25 (see Anderson et al. 2008). Pairwise tests (9999 permutations) and similarity analyses (SIMPER) were then used to detail differences when applicable. The model was modified (no species or location factors) for the FA composition of the hexacoral group due to (1) the small number of samples (n = 6) and location (NAFO division, n = 2), and because (2) each group is constituted by a single species precluding a test of the species effect. The PERMANOVA on hexacorals was run with Monte Carlo (MC) tests and designed with depth as covariate and group as a fixed factor.
Results
Specimen collection and distribution
A total of 75 samples were collected over the three geographic areas (Table 1; Fig. 1). Corals sampled belonged to (1) the Hexacorallia subclass: stony-cup corals (Order Scleractinia) and black-thorny corals (Order Antipatharia) specimens and to (2) the Octocorallia subclass: sea pens (Order Pennatulacea), soft corals, and gorgonians (Order Alcyonacea). Taxon groups are referred to as five coral groups by their common name (Table 1), reflecting the particular shapes of the animals, i.e., soft corals (lack of hard consolidated skeleton), gorgonians (presence of solid axis), sea pens (feather-like or whip-like appearance), stony-cup corals (calcareous skeleton), and black-thorny corals (non-calcareous skeletons composed of protein and chitin). Each group was comprised, respectively, of 3, 7, 6, 1, and 1 species (Table 1). FA analyses were completed on the entire sample collection, whereas lipid class analyses were run on a subsample of 19 specimens: three black-thorny corals (Stauropathes arctica), one gorgonian (Acanthogorgia armata), ten sea pens (five Anthoptilum grandiflorum, one Funiculinia quandrangularis, two Pennatula grandis, two Halipteris finmarchica), five soft corals (two Duva florida, three Nephtheidae sp.) and none on stony-cup corals.
There was considerable variability in coral presence among trawls, sampling stations, and geographic locations (Table 1; Fig. 1). The two stations in Division 3N had 2–3 species each for a total richness of 4. The same number of species was recorded in Divisions 3L and 3M, but with some species differences; Keratoisis grayi, Paramuricea sp., Radicipes gracilis, Funiculinia quandrangularis were absent from 3M and Paramuricea placomus, Distichoptilum gracile, Pennatula grandis, Flabellum alabastrum absent from Division 3L. Interestingly, the three specimens of stony-cup corals were found only at the three stations in Division 3M.
Lipid classes
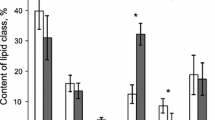
The total individual lipid content for all species varied between 0.29 and 7.71% with a mean of 3.08 ± 2.15% (± SD). Overall, the main lipid groups found in deep-sea corals were the phospholipids (34.6 ± 12.1%) followed by steryl and wax esters (15.2 ± 8.7%), AMPL (10.6 ± 5.4%), sterols (10.2 ± 2.9%), triacylglycerols (8.5 ± 5.5%), free FAs (6.6 ± 4.3%), and hydrocarbons (3.8 ± 5.7%) (Fig. 2). Black-thorny corals diverged from this pattern exhibiting a higher concentration of steryl and wax esters than the other taxon groups (Fig. 2). Total lipid percentages were significantly different between taxon groups (Kuskal–Wallis, p < 0.05) but with no significant distinction among groups. The nonsignificance of taxon group comparisons was mainly due to the low number of samples analyzed (only one specimen of gorgonian, for example; Table 1). Similarly, we could not test differences among species, depth ranges and/or locations because specimen numbers varied from 1 to a maximum of 5 for every group.
There was no effect of depth or geographic locations on lipid classes, but a significant (PERMANOVA, p < 0.01) difference between taxon groups (Table 2). Differences between taxa were mostly between black-thorny corals and sea pens (p < 0.01, 36.1% average dissimilarity), and sea pens and soft corals (p < 0.05, 30.5% dissimilarity) (Table 3). Black-thorny corals had lower content of phospholipids than sea pens (25.8% contribution to dissimilarity) and higher steryl and wax esters (23.6% contribution to dissimilarity). Similarly, soft corals had lower percentages of phospholipids than sea pens (20.3% contribution to dissimilarity) and higher AMPL (13.5% contribution to dissimilarity) (Fig. 2). Comparisons of lipid classes between taxon groups and gorgonians were limited by the single sample of gorgonian collected.
Fatty acid composition
Deep-sea corals collected as part of this study contained a high proportion of MUFA and PUFA as well as ω3 and ω6 FA with few differences between taxon groups in total SAFA, PUFA, and MUFA (Fig. 3). The main FAs identified (based on the mean per taxon group) were 16:0, 16:1ω7, 18:1ω7, 18:0, 18:1ω9, 20:1ω9, 20:1ω7, 20:4ω6, 20:5ω3 (EPA), 22:1ω11, 22:1ω7, 22:4ω6, 22:5ω3, and 22:6ω3 (DHA) (Fig. 4). The dominant FAs within each taxon group and species are presented in electronic supplementary material (ESM Tables S1, S2).
DHA/EPA and 18:1ω9/18:1ω7 ratios are often used to characterize level of carnivory (e.g., Graeve et al. 1997; Dalsgaard et al. 2003). The highest values of DHA/EPA were recorded in gorgonians (0.53) and soft corals (0.42) followed by a tendency for sea pens (0.27) to be more carnivorous. In contrast, ratios suggest a low level of carnivory in stony-cup corals (0.16) and black-thorny corals (0.15). Similarly, the lowest values for the ratio 18:1ω9/18:1ω7 were recorded in black-thorny corals. However, even though taxon group was significant in Kruskal–Wallis ANOVAs (p < 0.01), there were no significant differences among taxon groups within each subclass in DHA/EPA or 18:1ω9/18:1ω7.
The hexacoral subclass
The main FAs for black-thorny (Stauropathes arctica) corals were 22:5ω3 (DPA) and 20:1ω9 followed by EPA; for stony-cup corals (F. alabastrum), the main FAs were 16:0, 20:4ω6, DPA, 22:1ω11, and EPA (ESM Table S1). Interestingly, stony-cup corals have proportionally higher levels of 22:4ω6 compared to the other coral taxon groups. The first two PCO axes explained 89.5% of the variation (Fig. 5), and differences among taxon groups were significant (PERMANOVA: p = 0.0016, p-corrected MC = 0.0356, pseudo-F = 6.83) with no effect of depth or interaction between depth and taxon group. Pairwise tests demonstrated that S. arctica specimens were similar at 77.0% and F. alabastrum specimens at 83% and that the two species were dissimilar at about 32.7%. Flabellum alabastrum individuals were grouped together by a high proportion of 16:0, 22:5ω3, 20:4ω6, and EPA and S. arctica by 22:5ω3, 20:1ω9, EPA, and 18:1ω7. They differed mainly by a higher content of 20:4ω6 and lower content of 22:5ω3 and 20:1ω9 in F. alabastrum in comparison with S. arctica individuals (Fig. 5; ESM Table S1, S2).
Principal coordinates ordination (PCO) using Bray–Curtis similarity distances on untransformed fatty acid data (only FAs representing more than 1% of the total FA composition of at least one individual were selected) from every specimen of hexacoral taxon group collected. Only FA correlation vectors up to 0.3 have been superimposed on the figure to illustrate their contribution toward data grouping revealed by the PCO
The octocoral subclass
The dominant FA differed between orders: 20:4ω6 in gorgonians and soft corals (Alcyonacea), and EPA in sea pens (Pennatulacea) (Fig. 3). The first PCO axis (PCO1) explained 30.6% of variation in FAs between individuals, whereas PCO2 explained 17.9% and PCO3 explained 15.3% (Fig. 6a, b). The three axes accounted for more than 63% of the total variability. The PCO1 axis was mainly driven by the proportion of three fatty acids: 20:4ω6, 22:1ω9, and EPA, with 20:4ω6 being opposed to the other two FAs (Fig. 6a). Along this axis, there was segregation between two groups corresponding to the two orders (Pennatulacea and Alcyonacea); the first group included all the sea pens except Pennatula sp. (Pennatulacea), and the second group included all gorgonians and soft corals (Alcyonacea). Pennatula sp. was grouped with the second group of species because of its high content of 20:4ω6 and lower content of EPA. Along PCO2, a clear distinction appeared between Halipteris finmarchia and the other sea pens, and along PCO3, contents of 20:2ω6 and saturated FAs differentiated soft coral groups (Fig. 6b). Intraspecies variations were more pronounced on PCO2 while the PCO3 axis discriminated among soft coral species. Within the gorgonian group, the Paramuricea genus had high inter-individual variability in FA profiles (Fig. 6). The PERMANOVA confirmed PCO results, highlighting a significant effect of the taxon groups (i.e., soft corals, gorgonians, and sea pens) and, to a lesser extent, an effect of species (estimate source of variation) on FA composition, with no significant impact of depth or geographic locations (Table 4). It is important to state that the small number of samples at the species level limited the statistical power of the analysis, especially within the gorgonian group. Differences between all three octocoral taxon groups were significant (Table 5). SIMPER analyses on pairwise comparison of octocoral taxon groups are detailed below (only the differences in FA contributing to more than 10.0% of the dissimilarity):
Principal coordinates ordination (PCO) on Bray–Curtis similarity distances of untransformed fatty acid data (only FAs representing more than 1% of the total FA composition of at least one individual were selected) from every specimen of octocorals collected. Ordination of coral species along a PCO1/PCO2 and b PCO1/PCO3 axes. Only FA correlation vectors up to a 0.2 and b 0.5 have been superimposed on the figure to illustrate their contribution toward data grouping revealed by the PCO
-
Sea pens differed from soft corals and gorgonians with 32% dissimilarity and had a higher proportion of 20:5ω3 and a lower proportion of 20:4ω6.
-
Gorgonians differed from soft corals with only 25% dissimilarity. They had the same dominant FAs with little variation in proportions of 20:4ω6 (17% in soft corals and 16% in gorgonians) and 16:0 (10% in soft corals and 13% in gorgonians).
The main significant differences among species within taxon group were (not all differences are listed):
-
Within sea pens, Pennatula sp. differed significantly from four sea pen species but not from Funiculina quadrangularis (n = 1) and Distichoptilum gracile (n = 1). Pennatula sp. had lower EPA concentrations than the other sea pens and different dominant FAs (20:4ω6 and 20:1ω9). On the other hand, Anthoptilum grandiflorum and Halipteris finmarchica differed significantly: A. grandiflorum had lower EPA and 20:1ω7.
-
Within soft corals, Anthomastus sp. was significantly different from Duva florida and Nephtheidae sp. These species differed in EPA (lower in Anthomastus sp.) and 20:4ω6 content.
-
Within gorgonians, only the two species Acanthogorgia armata and Keratoisis grayi differed because of a low proportion of 20:1ω11 in A. armata, as well as lower EPA and slightly higher 20:4ω6.
Discussion
Considerable heterogeneity was observed in coral presence among trawl sampling stations and geographic locations. Like most organisms, deep-sea corals have preferred depth distributions and habitat characteristics. Food availability, temperature, salinity, substrate, currents, and slope contribute to distributional trends and level of abundance (e.g., Roberts et al. 2006; Wareham and Edinger 2007). Temperature was stable at all locations and is not likely to be a factor driving species distribution in our study. This study is based on coral lipid content to determine feeding types and behavior; no data on seasonal patterns (one sampling point in time) or suspended particulate and/or sedimentary organic matter were available.
Lipid classes
Lipid percentages in NL CWCs were lower than in shallow-water corals with values varying between 6 and 47% (Harland et al. 1993; Grottoli et al. 2004), as was also found by Hamoutene et al. (2008). The total lipid content measured in this study varied between 0.3 and 7.7%, while in Hamoutene et al. (2008), values ranged from 2.4 to 38.8% in soft corals and gorgonians. Taxa, species, and depth ranges considered in the two studies are different, likely explaining range differences.
In their study, Hamoutene et al. (2008) showed patterns in total lipid and lipid classes with depth, location, and species. Patterns with depth and location were attributed to lipid storage constraints due to a decrease in food availability. No effect of depth, species, or geographic location were evidenced here, but numbers of samples, depth ranges, and sampling areas were smaller in the present study (800–1400 m as opposed to 100–1300 m in Hamoutene et al. 2008). There are conflicting findings on the effect of depth and/or geographic locations on lipid classes in CWCs. Kiriakoulakis et al. (2005) found that the level of sterols in the scleractinian corals Lophelia pertusa and Madrepora oculata was highly variable (0.6–12.4% of total lipids) but independent of depth. Imbs et al. (2016) did not find significant differences in the lipid class content between specimens of the same species collected from different depths in the sea of Okhotsk (Russia).
NL CWC lipid classes were highly variable among taxon groups; phospholipids were the dominant lipid class in all taxon groups except the black-thorny coral species (S. arctica), which was dominated by steryl and wax esters. Monoalkyldiacyl glycerol and wax esters were the most represented classes in the coral samples investigated in Hamoutene et al. (2008) while Kiriakoulakis et al. (2005) showed that FAs and alcohols prevailed in two deep-sea stony-cup coral species: L. pertusa and M. oculata. In this study, black-thorny corals had higher levels of storage lipids and lower amounts of structural lipids than any other coral species. Physiology and morphological characteristics of S. arctica (stiff axes) may require less axial thickening (Sherwood and Edinger 2009) than gorgonians and less investment in new tissue/material production than other coral taxon groups. Moreover, black-thorny corals are known for their extreme longevity and very slow growth rates (e.g., Roark et al. 2009). Distinct morphological characteristics, longevity and slow growth rates, may influence lipid storage and metabolic demand and can explain the differences observed in lipid classes in S. arctica and highlight the taxonomic divergence of black-thorny coral from the other taxon groups. Oku et al. (2002) showed that decreased storage lipid (triacylglycerols and wax esters) in shallow-water Montipora digitata was associated with increased energy expended by proliferating cells for growth. In addition, authors suggest that as a strategy to respond to stress (Grottoli et al. 2004) or differential access to food (Hamoutene et al. 2008) for growth, corals adapt by changing the proportion of lipid classes but maintaining equivalent lipid levels.
Fatty acid composition
Trophic origins of fatty acids
Deep-sea corals collected for this study contained a high proportion of MUFA and PUFA as well as ω3 and ω6 FAs. Trophic markers, such as markers of diatoms (EPA, 18:1ω7), zooplankton (20:1ω9, 22:1ω9, 22:1ω11), dinoflagellates (DHA), and bacterial markers (see “Materials and methods”; Graeve et al. 1994; Dalsgaard et al. 2003), were measured in all the CWC samples studied. Other dominant FAs were 20:4ω6 and 22:5ω3 (docosapentaenoic acid: ω3DPA is an intermediary product between EPA and DHA; Kaur et al. 2011).
Knowledge of C20 PUFA metabolism in CWCs (i.e., azooxanthellate corals) is limited, but many animals are known to be restricted in the synthesis of 20:4ω6 and EPA (Monroig et al. 2013). Hence, a hypothesis about predominance of the trophic origin of these PUFAs in CWCs is reasonable (Imbs et al. 2016), and despite the potential removal of some PUFAs during passage through the water column (Budge and Parrish 1998; Parrish et al. 2005), it seems adequate to use the FA profiles of CWCs to distinguish diets and infer pelagic food sources.
Potential food sources of EPA in the context of deep-sea ecosystems are likely the result of sedimentation from surface waters since there is no primary productivity at depths > 100 m, or are contained in herbivorous zooplankton (Parrish et al. 2005; Dodds et al. 2009). Phytoplankton cells that reach deep seas are mostly dominated by diatoms with a small abundance of dinoflagellates (Agusti et al. 2015). If those results were applicable to the NL deep-sea environment, we could hypothesize that a proportion of the EPA measured in the NL CWCs might originate from diatoms. On the other hand, the lower availability of dinoflagellates (Agusti et al. 2015) might limit CWC access to DHA. However, the proportion of diatoms and dinoflagellates reaching the seafloor can vary seasonally with surface productivity and advection (Agusti et al. 2015; Parrish et al. 2005).
Known food sources of 20:4ω6 are mesozoobenthos and marine macrophytes (brown and red algae) inhabiting littoral and sublittoral zones, bacteria and foraminiferans (Graeve et al. 2002; Suhr et al. 2003; Würzberg et al. 2011a, b). Potential trophic transfer from macroalgae to CWCs is likely the result of long-distance advection of nutrients to offshore habitats, considering the distance of macrophyte-dominated habitats from the Flemish Cap area. Another source of 20:4ω6 could be micro-heterotrophs that are present in the sediment and potentially feed on ω6 PUFA-rich phytodetritus (Chang et al. 2012). DPA, on the other hand, has not been extensively studied because of the limited availability of the pure compound (Kaur et al. 2011). Corals without zooxanthellae, such as CWCs, have lower content of polyenoic C18 and 20:3ω6 acids, but higher levels of 18:lω9 and DPA acids than species with zooxanthellae (Latyshev et al. 1991). Further understanding of the role and origin of DPA needs to be acquired before discussing in detail the significance of higher values of DPA in some CWCs relative to others.
Fatty acid composition of NL cold-water corals
Overall, no effect of depth or geographic location was measured on FA composition for the hexacoral or octocoral subclasses, but effects of taxon groups and in some instances differences between species were significant. All the FAs cited above as characterizing a particular trophic behavior were measured in all specimens studied, with some differences in FA dominance/proportions patterns suggesting feeding preferences.
The hexacoral subclass
The restricted number of specimens within the two hexacoral groups studied here (i.e., three individuals for each species of S. arctica [black-thorny coral] and of Flabellum alabastrum [stony-cup coral]) limits the generalization of our findings to other hexacoral taxa. In S. arctica, DPA was the dominant FA but information on the trophic role/origin of this FA is limited. After DPA, zooplankton markers (20:1ω9, 22:1ω9, 22:1ω11) had the second highest proportions, highlighting the importance of carnivory for this species. Other FA markers for diatoms (EPA or 18:1ω7; Dalsgaard et al. 2003) were also abundant and may originate from herbivorous zooplankton (Parrish et al. 2005; Dodds et al. 2009). Conversely, in F. alabastrum, similar proportions of EPA (diatoms), DPA, 22:4ω6, 22:5ω11 (zooplankton), and 20:4ω6 suggest that they prey on multiple food sources. In addition, the two hexacoral species are significantly different: F. alabastrum had a higher content of 20:4ω6 (phytodetritus, foraminifera) and a lower content of zooplankton markers, suggesting more omnivorous behavior compared to carnivorous behavior in S. arctica. Importance of carnivory is also expressed as a high level of PUFA/SAFA (Cripps and Atkinson 2000). However, it was not confirmed by other traditional trophic ratios such as DHA (dinoflagellate marker)/EPA (diatom) or 18:1ω9 (higher in carnivores)/18:1ω7 also used to determine trophic levels (Graeve et al. 1994, 1997; Dalsgaard et al. 2003). These two ratios could be inadequate for CWCs for the following reasons: (1) few dinoflagellates occur in the deep sea (Agusti et al. 2015), limiting the use of DHA as a trophic marker and (2) the origin of 18:1ω7 could be associated not only with phytoplankton but also with bacterial markers of degrading organic matter settling from the surface (Dalsgaard et al. 2003). Likewise, the low level of carnivory inferred using those ratios contradicts the findings of other authors for F. alabastrum. With large unitary polyps, F. alabastrum occupy higher trophic levels, suggesting the ability to catch larger prey (Buhl-Mortensen et al. 2007; Sherwood et al. 2008). Similarly, polyp sizes in S. arctica are relatively large (diametric polyp size ranges from 3.5 to 7 mm; Molodtsova 2006) compared to other species studied here except for stony-cup corals (Sherwood et al. 2008) and support the hypothesis of feeding on bigger prey. The applicability of DHA/EPA and 18:1ω9/18:1ω7 as trophic markers needs to be further verified by determining composition of deepwater food sources.
The octocoral subclass
Despite high variability among and within species, significant differences were observed in FA profiles with a separation of the sea pen (Order Pennatulacea) species except Pennatula sp. from the Alcyonacea (soft corals and gorgonians). To a lesser extent, soft corals and gorgonians were also significantly different from each other: differences between orders were higher (> 32%) in dissimilarity percentages than differences between taxa within the Order Alcyonacea (25%).
With EPA being the dominant FA, sea pens feed mostly on diatoms derived from sinking organic matter or on herbivorous zooplankton prey, except Pennatula sp., which was found closer in composition to the soft corals and gorgonians (high 20:4ω6). Even though amounts of C16 PUFAs (primary producers) were low for all octocoral groups, the 16:1ω7/16:0 ratio, which is an additional indicator of diatoms (Dalsgaard et al. 2003), was higher in the sea pens (0.45, excluding Pennatula sp.) than the other taxon groups (0.30). Our results are consistent with Sherwood et al. (2008), who concluded that sea pens consumed the degraded fraction of particulate organic matter. Within the sea pens (other than Pennatula sp.), we also found significant interspecies differences between Anthoptilum grandiflorum and Halipteris finmarchica, principally due to variation in the two main FA proportions (EPA and 16:0). Interspecies differences within the sea pens have also been documented by other authors. Using stable isotopes on NL sea pens, Baillon et al. (2015) hypothesized that sea pen diets differed enough to preclude the pooling of species in trophic studies; they showed that A. grandiflorum, H. finmarchica, and P. aculeata had different carbon and nitrogen stable isotope signatures that could be explained primarily by their different polyp diameters and colony shapes, suggesting that they rely on slightly different food sources (varying proportions of phytodetritus and zooplankton).
Within the Order Alcyonacea (soft corals and gorgonians), the dominant FA was 20:4ω6, suggesting a higher reliance than sea pens on phytodetritus resulting from macrophytes and/or degradation of foraminifera. In addition, Baptista et al. (2012) found that 20:4ω6 was the most important FA in soft corals. Differences between soft coral and gorgonian groups were low (25%) and likely the result of variability in 20:4ω6 proportions. Within both soft corals and gorgonians, we also detected small interspecies differences suggesting some variation in feeding selection.
Tetracosapolyenoic acids
The analytical method used for FA analysis did not assess TPA concentrations as the analyses predate a series of articles on the importance of TPAs (24:5ω6 and 24:6ω3). TPAs are specific of octocorallia (sea pens, soft corals, and gorgonians) and absent from hexacorals (stony-cup corals and black-thorny corals); they are used as chemotaxonomic markers of polyp tissues, being independent of the presence of zooxanthellae and exoskeleton, and thus help in the discrimination of octocorals from other coral species (Imbs and Dautova 2008; Imbs et al. 2010, 2016; Baptista et al. 2012). They are key compounds in FA metabolism of octocorals and important for the explanation of sources and levels of C20–C22 PUFAs in soft corals. Information on TPA content may have modified the trends observed here in the calculation of the relative concentration of other FAs (Baptista et al. 2012) and required that statistical analyses for hexacorals and octocorals be completed separately. According to Sprecher’s pathway (Sprecher 2000), 24:5ω6 can be derived from DHA (22:6ω3) that was first elongated from arachidonic acid (20:4ω6), and 24:6ω3 from EPA. The low concentrations of DHA (< 5%) and docosapentaenoic (< 1%) in octocorallia in this study suggest that TPAs were not metabolized by Sprecher’s pathway, and thus if TPAs were present, they may originate from 20:4ω6 and EPA. The disregard of TPAs when calculating the relative concentration of FAs could result in an amplification of the percentage of such FAs when compared to species that may exhibit TPAs.
To conclude, lipid and FA profiles of NL CWCs differed among taxon groups. The sampling area was deep (> 800 m) and distribution of coral sparse and heterogeneous; no trend with location or depth was detected in FA or lipid profiles. However, the lipid and FA profiles differed significantly among taxon groups. Phospholipids were dominant in all taxon groups except for Stauropathes arctica (black-thorny coral), likely related to coral morphology and growth rates. Within the hexacoral subclass, the dominant FA in black-thorny corals (S. arctica) was DPA; they were also rich in FA markers of zooplankton suggesting a degree of carnivory. Conversely in stony-cup coral, individuals (F. alabastrum) appeared more omnivorous with no specific dominant FA. Differences in carnivory were not captured by traditional markers of trophic ecology (DHA/EPA, 18:1w9/18:1w7), suggesting a need for analyses of deepwater food sources and potential reconsideration of the value of these markers within deep-sea ecosystems. Within the octocoral subclass, differences between orders were greater than within taxon groups of the same order. Soft corals and gorgonians both had higher amounts of 20:4ω6, suggesting reliance on phytodetritus resulting from algae, macrophytes and/or foraminifera while sea pens likely consumed more diatoms derived food and/or zooplankton consuming diatoms with the exception of Pennatula sp.. The latter had FA profiles closer to the Order Alcyonacea, confirming differences already documented by authors between species within the sea pen group.
This study provides a snapshot of CWC trophic choices in the NL deep-sea environment. The limited food availability characterizing deep-sea ecosystems requires opportunist feeding. We observed differences in trophic behaviors mostly driven by taxonomy (differences in morphology), suggesting food selection and less opportunistic feeding than expected. More studies on the nature of food sources present in the deep sea in a wider range of depths are necessary to properly characterize trophic behaviors of deep-sea CWCs and better describe taxonomic differences.
References
Agusti S, González-Gordillo JI, Vaqué D, Estrada M, Cerezo MI, Salazar G, Gasol JM, Duarte CM (2015) Ubiquitous healthy diatoms in the deep-sea confirm deep carbon injection by the biological pump. Nat Commun 6:7608
Anderson MJ, Gorley RN, Clarke KR (2008) PEMANOVA+ for PRIMER. Guide to software and statistical methods. Primer-E Ltd., Plymouth, UK
Baillon S, Hamel J-F, Wareham VE, Mercier A (2014) Seasonality in reproduction of the deep-water pennatulacean coral Anthoptilum grandiflorum. Mar Biol 161:29–43
Baillon S, English M, Hamel JF, Mercier A (2015) Comparative biometry and isotopy of three dominant pennatulacean corals in the Northwest Atlantic. Acta Zool 97:475–493
Baker KD, Wareham VE, Snelgrove PV, Haedrich RL, Fifield DA, Edinger EN, Gilkinson KD (2012) Distributional patterns of deep-sea coral assemblages in three submarine canyons off Newfoundland, Canada. Mar Ecol Prog Ser 445:235–249
Baptista M, Lopes VM, Pimentel MS, Bandarra N, Narciso L, Marques A, Rosa R (2012) Temporal fatty acid dynamics of the octocoral Veretillum cynomorium. Comp Biochem Physiol B Biochem Mol Biol 161:178–187
Budge SM, Parrish CC (1998) Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland. II. Fatty acids. Org Geochem 29:1547–1559
Buhl-Mortensen L, Mortensen PB (2005) Distribution and diversity of species associated with deep-sea gorgonian corals off Atlantic Canada. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Netherlands, pp 849–879
Buhl-Mortensen L, Mortensen PB, Armsworthy S, Jackson D (2007) Field observations of Flabellum spp. and laboratory study of the behavior and respiration of Flabellum alabastrum. Bull Mar Sci 81:543–552
Buhl-Mortensen P, Buhl-Mortensen L, Purser A (2015a) Trophic ecology and habitat provision in cold-water coral ecosystems. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine animal forests: the ecology of benthic biodiversity hotspots. Springer, Netherlands, pp 1–26
Buhl-Mortensen L, Olafsdottir SH, Buhl-Mortensen P, Burgos JM, Ragnarsson SA (2015b) Distribution of nine cold-water coral species (Scleractinia and Gorgonacea) in the cold temperate North Atlantic: effects of bathymetry and hydrography. Hydrobiologia 759:39–61
Buhl-Mortensen L, Ellingsen KE, Buhl-Mortensen P, Skaar KL, Gonzalez-Mirelis G (2016) Trawling disturbance on megabenthos and sediment in the Barents Sea: chronic effects on density, diversity, and composition. ICES J Mar Sci 73:i98–i114
Chang KJL, Dunstan GA, Abell GC, Clementson LA, Blackburn SI, Nichols PD, Koutoulis A (2012) Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl Microbiol Biotechnol 93:2215–2231
Clarke KR, Gorley RN (2015) Primer. Primer-e Ltd., Plymouth, UK
Cripps GC, Atkinson A (2000) Fatty acid composition as an indicator of carnivory in Antarctic krill, Euphausia superba. Can J Fish Aquat Sci 57:31–37
Dalsgaard J, St John M, Kattner G, Dr Müller-Navarra, Hagen W (2003) Fatty acid trophic markers in the pelagic marine environment. Adv Mar Biol 46:225–340
Dodds L, Black K, Orr H, Roberts J (2009) Lipid biomarkers reveal geographical differences in food supply to the cold-water coral Lophelia pertusa (Scleractinia). Mar Ecol Prog Ser 397:113–124
Dullo WC, Flögel S, Rüggeberg A (2008) Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar Ecol Prog Ser 371:165–176
Edinger EN, Wareham VE, Haedrich RL (2007) Patterns of groundfish diversity and abundance in relation to deep-sea coral distributions in Newfoundland and Labrador waters. Bull Mar Sci 81:101–122
Gilkinson KD, Edinger E (2009) The ecology of deep-sea corals of Newfoundland and Labrador waters: biogeography, life history, biogeochemistry, and relation to fishes. Canadian Technical Report of Fisheries and Aquatic Science, vol. 2830, Fisheries and Oceans Canada, St Johns, NL
Graeve M, Kattner G, Hagen W (1994) Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: experimental evidence of trophic markers. J Exp Mar Bio Ecol 182:97–110
Graeve M, Kattner G, Piepenburg D (1997) Lipids in Arctic benthos: does the fatty acid and alcohol composition reflect feeding and trophic interactions? Polar Biol 18:53–61
Graeve M, Kattner G, Wiencke C, Karsten U (2002) Fatty acid composition of Arctic and Antarctic macroalgae: indicators for phylogenetic and trophic relationships. Mar Ecol Prog Ser 231:67–74
Grottoli A, Rodrigues L, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar Biol 145:621–631
Hamoutene D, Puestow T, Miller-Banoub J, Wareham V (2008) Main lipid classes in some species of deep-sea corals in the Newfoundland and Labrador region (Northwest Atlantic Ocean). Coral Reefs 27:237–246
Harland AD, Navarro JC, Spencer Davies P, Fixter LM (1993) Lipids of some Caribbean and Red Sea corals: total lipid, wax esters, triglycerides and fatty acids. Mar Biol 117:113–117
Huvenne V, Bett B, Masson D, Le Bas T, Wheeler A (2016) Effectiveness of a deep-sea cold-water coral marine protected area, following eight years of fisheries closure. Biol Conserv 200:60–69
Iken K, Brey T, Wand U, Voigt J, Junghans P (2001) Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): a stable isotope analysis. Prog Oceanogr 50:383–405
Imbs AB, Dautova TN (2008) Use of lipids for chemotaxonomy of octocorals (Cnidaria: Alcyonaria). Russ J Mar Biol 34:174–178
Imbs AB, Demidkova DA, Dautova TN (2016) Lipids and fatty acids of cold-water soft corals and hydrocorals: a comparison with tropical species and implications for coral nutrition. Mar Biol 163:1–12
Imbs AB, Latyshev NA, Dautova TN, Latypov YY (2010) Distribution of lipids and fatty acids in corals by their taxonomic position and presence of zooxanthellae. Mar Ecol Prog Ser 409:65–75
Jordán F, Scheuring I (2004) Network ecology: topological constraints on ecosystem dynamics. Phys Life Rev 1:139–172
Kaur G, Cameron-Smith D, Garg M, Sinclair AJ (2011) Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res 50:28–34
Kiriakoulakis K, Fisher E, Wolff GA, Freiwald A, Grehan A, Roberts JM (2005) Lipids and nitrogen isotopes of two deep-water corals from the north-east Atlantic: initial results and implications for their nutrition. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Netherlands, pp 715–729
Lacharité M, Metaxas A (2013) Early life history of deep-water gorgonian corals may limit their abundance. PLoS One 8:e65394
Latyshev N, Naumenko N, Svetashev V, Latypov YY (1991) Fatty acids of reef-building corals. Mar Ecol Prog Ser 76:295–301
Molodtsova TN (2006) Black corals (Antipatharia: Anthozoa: Cnidaria) of the north-eastern Atlantic. In: Mironov AN, Gebruk AV, Southward AJ (eds) Biogeography of the Atlantic seamounts. KMK Scientific Press, Moscow, pp 141–151
Monroig Ó, Tocher DR, Navarro JC (2013) Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar Drugs 11:3998–4018
Mortensen PB, Buhl-Mortensen L (2004) Distribution of deep-water gorgonian corals in relation to benthic habitat features in the Northeast Channel (Atlantic Canada). Mar Biol 144:1223–1238
Mortensen PB, Buhl-Mortensen L (2005) Morphology and growth of the deep-water gorgonians Primnoa resedaeformis and Paragorgia arborea. Mar Biol 147:775–788
Mortensen PB, Hovland T, Fosså JH, Furevik DM (2001) Distribution, abundance and size of Lophelia pertusa coral reefs in mid-Norway in relation to seabed characteristics. J Mar Biol Assoc UK 81:581–597
Mueller C, Larsson AI, Veuger B, Middelburg J, van Oevelen D (2014) Opportunistic feeding on various organic food sources by the cold-water coral Lophelia pertusa. Biogeosciences 11:123–133
Naumann MS, Orejas C, Wild C, Ferrier-Pagès C (2011) First evidence for zooplankton feeding sustaining key physiological processes in a scleractinian cold-water coral. J Exp Biol 214:3570–3576
Neves BdeM (2016) Growth in cold-water octocorals: rates, morphology and environmental controls. PhD thesis, Memorial University, St Johns, NL, p 433
Neves BdeM, Edinger E, Layne GD, Wareham VE (2015) Decadal longevity and slow growth rates in the deep-water sea pen Halipteris finmarchica (Sars, 1851) (Octocorallia: Pennatulacea): implications for vulnerability and recovery from anthropogenic disturbance. Hydrobiologia 759:147–170
Oku H, Yamashiro H, Onaga K, Iwasaki H, Takara K (2002) Lipid distribution in branching coral Montipora digitata. Fish Sci 68:517–522
Parrish CC (1987) Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by latroscan flame ionization detection. Can J Fish Aquat Sci 44:722–731
Parrish CC (1999) Determination of total lipid, lipid classes, and fatty acids in aquatic samples. In: Arts MT, Wainmann BC (eds) Lipids in freshwater ecosystems. Springer, New York, pp 4–20
Parrish CC, Thompson RJ, Deibel D (2005) Lipid classes and fatty acids in plankton and settling matter during the spring bloom in a cold ocean coastal environment. Mar Ecol Prog Ser 286:57–68
Radice VZ, Quattrini AM, Wareham VE, Edinger EN, Cordes EE (2016) Vertical water mass structure in the North Atlantic influences the bathymetric distribution of species in the deep-sea coral genus Paramuricea. Deep Sea Res Part 1 Oceanogr Res Pap 116:253–263
Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA (2009) Extreme longevity in proteinaceous deep-sea corals. Proc Natl Acad Sci U S A 106:5204–5208
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Sherwood OA, Edinger EN (2009) Ages and growth rates of some deep-sea gorgonian and antipatharian corals of Newfoundland and Labrador. Can J Fish Aquat Sci 66:142–152
Sherwood OA, Jamieson RE, Edinger EN, Wareham VE (2008) Stable C and N isotopic composition of cold-water corals from the Newfoundland and Labrador continental slope: Examination of trophic, depth and spatial effects. Deep Sea Res Part 1 Oceanogr Res Pap 55:1392–1402
Sprecher H (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta 1486:219–231
Suhr SB, Pond DW, Gooday AJ, Smith CR (2003) Selective feeding by benthic foraminifera on phytodetritus on the western Antarctic Peninsula shelf: evidence from fatty acid biomarker analysis. Mar Ecol Prog Ser 262:153–162
Wareham VE, Edinger EN (2007) Distribution of deep-sea corals in the Newfoundland and Labrador region, Northwest Atlantic Ocean. Bull Mar Sci 81:289–313
Würzberg L, Peters J, Brandt A (2011a) Fatty acid patterns of Southern Ocean shelf and deep-sea peracarid crustaceans and a possible food source, foraminiferans. Deep Sea Res Part 2 Top Stud Oceanogr 58:2027–2035
Würzberg L, Peters J, Schüller M, Brandt A (2011b) Diet insights of deep-sea polychaetes derived from fatty acid analyses. Deep Sea Res Part 2 Top Stud Oceanogr 58:153–162
Zedel L, Fowler WA (2009) Comparison of boundary layer current profiles in locations with and without corals in Haddock Channels, Southwest Grand Banks. In: Gilkinson KD, Edinger E (2009) The ecology of deep-sea corals of Newfoundland and Labrador waters: biogeography, life history, biogeochemistry, and relation to fishes. Canadian Technical Report of Fisheries and Aquatic Science, vol. 2830, Fisheries and Oceans Canada, St Johns, NL, pp 97–104
Acknowledgements
This project was funded by International Governance Program fund. We would like to thank Jeannette Wells from CREAIT laboratory for lipid classes and fatty acid analyses and Barbara Neves for completing the map.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Mark J. A. Vermeij
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salvo, F., Hamoutene, D., Hayes, V.E.W. et al. Investigation of trophic ecology in Newfoundland cold-water deep-sea corals using lipid class and fatty acid analyses. Coral Reefs 37, 157–171 (2018). https://doi.org/10.1007/s00338-017-1644-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-017-1644-z