Abstract
Objectives
To predict tumor grade (G1 vs. G2/3), presence of distant metastasis (M+), metastatic lymph nodes (N+), and microvascular invasion (VI) of pancreatic neuroendocrine neoplasms (PanNEN) based on preoperative CT radiomic features (RFs), by applying a machine learning approach aimed to limit overfit.
Methods
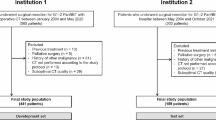
This retrospective study included 101 patients who underwent surgery for PanNEN; the entire population was split into training (n = 70) and validation cohort (n = 31). Based on a previously validated methodology, after tumor segmentation on contrast-enhanced CT, RFs were extracted from unenhanced CT images. In addition, conventional radiological and clinical features were combined with RFs into multivariate logistic regression models using minimum redundancy and a bootstrap-based machine learning approach. For each endpoint, models were trained and validated including only RFs (RF_model), and both (radiomic and clinicoradiological) features (COMB_model).
Results
Twenty-five patients had G2/G3 tumor, 37 N+, and 14 M+ and 38 were shown to have VI. From a total of 182 RFs initially extracted, few independent radiomic and clinicoradiological features were identified. For M+ and G, the resulting models showed moderate to high performances: areas under the curve (AUC) for training/validation cohorts were 0.85/0.77 (RF_model) and 0.81/0.81 (COMB_model) for M+ and 0.67/0.72 and 0.68/0.70 for G. Concerning N+ and VI, only the COMB_model could be built, with poorer performance for N+ (AUC = 0.72/0.61) compared to VI (0.82/0.75). For all endpoints, the negative predictive value was good (≥ 0.75).
Conclusions
Combining few radiomic and clinicoradiological features resulted in presurgical prediction of histological characteristics of PanNENs. Despite the limited risk of overfit, external validations are warranted.
Key Points
• Histology is the only tool currently available allowing characterization of PanNEN biological characteristics important for prognostic assessment; significant limitations to this approach exist.
• Based upon preoperative contrast-enhanced CT images, a machine learning approach optimized to favor models’ generalizability was successfully applied to train predictive models for tumor grading (G1 vs. G2/3), microvascular invasion, metastatic lymph nodes, and distant metastatic spread.
• Moderate to high discriminative models (AUC: 0.67–0.85) based on few parameters (≤ 3) showing high negative predictive value (0.75–0.98) were generated and then successfully validated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine neoplasms (PanNENs) are relatively rare tumors, representing approximately 2% of pancreatic neoplasms [1, 2], though with increasing incidence, mainly due to their more frequent incidental diagnosis. Importantly, PanNENs comprise a wide range of entities, both histologically and macroscopically [2, 3]. In this respect, histology is the only validated methodology currently available that allows tumor grading, distinguishing low grade (G1) from more aggressive (G2/G3) neoplasms, mainly based on Ki67 proliferative index, and to assess other biological characteristics important for prognostic assessment [3,4,5]. However, endoscopic ultrasound-guided fine-needle aspiration biopsy (FNAB), an invasive procedure constrained by potential risks, has limited accuracy [6, 7], so that reliable histology may be obtained only after surgery.
On the other hand, macroscopic imaging features have been associated with the outcome of PanNENs, specifically size of the lesion, presence of necrosis, nodal involvement, or distant metastases [8,9,10], but these cannot be considered sufficiently reliable to drive the therapeutic decision [11, 12]
Recently, radiomics emerged as a promising tool in characterizing tumors [13]: it consists in extracting quantitative data from medical images, aiming to build models predicting histological characteristics and/or clinical outcomes [14,15,16,17]. Few studies explored the potential of radiomics in the setting of PanNENs, often showing contrasting results since limited by small sample size issues and potentially biased methodologies [18,19,20,21,22,23,24,25,26].
The aim of the current study, based on a sufficiently large population, was to assess the association between PanNEN histological characteristics and computed tomography (CT) radiomic features (RFs), by applying a machine learning approach optimized to avoid/limit the risk of overfit. Clinicoradiological features were also tested.
Materials and methods
Patients’ cohort
This is a monocentric, retrospective, observational study including patients who underwent upfront surgery for PanNEN at San Raffaele Scientific Institute (Milan, Italy) from January 2015 to December 2021; data were collected within the context of an Ethics Committee–approved study in patients who had signed an institutional procedure–specific informed consent. From a prospectively acquired database, adult patients without visible distant metastases who underwent adequate quality abdominal CT imaging within 1 month before index surgery were enrolled. The resulting population (n = 101) was then randomly split into training (n = 70) and validation cohorts (n = 31) according to the second level of the TRIPOD guidelines for the validation of predictive models in oncology [27].
The histological endpoints considered, as defined by postoperative histological specimens, were tumor grade (G) (G1 vs. G2/3), the presence of distant metastases (M+), metastatic lymph nodes (N+), and microvascular invasion (VI).
Clinical variables
Demographic variables were retrospectively reviewed from an electronic database.
Radiological variables and radiomic features
In patients who underwent multiple preoperative CT scan, the last examination closest to the date of surgery was used for review.
CT protocol
Three different CT scanners were used (Philips Brilliance [64 slices]; Toshiba Aquilion [16 slices]; Siemens Somatom Definition Flash [128 slices]). Apart from beam collimation, which relies upon the number of detector rows, scanning parameters were as follows: 0.938–0.983 (pitch), 100–120 kVp (tube voltage), 138–534 mAs (tube current), 2–3 mm (slice thickness), 1 mm (gap). The matrix size of reconstructed CT images was 512 × 512 pixels, and pixel/voxel size ranges were 0.598–0.888 mm and 0.819–2.206 mm3, respectively. CT protocol included administration of intravenous non-ionic iodine contrast medium (Iopromide, Ultravist 370 mg iodine/mL (Bayer HealthCare), 120 mL at a rate of 4 mL/s) and consisted of a multiphase acquisition (unenhanced, arterial and portal venous axial scans of the abdomen).
The variability of the features between the three scanners was tested through the Mann-Whitney test, finding no significant inter-scanner variations: this result, together with the careful application of the abovementioned acquisition protocols, should limit any potential bias due to feature repeatability.
Conventional imaging parameters
CT findings were selected for analysis by two radiologists and two senior consultant pancreatic surgeons on the basis of their clinical experience; variables previously described in the literature were also considered. The selected CT findings included the followings: (i) presence of necrosis, defined as tumoral tissue that did not enhance in the arterial phase and PVP [28, 29], (ii) presence of cystic/liquid component, (iii) pancreatic parenchyma atrophy, defined as a significant reduction in the volume of the gland, (iv) macroscopic arterial/venous infiltration, and (v) contiguous organs invasion.
Delineation
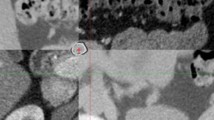
The robustness of CT RFs against interobserver contouring variability was already assessed by our group [30, 31], showing high intra-correlation coefficient (ICC) for all features. As previously reported, since PanNENs show the greatest conspicuity on arterial phase images, it was established to contour tumors in this phase only. Then, a rigid registration, based upon a box ROI (region of interest) surrounding the pancreas and including the nearest structures, was performed between arterial and unenhanced CT images: a mutual information algorithm was applied followed by manual fine-tuning, by visually overlaying images with and without contrast. The contours were transferred on the co-registered unenhanced CT images, and possibly adjusted for minor local anatomical discrepancies due to respiration and organ motion between different phases (Fig. 1). The contouring and rigid registration of images were performed using the Eclipse System (Varian Medical Systems Inc.).
The tumor is delineated on contrast-enhanced CT, arterial phase (on the left), and then projected onto the corresponding unenhanced image (at the middle) using a registration based on a box ROI surrounding the pancreas: if necessary, the contours were then manually adjusted to correct small anatomical discrepancies in the resulting co-registered image (on the right)
The choice of unenhanced images for radiomic features extraction was due to the fact that contrast medium administration could modify tissue heterogeneity with respect to the intrinsic inter-patient variability of contrast administration.
Radiomic feature extraction
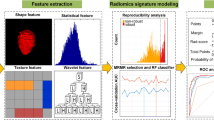
All images were resampled at cubic voxels of 0.78 × 0.78 × 0.78 mm3 with bilinear interpolation using an automatic workflow expressly developed in commercially available software (MIM Software Inc., version 6.5.5). This procedure was implemented to reduce directional bias, according to the specific recommendation of the International Biomarker Standardization Initiative (IBSI) [32, 33]. Image rebinning was also necessary, not only to speed up the process of RF extraction, but also to limit noise: we chose 64 bins, as reported in the literature [34, 35]. DICOM files were then imported into MATLAB using the Computational Environment for Radiological Research [36, 37]. One hundred eighty-two RFs of first and higher order were extracted using SPAARC Pipeline for Automated Analysis and Radiomics Computing (IBSI complying) [32,33,34,35].
Statistical analysis
As previously stated, the original population was randomly split into training (n = 70) and validation (n = 31) cohorts.
RF redundancy limitation
To limit the risk of redundancy, we first applied, for each endpoint, a correlation-based filter: in short, a Spearman coefficient threshold equal to 0.80 was fixed to select redundant (≥ 0.80) and independent RFs (< 0.80). The independent variables were retained for further analysis; among redundant variables, the ones with the best p value resulting from univariate logistic regression were selected.
Model development
To assess the best combination of the previously selected clinical, radiological, and radiomic variables, a machine learning bootstrap-based method was used. In short, the training set of data was bootstrapped 1000 times and a backward multivariate logistic regression was run for each sample including two (for G and M+) or three (for N+ and VI) variables according to the number of events for each endpoint [38,39,40,41]. Three models were then developed for each endpoint: a “conventional” radiological model, a strictly radiomic model, and a combined model considering information from radiomic, conventional radiologic, and clinical variables. Finally, a prognostic index was derived according to the following formula:PI = 1/(1 + EXP(−(Σbi ∗ xi)) where bi are the MLR coefficients associated with the covariates xi.
Model validation
To assess the ability of the prognostic index in stratifying patients according to the histological endpoints, the coefficients of the prognostic index were directly entered into a new univariate logistic regression considering data from validation set. For each model, a p < 0.05 was required for considering it validated.
Analyses were performed using homemade Matlab codes.
Results
Patients’ characteristics are summarized in Table 1. Twenty-five patients (24.7%) had G2/G3 tumor (specifically, three patients only [2.9%] had undifferentiated neoplasm), 37 (36.6%) were shown to have nodal involvement, 14 (13.8%) suffered from distant metastases (mostly in the liver), and 38 (37.6%) had microvascular spread of the disease at pathological specimen. Between training and validation cohorts, no significant differences were found when considering both radiological and clinical variables, nor pathological data. Median tumor volume was 6 cc [1.3–19.9].
The combination of two variables only (one radiomic and one clinicoradiological feature) resulted in good prediction of the risk of M+ and G with AUC = 0.85 and 0.67, respectively; these results were confirmed in the validation cohort (AUC = 0.77 and 0.72, respectively). The models predicting the risk of IV and N+ (both comprising two radiomic and one clinicoradiological feature) showed AUC = 0.82 and 0.72, respectively, in the training set; these results were confirmed in the validation cohort (0.75 and 0.62, respectively). A pure RF_model could be generated only when considering M+ and G as endpoints, with similar performances of the corresponding COMB_models (AUC = 0.81 and 0.68, respectively, in the training cohort; AUC = 0.81 and 0.70 in the validation set). A pure “conventional” radiological model failed to be confirmed in the validation set for all endpoints with the only exception of microvascular invasion.
Negative predictive values resulted moderate to high for all validated models for the different endpoints, ranging between 77.8% (G+ COMB_model) and 97% (M+ RF_model).
The performances of the models for each endpoint are reported in Table 2 and summarized in Fig. 2. In Fig. 3, ROC curves of models are shown.
Discussion
Few studies explored the potential of radiomics in the setting of pancreatic neuroendocrine neoplasms (PanNENs), often showing contrasting results since limited by small sample size issues and potentially biased methodologies [18,19,20,21,22,23,24,25,26]. In the present study, we applied, in a relatively large cohort of patients, a machine learning approach optimized to limit/avoid the risk of overfit; in doing so, we sought to develop and validate preoperative models (including a maximum of three variables) based upon CT images to predict tumor grade (G1 vs. G2/3), presence of distant metastasis, metastatic lymph nodes, and microvascular invasion at pathological specimen.
The vast majority of the present literature focuses on tumor grade prediction [10, 18,19,20, 22, 23, 42,43,44,45], which is indeed a crucial cornerstone for treatment planning being a surrogate for biological aggressiveness. In this respect, an image-based biomarker able to accurately predict grading could be of great impact, especially when considering patients with small lesions (< 2 cm) which are generally thought to correspond to well-differentiated (G1) tumors to be conservatively managed [11, 46]. Nevertheless, these small tumors do sometimes reveal aggressive biological behavior and need a more aggressive approach [2, 47]. Importantly, endoscopic ultrasound-guided fine-needle aspiration biopsy is not always reliable in determining tumor grading of small tumors [6, 7, 48]. A possible solution could come from radiomics, since radiomic features (RFs), being derived from the whole volume of the lesion, are paradoxically more representative of the heterogeneity of the entire lesion than histology itself (from bioptic samples). In the present study, we found that coupling one robust RF (Morphology_areaDensity_aabb) with one conventional radiological finding (tumor necrosis) resulted in good negative predictive value (77.8%) and area under the curve (0.72) for grade prediction.
These results corroborated our previous findings in a pilot study on 39 patients [26] and highlight the importance of a rigorous radiomic workflow based upon (i) a strict selection of few robust RFs and (ii) availability of an independent validation cohort to reduce any risk of overfitting. Other studies tried to avoid this issue by restricting the number of variables [20], or splitting the cohort into training and validation sets [18, 19, 43]; however, with this second approach only, if no attempt is made to achieve optimally robust models in the training group, the performances may significantly reduce in course of validation, and the proposed findings may lack interpretability. In this respect, our findings are in good agreement with the results obtained by Bian et al [20] in a group of 102 PanNEN patients applying LASSO for tumor grade prediction.
Apart from G, to our knowledge, few other studies explored the value of radiomics to predict other histological characteristics of PanNENs [25, 49].
Accurate preoperative N staging represents indeed a major cornerstone in the treatment algorithm of PanNENs, since patients with different N stages have different prognosis and may need a different extent of lymphadenectomy or neoadjuvant treatment [50]; specifically, the number of positive lymph nodes is accurate in predicting recurrence for PanNENs after surgery [51]. A recent report by Mapelli and colleagues [49] found that second-order RFs extracted from T2 MR sequences have good predictive performance (AUC = 0.992) with respect to lymph nodal involvement. Our model has lower accuracy, but with the clear advantage of relying upon RFs derived from CT images, which are, actually, the standard of care when dealing with non-invasive staging of PanNENs.
Our models also show good results with respect to prediction of distant metastases and microvascular invasion, providing a further insight into disease biological behavior; these findings are indeed in agreement with previous literature [25].
Interestingly, the degree of clinical/biological interpretability of the RFs finally retained in each validated model is promising, being related to tumor irregular shape (as frequently observed in the setting of pancreatic adenocarcinoma) [52] and/or HU value heterogeneity. With regard to this last point, our group recently found an intriguing explanation connecting, in nonfunctioning PanNENs, microvessel density, radiological appearance in terms of HU values, and biological behavior [53]: in short, low microvessel density (assessed by CD34 staining), corresponding to hypoenhancement in arterial phase, has been found to be associated with pathological features of aggressiveness.
Finally, to objectify the incremental value of radiomics with respect to radiologists’ subjective assessment, we demonstrated that a model based upon, specifically, conventional radiological parameters failed to be confirmed in the validation set.
The present study has several limitations, the most important ones being its retrospective nature and the relatively small number of events observed. External validation is also warranted and already planned. Furthermore, our model has been thought not for a standalone usage but rather to be embedded in the multidisciplinary assessment of the patient.
In conclusion, despite the abovementioned limitations, the combination of few radiomic and clinicoradiological features by means of robust methodology that avoid/limit the risk of overfit resulted in robust presurgical prediction of histological characteristics of PanNENs, potentially providing a tool for patients’ personalized management, once more extensive external validation will be accomplished.
Abbreviations
- CT:
-
Computer tomography
- FNAB:
-
Fine-needle aspiration biopsy
- G:
-
Tumor grade
- NEN:
-
Neuroendocrine neoplasm
- PanNEN:
-
Pancreatic neuroendocrine neoplasm
- PVP:
-
Portal venous phase
- RF:
-
Radiomic feature
- VI:
-
Vascular invasion
References
Taskin OC, Clarke CN, Erkan M et al (2020) Pancreatic neuroendocrine neoplasms: current state and ongoing controversies on terminology, classification and prognostication. J Gastrointest Oncol. https://doi.org/10.21037/jgo.2020.03.07
Partelli S, Bartsch DK, Capdevila J et al (2017) ENETS consensus guidelines for the standards of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. https://doi.org/10.1159/000464292
Klimstra DS (2016) Pathologic classification of neuroendocrine neoplasms. Hematol Oncol Clin North Am. https://doi.org/10.1016/j.hoc.2015.08.005
Pasaoglu E, Dursun N, Ozyalvacli G et al (2015) Comparison of World Health Organization 2000/2004 and World Health Organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol 19: https://doi.org/10.1016/j.anndiagpath.2015.01.001
Kim JY, Hong SM, Ro JY (2017) Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. https://doi.org/10.1016/j.anndiagpath.2017.04.005
Rebours V, Cordova J, Couvelard A, et al (2015) Can pancreatic neuroendocrine tumour biopsy accurately determine pathological characteristics? Dig Liver Dis 47: https://doi.org/10.1016/j.dld.2015.06.005
Fujimori N, Osoegawa T, Lee L et al (2016) Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand J Gastroenterol 51: https://doi.org/10.3109/00365521.2015.1083050
Pavel M, De Herder WW (2017) ENETS consensus guidelines for the standards of care in neuroendocrine tumors. Neuroendocrinology. https://doi.org/10.1159/000457957
Tamm EP, Bhosale P, Lee JH, Rohren EM (2016) State-of-the-art imaging of pancreatic neuroendocrine tumors. Surg Oncol Clin N Am. https://doi.org/10.1016/j.soc.2015.11.007
Choi TW, Kim JH, Yu MH et al (2018) Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol 59: https://doi.org/10.1177/0284185117725367
Partelli S, Cirocchi R, Crippa S et al (2017) Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br J Surg. https://doi.org/10.1002/bjs.10312
Rinke A, Gress TM (2017) Neuroendocrine cancer, therapeutic strategies in G3 cancers. Digestion 95: https://doi.org/10.1159/000454761
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology. https://doi.org/10.1148/radiol.2015151169
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48: https://doi.org/10.1016/j.ejca.2011.11.036
Wilson R, Devaraj A (2017) Radiomics of pulmonary nodules and lung cancer. Transl Lung Cancer Res. https://doi.org/10.21037/tlcr.2017.01.04
Altazi BA, Fernandez DC, Zhang GG et al (2018) Investigating multi-radiomic models for enhancing prediction power of cervical cancer treatment outcomes. Phys Med 46: https://doi.org/10.1016/j.ejmp.2017.10.009
Giganti F, Marra P, Ambrosi A et al (2017) Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: comparison with tumour regression grade at final histology. Eur J Radiol 90: https://doi.org/10.1016/j.ejrad.2017.02.043
Gu D, Hu Y, Ding H et al (2019) CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur Radiol 29: https://doi.org/10.1007/s00330-019-06176-x
Liang W, Yang P, Huang R et al (2019) A combined nomogram model to preoperatively predict histologic grade in pancreatic neuroendocrine tumors. Clin Cancer Res 25: https://doi.org/10.1158/1078-0432.CCR-18-1305
Bian Y, Zhao Z, Jiang H et al (2020) Noncontrast radiomics approach for predicting grades of nonfunctional pancreatic neuroendocrine tumors. J Magn Reson Imaging 52: https://doi.org/10.1002/jmri.27176
Shi YJ, Zhu HT, Liu YL et al (2020) Radiomics analysis based on diffusion kurtosis imaging and T2 weighted imaging for differentiation of pancreatic neuroendocrine tumors from solid pseudopapillary tumors. Front Oncol 10: https://doi.org/10.3389/fonc.2020.01624
Izumiya M (2020) Editorial for “Noncontrast radiomics approach for predicting grades of nonfunctional pancreatic neuroendocrine tumors.” J Magn Reson Imaging. https://doi.org/10.1002/jmri.27280
Bian Y, Jiang H, Ma C et al (2020) CT-based radiomics score for distinguishing between grade 1 and grade 2 nonfunctioning pancreatic neuroendocrine tumors. AJR Am J Roentgenol 215: https://doi.org/10.2214/AJR.19.22123
McGovern JM, Singhi AD, Borhani AA et al (2018) CT radiogenomic characterization of the alternative lengthening of telomeres phenotype in pancreatic neuroendocrine tumors. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.17.19490
Kulali F, Semiz-Oysu A, Demir M et al (2018) Role of diffusion-weighted MR imaging in predicting the grade of nonfunctional pancreatic neuroendocrine tumors. Diagn Interv Imaging 99: https://doi.org/10.1016/j.diii.2017.10.012
Benedetti G, Mori M, Panzeri MM et al (2021) CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol Med. https://doi.org/10.1007/s11547-021-01333-z
Collins GS, Reitsma JB, Altman DG, Moons KGM (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. Eur Urol 67: https://doi.org/10.1016/j.eururo.2014.11.025
Faivre S, Zappa M, Vilgrain V et al (2011) Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res 17: https://doi.org/10.1158/1078-0432.CCR-10-1708
Kim DW, Lee SS, Kim SO et al (2020) Estimating recurrence after upfront surgery in patients with resectable pancreatic ductal adenocarcinoma by using pancreatic CT: development and validation of a risk score. Radiology. https://doi.org/10.1148/radiol.2020200281
Loi S, Mori M, Benedetti G et al (2020) Robustness of CT radiomic features against image discretization and interpolation in characterizing pancreatic neuroendocrine neoplasms. Phys Med. https://doi.org/10.1016/j.ejmp.2020.06.025
Mori M, Benedetti G, Partelli S et al (2019) Ct radiomic features of pancreatic neuroendocrine neoplasms (PanNEN) are robust against delineation uncertainty. Phys Med. https://doi.org/10.1016/j.ejmp.2018.12.005
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295: https://doi.org/10.1148/radiol.2020191145
Spadarella G, Stanzione A, Akinci D'Antonoli T et al (2022) Systematic review of the radiomics quality score applications: an EuSoMII Radiomics Auditing Group Initiative. Eur Radiol. https://doi.org/10.1007/s00330-022-09187-3
Desseroit MC, Tixier F, Weber WA, et al (2017) Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med 58: https://doi.org/10.2967/jnumed.116.180919
Mori M, Palumbo D, De Cobelli F, Fiorino C (2022) Does radiomics play a role in the diagnosis, staging and re-staging of gastroesophageal junction adenocarcinoma? Updates Surg. https://doi.org/10.1007/s13304-022-01377-4
Deasy JO, Blanco AI, Clark VH (2003) CERR: a computational environment for radiotherapy research. Med Phys 30: https://doi.org/10.1118/1.1568978
Apte AP, Iyer A, Crispin-Ortuzar M et al (2018) Technical note: extension of CERR for computational radiomics: a comprehensive MATLAB platform for reproducible radiomics research. Med Phys 45: https://doi.org/10.1002/mp.13046
Peduzzi P, Concato J, Kemper E et al (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49: https://doi.org/10.1016/S0895-4356(96)00236-3
Neeman T (2009) Clinical prediction models: a practical approach to development, validation, and updating by Ewout W. Steyerberg. Int Stat Rev 77: https://doi.org/10.1111/j.1751-5823.2009.00085_22.x
Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol 165: https://doi.org/10.1093/aje/kwk052
Mori M, Passoni P, Incerti E et al (2020) Training and validation of a robust PET radiomic-based index to predict distant-relapse-free-survival after radio-chemotherapy for locally advanced pancreatic cancer. Radiother Oncol 153: https://doi.org/10.1016/j.radonc.2020.07.003
Canellas R, Burk KS, Parakh A, Sahani DV (2018) Prediction of pancreatic neuroendocrine tumor grade based on CT features and texture analysis. AJR Am J Roentgenol 210: https://doi.org/10.2214/AJR.17.18417
Zhao Z, Bian Y, Jiang H et al (2020) CT-radiomic approach to predict G1/2 nonfunctional pancreatic neuroendocrine tumor. Acad Radiol 27: https://doi.org/10.1016/j.acra.2020.01.002
Karmazanovsky G, Gruzdev I, Tikhonova V, Kondratyev E, Revishvili A et al (2021) Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol Med. https://doi.org/10.1007/s11547-021-01405-0
Bezzi C, Mapelli P, Presotto L et al (2021) Radiomics in pancreatic neuroendocrine tumors: methodological issues and clinical significance. Eur J Nucl Med Mol Imaging 48(12):4002–4015. https://doi.org/10.1007/s00259-021-05338-8
Smith JK, Ng SC, Hill JS et al (2010) Complications after pancreatectomy for neuroendocrine tumors: a national study. J Surg Res 163: https://doi.org/10.1016/j.jss.2010.04.017
Yang G, Ji M, Chen J et al (2017) Surgery management for sporadic small (≤ 2 cm), non-functioning pancreatic neuroendocrine tumors: a consensus statement by the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int J Oncol 50: https://doi.org/10.3892/ijo.2016.3826
Falconi M, Eriksson B, Kaltsas G et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. https://doi.org/10.1159/000443171
Mapelli P, Bezzi C, Palumbo D et al (2022) 68Ga-DOTATOC PET/MR imaging and radiomic parameters in predicting histopathological prognostic factors in patients with pancreatic neuroendocrine well-differentiated tumours. Eur J Nucl Med Mol Imaging 49: https://doi.org/10.1007/s00259-022-05677-0
Guarneri G, De Mestier L, Landoni L et al (2021) Prognostic role of examined and positive lymph nodes after distal pancreatectomy for non-functioning neuroendocrine neoplasms. Neuroendocrinology 111: https://doi.org/10.1159/000509709
Partelli S, Muffatti F, Andreasi V et al (2022) A single-center prospective observational study investigating the accuracy of preoperative diagnostic procedures in the assessment of lymph node metastases in nonfunctioning pancreatic neuroendocrine tumors. Ann Surg 276(5):921–928. https://doi.org/10.1097/SLA.0000000000005615
Palumbo D, Mori M, Prato F et al (2021) Prediction of early distant recurrence in upfront resectable pancreatic adenocarcinoma: a multidisciplinary, machine learning-based approach. Cancers (Basel) 13(19):4938. https://doi.org/10.3390/cancers13194938
Battistella A, Partelli S, Andreasi V et al (2022) Preoperative assessment of microvessel density in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). Surgery. https://doi.org/10.1016/j.surg.2022.06.017
Acknowledgements
Dr. Martina Mori was funded by an AIRC grant (IG-23015). E. Spezi and P. Whybra (Cardiff University) are kindly acknowledged for their support in the implementation and use of SpAARC software. Claudio Fiorino is the scientific guarantor of the present study.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Claudio Fiorino.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Martina Mori) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mori, M., Palumbo, D., Muffatti, F. et al. Prediction of the characteristics of aggressiveness of pancreatic neuroendocrine neoplasms (PanNENs) based on CT radiomic features. Eur Radiol 33, 4412–4421 (2023). https://doi.org/10.1007/s00330-022-09351-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09351-9