Abstract
Objectives
Placenta accreta spectrum (PAS) can induce severe life-threatening obstetric hemorrhage. Herein, we conducted a Bayesian network meta-analysis of previous studies to evaluate the relative benefits of different prophylactic balloon occlusion (PBO) procedures.
Methods
PubMed, Embase, Cochrane Library, and Web of Science were searched from inception to July 2021. Blood loss volume, blood transfusion volume, and hysterectomy rate were regarded as the primary endpoints. The data were pooled using a Bayesian network and traditional pairwise meta-analysis.
Results
Fifty-nine articles with a total sample size of 5150 patients were included. Compared with no PBO (non-PBO) intervention, PBO of the abdominal aorta (PBOAA, mean difference(MD) − 1.02, 95% credible interval (CrI) − 1.4 to − 0.67), common iliac artery (PBOCIA, MD − 0.84; 95%CrI − 1.36 to − 0.06) and internal iliac artery (PBOIIA, MD − 0.42; 95%CrI − 0.72 to − 0.13) significantly lowered blood loss volume, with PBOAA being more effective than PBOIIA (MD − 0.60; 95%CrI − 1.05 to − 0.17). PBOAA and PBOIIA also significantly decreased blood loss volume (MD − 2.33; 95%CrI − 3.74 to − 0.94, MD − 1.57; 95%CrI − 2.77 to − 0.47 respectively) and hysterectomy rate (OR 0.31; 95%CrI 0.16 to 0.54, OR 0.53; 95%CrI 0.29 to 0.92 respectively). PBOAA has the highest probability of being more effective in reducing the blood loss volume, blood transfusion volume, and hysterectomy rate.
Conclusions
Performing PBOAA, PBOCIA, or PBOIIA in PAS patients is an effective way to minimize blood loss volume, while PBOAA and PBOIIA also reduce blood transfusion volume and hysterectomy rate. PBOAA is a notably more effective strategy to reduce blood loss volume than PBOIIA.
Key Points
• PBOAA, PBOCIA, and PBOIIA procedures can significantly reduce the blood loss volume compared to non-PBO intervention in PAS patients, of which PBOAA was more effective than the PBOIIA procedure.
• PBOAA and PBOIIA could significantly reduce the blood transfusion volume and hysterectomy rate in contrast to the non-PBO intervention in patients with PAS.
• According to our statistical treatment ranking, PBOAA was statistically superior in reducing blood transfusion volume, blood transfusion volume, and hysterectomy rate than other PBO procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Placenta accreta spectrum (PAS) is a generalized term for placenta abnormally and firmly adhered to the uterine wall, which is associated with significant maternal morbidity as well as mortality and is a leading cause of severe obstetric hemorrhage or even peripartum hysterectomy [1]. Depending on the invasiveness of the placental chorionic villi, there are three major pathological subtypes: placenta accreta, placenta increta, and placenta percreta. A history of previous cesarean section is considered a major risk factor for PAS. Due to the rising global cesarean section rate, the morbidity of PAS has also increased from 0.8/1000 deliveries in the 1980s to 3/1000 deliveries during recent years [2].
Existing research shows that PAS has the highest emergency hysterectomy rate among all risk factors of postpartum hemorrhage and 90% of PAS patients will require blood transfusions (with 40% of patients even requiring massive transfusion). The average blood loss volume in patients with placenta increta or percreta has been reported to be up to 3000 ml and even exceeding 5000 ml in around 20% of patients [3,4,5]. Given the severity of PAS, further research and new interventional techniques are warranted to better manage and reduce bleeding, blood transfusion, and hysterectomy rates.
In the last few decades, endovascular interventional management strategies for bleeding control have been on the rise, such as prophylactic balloon occlusion (PBO). PBO can reduce the distal pulse pressure in the occluded arteries and reduce blood perfusion to the uterus, minimizing intraoperative bleeding. Based on the position of the balloon occlusion, PBO can be classified into different subtypes: prophylactic balloon occlusion of the abdominal aorta (PBOAA), common iliac artery (PBOCIA), internal iliac artery (PBOIIA), and uterine artery (PBOUA). However, the efficacy of PBO remains controversial, and there is no clear consensus on which artery should be chosen for occlusion intervention. There are only a few meta-analyses in the existing literature, and all of them are pairwise designs that compare non-PBO to PBOAA or PBOIIA. To this date, there is no comprehensive network meta-analysis to compare these four kinds of PBO interventions simultaneously.
To that end, we employed a Bayesian network meta-analysis to investigate the effectiveness of different PBO procedures for controlling PAS-related hemorrhage. The primary endpoints included blood loss volume, blood transfusion volume, and incidence of hysterectomy. The secondary endpoints comprised of the timing of balloon occlusion, radiation doses, and PBO-related complications.
Materials and methods
Search strategy
The literature was retrieved from PubMed, Embase, Cochrane Library, and Web of Science by Mesh terms and free keywords from the time of inception to July 2021. The following Mesh terms and free keywords were used in literature search: “placenta accreta,” “placenta increta,” “placenta percreta,” “placental abnormalities,” “prophylactic,” “balloon,” “catheter,” “occlusion,” “abdominal aorta,” “iliac artery,” “uterine artery,” and “endovascular”. The retrieved articles were imported into the Endnote X8 software and subsequently extracted by first screening the titles and abstracts. After preliminary screening, nonconforming literature was excluded by reading the full text, while the final remaining articles were selected for this study.
Inclusion and exclusion criteria
Studies that meet the following criteria included the following: (1) Studies comparing outcomes of patients with PAS who underwent PBO procedure before cesarean section versus those who did not; (2) Studies reporting on at least one outcome of our primary or secondary endpoints; (3) Studies published in English from inception to July 2021.
Studies with the following criteria excluded the following: (1) Studies not reporting a control group, without a clear confirmation of PAS, or reporting the use of PBO procedure in emergency setting; (2) case report, review articles, editorials, letters, and conference abstracts; (3) duplicated reports; (4) incomplete data and undetermined outcome endpoints.
Data extraction
Data extraction was performed independently by two reviewers. Disagreements were solved by discussion and following a third reviewer’s opinion. The main data extracted included: First author, year of publication, country of publication, patient characteristics, treatment methods, primary and secondary endpoints, and quality assessment. If the included studies did not provide standard deviations, the standard deviations were recalculated using the formula that was suggested in the Cochrane Handbook [6].
Quality evaluation
The quality assessment was completed by two independent researchers using the Newcastle–Ottawa Quality Assessment Scale. According to this scale, a study can be awarded a maximum of nine stars; four stars for study selection, two stars for comparability, and three stars for outcome sections. However, this scale does not provide a threshold score for distinguishing a good from a poor study. Publication bias was assessed using the comparison-adjusted funnel plots.
Statistical analysis
Firstly, we did a traditional pairwise meta-analysis for all available direct evidence using RevMan-5.4 software (the Cochrane Collaboration). Heterogeneity between studies was estimated using the chi-squared test (significant when the p value < 0.1) and the I2 test (substantial heterogeneity when I2 value > 50%). If studies showed a significant heterogeneity (I2 > 0.5, p < 0.1), a random-effects model was used to analyze the pooled effect sizes; if no significant heterogeneity (I2 < 0.5, p > 0.1) was detected, a fixed-effects model was used. The mean difference (MD) and 95% confidence interval (95% CI) were calculated for continuous variables; odds ratios (OR) and 95% CI were calculated for dichotomous variables.
A Bayesian network meta-analysis was then performed using R-3.5 software (R Foundation for Statistical Computing) via the gemtc package. The statistical heterogeneity in the entire network was assessed based on the value of the heterogeneity parameter (I2). The posterior distribution results were reported using its median (MD or OR), accompanied by the 95% credibility intervals (95% CrIs). The model’s overall fit was assessed by calculating the ratio value of the posterior mean residual deviance with a random effect model and the number of independent data points [7; 8]. The consistency was evaluated by comparing the fit of consistency and inconsistency models using deviance information criteria (differences < 5 are considered consistency fitting) [9].
A network meta-regression analysis for the information of procedures operators (interventional radiologists or vascular surgeons) was conducted to address heterogeneity or confounding in the entire network. For each outcome, we evaluated the probability that each intervention is the first best, second best, third best, etc., based on the rank order of this intervention in each iteration of the Markov chain. p ≤ 0.05 was considered statistically significant.
Results
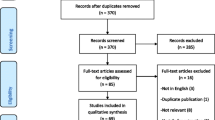
As shown in Fig. 1, a total of 1010 articles were identified initially, 942 of which were excluded based on the title and abstract. The full texts of the remaining 68 articles were read. After re-excluding 9 articles, 59 articles were finally included in the network meta-analysis. The flow diagram of study selection is shown in Fig. 1.
Study characteristics
Fifty nine studies were incorporated in this network meta-analysis, including 5150 PAS patients undergoing cesarean section. Among them, 2913 patients underwent the PBO procedure, and the remaining 2237 patients did not undergo any PBO procedure. Of those patients who underwent the PBO procedure, 1605 were included in the PBOIIA management group, 137 were included in the PBOCIA management group, 1112 were included in the PBOAA management group, and 59 were included in the PBOUA management group. Apart from one study [10], the other 21 studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] reported radiation doses of less than 100 mGy. PBO procedure–related complications were reported in 65 (2.2%) patients. Artery thrombosis, a most common balloon-related vascular complication, was reported in 37 (1.2%) patients. Other PBO-related complications included dissection of an artery (4 patients), groin hematoma (7 patients), abdominal aortic dissection leading to death (1 patient), balloon rupture (2 patients), pseudoaneurysm in the common femoral artery (3 patients), balloon migration (1 patient), buttock claudication (1 patient), and lower limb paraesthesia (2 patients). The characteristics of the patients in the included studies are shown in Table 1.
Bayesian network meta-analysis
The network evidence maps of eligible comparisons are displayed in Supplementary Fig. 1.
Blood loss volume
A total of 55 articles compared the blood loss volume between the PBO group and the non-PBO group. PBOAA (MD − 1.02; 95%CrI − 1.4 to − 0.67), PBOCIA (MD − 0.84; 95%CrI − 1.36 to − 0.06), and PBOIIA (MD − 0.42; 95%CrI − 0.72 to − 0.13) significantly lowered the blood loss volume compared to the non-PBO treatment (Table 2). Moreover, PBOAA procedures encountered significantly less blood loss volume than those who underwent PBOIIA (MD − 0.60; 95%CrI − 1.05 to − 0.17). From the treatment ranking gram (Fig. 2), PBOAA had the highest probability (46%) to be the best treatment for reducing blood loss volume.
Blood transfusion volume
Forty-six studies involving 4161 patients compared the blood transfusion volume between the PBO group and the non-PBO group. In contrast to the non-PBO group, the PBOAA (MD − 2.33; 95%CrI − 3.74 to − 0.94) and PBOIIA (MD − 1.57; 95%CrI − 2.77 to − 0.47) treatment groups had a notably lower blood transfusion volume (Table 2). However, there was no significant difference among the PBOIIA, PBOCIA, PBOAA, and PBOUA subgroups (p > 0.05). According to the treatment ranking gram (Fig. 2), PBOAA was ranked first in the probability of reduced blood transfusion volume.
Hysterectomy
Excluding the studies that had patients undergoing a planned cesarean hysterectomy after the PBO procedure, 42 studies compared the hysterectomy rate between the PBO and non-PBO groups. PBOAA (OR 0.31; 95%CrI 0.16 to 0.54) and PBOIIA groups (OR 0.53; 95%CrI 0.29 to 0.92) showed a reduced hysterectomy rate compared to the non-PBO group (Table 2). No significant difference was observed among the PBOIIA, PBOCIA, and PBOAA groups (p > 0.05). According to the treatment ranking gram (Fig. 2), PBOCIA had the highest probability to be the best treatment, followed by PBOAA. But considering that the difference between PBOCIA and non-PBO groups was not statistically significant (OR 0.23; 95%CI 0.04 to 1.2; p > 0.05), we concluded that it was PBOAA that ranked first as the intervention with statistically the lowest risk of hysterectomy.
Occlusion time and radiation doses
Seven studies compared the timing of balloon occlusion and radiation doses between PBOIIA and PBOAA. Bayesian network meta-analysis could not be performed in this case, so we just performed a traditional pairwise meta-analysis (see later).
Quality assessment
The corresponding Newcastle–Ottawa Quality Assessment results were presented in Supplementary Table 1. The quality scores of studies ranged from six to nine stars with a median of seven stars.
Heterogeneity, model fit, and inconsistency
The Brooks-Gelman-Rubin plots showed that the model had a sufficient convergence in the entire network (Supplementary Fig. 2). Global I2 was used to evaluate heterogeneity which was 1% for blood loss volume, 2% for blood transfusion volume, and 7% for hysterectomy. The model fit was estimated by comparing the posterior mean residual deviance with the number of data points, and the results were similar (Supplementary Table 2). The DICs values were analogous between consistency and inconsistency models, suggesting that the data was therefore consistent.
Traditional pairwise meta-analysis
In addition, we conducted a standard pairwise meta-analysis to complement the Bayesian network meta-analytical results. The results are shown in Table 3. In brief, PBOAA, PBOIIA, and PBOUA procedures significantly reduced the blood loss volume as well as blood transfusion volume when compared to the non-PBO intervention. Similar to the results of Bayesian network analysis, PBOAA procedures significantly reduced the blood loss volume than those who underwent PBOIIA (MD − 0.73; 95%CI − 1.43 to − 0.02). PBOCIA only displayed a notable reduction in the hysterectomy rate, as compared with the non-PBO intervention. Taken together, the findings of our Traditional pairwise meta-analysis were almost similar to those of our network meta-analysis. Furthermore, the random effect model results matched the fixed-effect model’s (Supplementary Table 3), suggesting that our results possess high consistency overall.
As for the balloon occlusion time and radiation doses, PBOAA was associated with a shorter occlusion duration (MD − 7.41; 95%CI − 11.78 to − 3.04) and a lower radiation dose (MD − 16.77; 95% CI − 29.57 to − 3.98) when compared to PBOIIA procedure.
Meta-regression analyses
The meta-regression analysis for the information of procedures operators (interventional radiologists or vascular surgeons) did not alter the outcomes regarding the blood loss volume, blood transfusion volume, and hysterectomy (Supplementary Table 4).
Publication bias
The comparison-adjusted funnel plots were used to evaluate the publication bias. Visual estimation of the funnel plot revealed no significant asymmetry in the endpoints of blood loss volume, blood transfusion volume, and hysterectomy rate (Supplementary Fig. 3), which means there was no obvious publication bias.
Discussion
Surgical management of PAS remains a clinical challenge. Endovascular interventional radiology techniques performed before surgery have been used extensively to treat postpartum hemorrhage, especially the PBO procedures. Placement of a balloon catheter in either the distal abdominal aorta, bilateral common iliac artery, internal iliac artery or uterine artery of PAS patients to temporarily reduce uterine blood flow would minimize bleeding and provide a clearer surgical field for obstetricians. However, there are different PBO techniques for PAS that vary considerably depending on the hospital and surgeon. To this date, the ideal PBO procedure remains up for debate, and there is a strong need for conformity.
Our study is the first comprehensive network meta-analysis of different PBO procedures in PAS patients undergoing a cesarean section. Herein we uncovered that in contrast to the non-PBO treatment, performing PBOIIA, PBOCIA, or PBOAA is associated with significantly reduced blood loss volume, blood transfusion volume, and lower hysterectomy rate in PAS patients. Interestingly, PBOAA had a better effect of reducing blood loss than PBOIIA procedures (mean reduction 600 ml). The poorer performance of PBOIIA than PBOAA may be due to the extensive collateral circulation between the arteries in the pelvis. Through the collateral circulation, the branches originating from other blood vessels (including external iliac artery or femoral artery) can quickly compensate for the occluded arteries of the uterus [32], which causes PBOIIA may not completely block the uterine blood supply. PBOAA had the highest probability of being the best treatment in reducing blood loss volume, blood transfusion volume, and lower hysterectomy rate.
Interventional procedures routinely necessitate the use of X-rays which are associated with an increased risk of harm to the fetus development. However, according to the International Commission on Radiological Protection (ICRP), the fetal teratogenic risk does not increase if the radiation dose is less than 100 mGy. Of the 22 included studies that mentioned radiation doses, 21 had radiation doses below 100 mGy. Since catheterization into the abdominal aorta is easier than the common iliac artery, internal iliac artery, or uterine artery; the procedural time for PBOAA was the shortest; and the radiation doses were the lowest accordingly. Our meta-analysis confirmed that PBOAA was associated with lower radiation doses compared to that of PBOIIA and no radiation-related neonatal complications were reported in these studies.
Artery thrombosis is one of the most notable and common vascular complications related to PBO procedures. The risk of thrombosis is generally associated with the hypercoagulability of maternity blood, vascular intimal injury when inserting the balloon catheter, and the blocking time of balloon occlusion [33]. One previous study pointed out that the balloon occlusion time should not be too long to avoid thrombotic complications and PBOAA should be intermittently inflated for a maximum duration of 40 min, whereas PBOIIA balloons be intermittently inflated for a maximum duration of 4 h [17]. Similarly, a too-long balloon occlusion duration might also lead to limb ischemia necrosis or even the multiple organ dysfunction syndromes [11]. Thus, if there is a need to prolong the balloon occlusion time, it is necessary to deflate the balloon intermittently for 10–15 min to restore the blood supply. This meta-analysis presented that PBOAA has a shorter occlusion duration than PBOIIA.
Previous meta-analyses focused mainly on a specific PBO management, for instance, occlusion of the internal iliac artery or abdominal aorta [34,35,36]. The studies of He, Q. (2019) [35] and Chen, L. (2019) [34] showed that the use of PBOAA was associated with reduced blood loss volume, blood transfusion volume, and hysterectomy rate. In comparison, Nankali, A. (2021) [32] concluded that PBOIIA was only associated with reduced blood loss volume and hysterectomy rate, with no significant difference in blood transfusion volume. Other studies have also summarized the role of interventional radiology treatment modalities in the management of PAS, including the use of PBO procedures, but there was no quantitative analysis of the outcomes [37,38,39].
Only two meta-analyses analyzed the efficacy of different balloon occlusion procedures for hemorrhage control in PAS [40; 41]. The study of Shahin, Y 2018 [40] showed that interventional radiology significantly reduced blood loss and red blood cell transfusions, with no difference in unplanned hysterectomy. In addition, the subgroup analysis suggested that PBOAA was related with the lowest blood loss volume, blood transfusion volume, and hysterectomy rate during a cesarean section while PBOIIA correlated with decreased blood loss volume and blood transfusion volume only. The other meta-analysis [41], which included 13 studies, revealed significantly lower intraoperative blood loss in patients with PBO procedures than non-PBO procedures, with no statistical difference in other outcomes. However, this author made a general synthetic analysis with high heterogeneity (I2 = 98%), and no further subgroup analysis was conducted on the other different types of occlusion artery.
Our Bayesian network meta-analysis study possessed low global heterogeneity: 1% for blood loss volume, 2% for blood transfusion volume, and 7% for hysterectomy. More importantly, we also conducted a traditional pairwise meta-analysis that yielded consistent results compared to our Bayesian network meta-analysis. These results further affirmed the robustness of our findings.
Nevertheless, our study has some limitations: firstly, we observed high heterogeneity for direct pairwise meta-analysis in blood loss volume and blood transfusion volume (I2 > 50%). We tried to lower the heterogeneity using a sensitivity analysis by removing studies one at a time and reanalyzing them but were unsuccessful. We speculate that it may originate at the obstetrician, patient characteristics assessments, and blood loss volume estimation level. Moreover, most studies did not detail the area and depth of placental implantation of PAS patients, which may also be one of the sources of heterogeneity. Secondly, although we compared four different PBO procedures, most of the included studies focused on PBOAA or PBOIIA. The limited number of PBOCIA or PBOUA studies might affect the robustness of our findings, especially in their respective outcomes. Thirdly, for the outcome of hysterectomy rate, some studies had no event rates. Thus, the corresponding CrIs were very wide, resulting in increased uncertainty. Finally, limited by the small number of RCT studies, we only included 3 RCT studies in our analysis. If analyzing using more RCT, it can strengthen the evidence grade.
In conclusion, our Bayesian network meta-analysis indicated that PBOAA and PBOIIA could significantly reduce the blood loss volume, blood transfusion volume as well as hysterectomy rate in contrast to the non-PBO intervention in patients with PAS. PBOAA was more effective in reducing blood loss volume, fetus radiation dose, and balloon occlusion duration compared with PBOIIA. There were few studies in the literature-reported PBOCIA and PBOUA procedures. These limited researches showed that PBOCIA could only significantly reduce the blood loss volume compared with non-PBO intervention. What’s more, according to our statistical treatment ranking, PBOAA was statistically superior in reducing blood transfusion volume, blood transfusion volume and hysterectomy rate than other PBO procedures.
Abbreviations
- CI:
-
Confidence interval
- CrI:
-
Credibility interval
- DIC:
-
Deviance information criterion
- MD:
-
Mean difference
- OR:
-
Odds ratios
- PAS:
-
Placenta accreta spectrum
- PBO:
-
Prophylactic balloon occlusion
- PBOAA:
-
Prophylactic balloon occlusion of the abdominal aorta
- PBOCIA:
-
Prophylactic balloon occlusion of the common iliac artery
- PBOIIA:
-
Prophylactic balloon occlusion of the internal iliac arteries
- PBOUA:
-
Prophylactic balloon occlusion of the uterine artery
References
Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S (2019) FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet 146:20–24
Bartels HC, Postle JD, Downey P, Brennan DJ (2018) Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis Markers 2018:1507674
Green L, Knight M, Seeney FM et al (2016) The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG 123:2164–2170
Erfani H, Fox KA, Clark SL et al (2019) Maternal outcomes in unexpected placenta accreta spectrum disorders: single-center experience with a multidisciplinary team. Am J Obstet Gynecol 221:337.e331-337.e335
Kassem GA, Alzahrani AK (2013) Maternal and neonatal outcomes of placenta previa and placenta accreta: three years of experience with a two-consultant approach. Int J Womens Health 5:803–810
Higgins JPT, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions. Wiley Online Library, New York, p 142
Dias S, Sutton AJ, Ades AE, Welton NJ (2013) Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 33:607–617
Mills EJ, Thorlund K, Ioannidis JP (2013) Demystifying trial networks and network meta-analysis. BMJ 346:2914
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE (2013) Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 33:641–656
Picel AC, Wolford B, Cochran RL, Ramos GA, Roberts AC (2018) Prophylactic internal iliac artery occlusion balloon placement to reduce operative blood loss in patients with invasive placenta. J Vasc Interv Radiol 29:219–224
Chen M, Xie L (2016) Clinical evaluation of balloon occlusion of the lower abdominal aorta in patients with placenta previa and previous cesarean section: a retrospective study on 43 cases. Int J Surg 34:6–9
Wu Q, Liu Z, Zhao X et al (2016) Outcome of pregnancies after balloon occlusion of the infrarenal abdominal aorta during caesarean in 230 patients with placenta praevia accreta. Cardiovasc Intervent Radiol 39:1573–1579
Cui S, Zhi Y, Cheng G, Zhang K, Zhang L, Shen L (2017) Retrospective analysis of placenta previa with abnormal placentation with and without prophylactic use of abdominal aorta balloon occlusion. Int J Gynaecol Obstet 137:265–270
Fan Y, Gong X, Wang N et al (2017) A prospective observational study evaluating the efficacy of prophylactic internal iliac artery balloon catheterization in the management of placenta previa-accreta: A STROBE compliant article. Medicine (Baltimore) 96:e8276
Xie L, Wang Y, Luo F-Y, Man Y-C, Zhao X-L (2017) Prophylactic use of an infrarenal abdominal aorta balloon catheter in pregnancies complicated by placenta accreta. J Obstet Gynaecol 37:557–561
Duan X, Chen P, Han X et al (2018) Intermittent aortic balloon occlusion combined with cesarean section for the treatment of patients with placenta previa complicated by placenta accreta: A retrospective study. J Obstet Gynaecol Res 44:1752–1760
Li K, Zou Y, Sun J, Wen H (2018) Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: a retrospective cohort study. Eur Radiol 28:4959–4967
Mei Y, Luo D, Lin Y (2018) Clinical application of prophylactic internal iliac artery balloon occlusion combined with uterine artery embolization in patients with abnormally invasive placenta. J Matern Fetal Neonatal Med 31:3287–3292
Ono Y, Murayama Y, Era S et al (2018) Study of the utility and problems of common iliac artery balloon occlusion for placenta previa with accreta. J Obstet Gynaecol Res 44:456–462
Sun W, Duan S, Xin G et al (2018) Safety and efficacy of preoperative abdominal Aortic balloon occlusion in placenta increta and/or percreta. J Surg Res 222:75–84
Mei Y, Zhao H, Zhou H, Jing H, Lin Y (2019) Comparison of infrarenal aortic balloon occlusion with internal iliac artery balloon occlusion for patients with placenta accreta. BMC Pregnancy Childbirth 19:147
Wei Y, Luo J, Luo D (2019) Comparison of efficacy between internal iliac artery and abdominal aorta balloon occlusions in pernicious placenta previa patients with placenta accrete. Gynecol Obstet Invest 84:343–349
Zheng ZR, Xie X, Hou Y, Xie P, Yu X, Xie L (2019) Intraoperative infrarenal aortic balloon occlusion in pregnancies with placenta accreta, increta, and percreta. Clin Exp Obstet Gynecol 46:704–708
Cho SB, Hong SJ, Lee S et al (2020) Preoperative Prophylactic Balloon-Assisted Occlusion of the Internal Iliac Arteries in the Management of Placenta Increta/Percreta. Medicina (Kaunas) 56:368
Dai M, Jin G, Lin J et al (2020) Control of postpartum hemorrhage in women with placenta accreta spectrum using prophylactic balloon occlusion combined with Pituitrin intra-arterial infusion. Eur Radiol 30:4524–4533
Lee AY, Ballah D, Moreno I et al (2020) Outcomes of balloon occlusion in the University of California Morbidly Adherent Placenta Registry. Am J Obstet Gynecol MFM 2:100065
Mei Y, Luo D, Wei S et al (2020) Comparison of emergency cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters in patients with placenta accreta spectrum. J Matern Fetal Neonatal Med. https://doi.org/10.1080/14767058.2020.1815187:1-6
Tokue H, Tokue A, Tsushima Y, Kameda T (2020) Safety and efficacy of aortic vs internal iliac balloon occlusion for cesarean delivery in coexisting placenta accreta and placenta previa. Cardiovasc Intervent Radiol 43:1277–1284
Fan Y, Gong X, Wang N et al (2021) A participant-assigned interventional research of precesarean internal iliac artery balloon catheterization for managing intraoperative hemorrhage of placenta previa and placenta accreta spectrum disorders after cesarean section. Curr Med Sci 41:336–341
Liu Y, Shan N, Yuan Y, Tan B, Qi H, Che P (2021) The clinical evaluation of preoperative abdominal aortic balloon occlusion for patients with placenta increta or percreta. J Matern Fetal Neonatal Med. https://doi.org/10.1080/14767058.2021.1906219:1-6
Zhou X, Sun X, Wang M, Huang L, Xiong W (2021) The effectiveness of prophylactic internal iliac artery balloon occlusion in the treatment of patients with pernicious placenta previa coexisting with placenta accreta. J Matern Fetal Neonatal Med 34:93–98
Palacios Jaraquemada JM, García Mónaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA (2007) Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand 86:228–234
Luo Y, Duan H, Liu W et al (2013) Clinical evaluation for lower abdominal aorta balloon occluding in the pelvic and sacral tumor resection. J Surg Oncol 108:148–151
Chen L, Wang X, Wang H, Li Q, Shan N, Qi H (2019) Clinical evaluation of prophylactic abdominal aortic balloon occlusion in patients with placenta accreta: a systematic review and meta-analysis. BMC Pregnancy Childbirth 19:30
He Q, Li Y-l, Zhu M-j et al (2019) Prophylactic abdominal aortic balloon occlusion in patients with pernicious placenta previa during cesarean section: a systematic review and meta-analysis from randomized controlled trials. Arch Gynecol Obstet 300:1131–1145
Nankali A, Salari N, Kazeminia M, Mohammadi M, Rasoulinya S, Hosseinian-Far M (2021) The effect prophylactic internal iliac artery balloon occlusion in patients with placenta previa or placental accreta spectrum: a systematic review and meta-analysis. Reprod Biol Endocrinol 19:40
Di Mascio D, Panici P, Nappi L, D’Antonio F (2019) The role of interventional radiology in the management of placenta accreta spectrum disorders. Curr Obstet Gynecol Rep 8:139–144
Dilauro MD, Dason S, Athreya S (2012) Prophylactic balloon occlusion of internal iliac arteries in women with placenta accreta: literature review and analysis. Clin Radiol 67:515–520
Soyer P, Barat M, Loffroy R et al (2020) The role of interventional radiology in the management of abnormally invasive placenta: a systematic review of current evidences. Quant Imaging Med Surg 10:1370–1391
Shahin Y, Pang CL (2018) Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. Eur Radiol 28:2713–2726
D’Antonio F, Iacovelli A, Liberati M et al (2019) Role of interventional radiology in pregnancy complicated by placenta accreta spectrum disorder: systematic review and meta-analysis. Ultrasound Obstet Gynecol 53:743–751
Acknowledgements
The authors gratefully acknowledge the financial supports of the Science and Technology Program of Science and Technology Commission Shanghai Municipality under Grant numbers 19441907000.
Funding
This study has received funding from the Science and Technology Program of Science and Technology Commission Shanghai Municipality under Grant numbers 19441907000.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Xuebin Zhang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Approval from the institutional animal care committee was not required because this is a meta-analysis based on published studies.
Ethical approval
Institutional Review Board approval was not required because this is a meta-analysis based on published studies.
Methodology
• Bayesian network meta-analysis.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dai, M., Zhang, F., Li, K. et al. The effect of prophylactic balloon occlusion in patients with placenta accreta spectrum: a Bayesian network meta-analysis. Eur Radiol 32, 3297–3308 (2022). https://doi.org/10.1007/s00330-021-08423-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08423-6