Abstract
Purpose
To investigate safety and efficacy of intra-aortic balloon occlusion (IABO) versus internal iliac artery balloon occlusion (IIABO) for cesarean delivery in coexisting placenta accreta and placenta previa.
Materials and Methods
From 2006 to 2019, 60 pregnant women who had undergone preoperative IABO (n = 28) and IIABO (n = 32) for cesarean delivery in coexisting placenta accreta and placenta previa were retrospectively identified, and their medical records and relevant imaging were reviewed.
Results
Maternal characteristics (age, gravidity, previous cesarean delivery, gestational age, and neonatal weight) were similar in both groups. Estimated blood loss, volume of blood transfusion, length of hospitalization, and rate of hysterectomy were not significantly different between the groups. Operation time (the duration of cesarean delivery and hysterectomy, p < 0.05), total time of balloon occlusion (p < 0.01), and fetal radiation dose (p < 0.001) in the IABO group were less than in the IIABO group. No severe complications related to the balloon occlusion procedure were noted in either group.
Conclusion
IABO and IIABO are safe and effective options for cesarean delivery in patients with combined placenta accreta and placenta previa. The average operation time, balloon occlusion time, and fetal radiation dose in patients with IABO are less than in patients with IIABO. There were no complications related to balloon occlusion of the aorta or internal iliac artery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Placenta previa classically presents as painless vaginal bleeding in the third trimester secondary to abnormal placentation near or covering the internal cervical os. The incidence of placenta previa is approximately 0.5% of all deliveries, and it has been increasing with the increased rate of cesarean delivery globally [1].
Morbidly adherent placenta accreta is also increasing for similar reasons. It is classified into three types based on the depth of invasion: placenta accreta, a morbid attachment of the placental villi to the surface of the myometrium; placenta increta, the placental villi implanting into or penetrating the myometrium; and placenta percreta, the placental villi penetrating the myometrium and reaching or invading the uterine serosa [2]. The incidence of placenta accreta disorders is approximately 0.3% with a reported mortality rate of approximately 7.0%. In the presence of placenta previa, placenta accreta will also be noted in 3–67% of cases, increasing with the number of prior uterine scars. [3]. The coexistence of placenta accreta and previa causes significant obstetrical morbidity via hemorrhage, and many women require emergent hysterectomy with its inherent surgical risks, additional blood loss, and subsequent loss of reproductive capacity.
Prophylactic uterine artery embolization immediately following cesarean delivery may be appropriate to manage the massive obstetrical hemorrhage during high-risk deliveries with these complications. Recently, the efficacy of prophylactic arterial balloon occlusion for cesarean delivery has been reported. However, its use remains controversial [4,5,6,7,8,9]. No randomized controlled clinical trials have been performed, and there is no consensus about which artery is most effective for prophylactic balloon occlusion (internal iliac, common iliac, or aorta). Furthermore, the timing of balloon inflation before cesarean delivery or after fetal delivery and what type of balloon to use are also controversial.
To evaluate the safety and efficacy of this new technique, we compared the use of intra aorta balloon occlusion (IABO) and internal iliac artery balloon occlusion (IIABO) in cases of coexisting placenta accreta and placenta previa. We also conducted a literature review for the comparison of IABO with IIABO for patients with placenta accreta.
Materials and Methods
Patients
Eligible patients were those who had undergone IABO (n = 28) and IIABO (n = 32) for coexisting placenta accreta and placenta previa during cesarean delivery from January 2006 to April 2019. We used IIABO from 2006 and also used IABO from 2013. Since 2013, small-size (7-French) aortic occlusion balloon catheter (Rescue BalloonR, Tokai Medical Product, Aichi, Japan) has become available in our country. In addition, many abnormal cases are participated to our hospital from neighboring hospitals.
The study included data obtained in a retrospective fashion and approved by the institutional ethic committee. Ultrasonography and/or MRI was obtained to confirm the diagnosis of placenta accreta and placenta previa during the antenatal period in all patients. Placenta previa was diagnosed using ultrasonography, when the internal cervical os was found to be completely or partially covered by the placenta (Fig. 1). In this study, placenta accreta was defined as patients with three grades of abnormal trophoblastic invasion unless otherwise specified.
Patient with combined placenta accreta and placenta previa. A Transabdominal ultrasonography image showed disruption of the uterine serosa–bladder interface (arrow) by invading placental tissue. (B: bladder, P: placenta). B Sagittal MR image (T2-weighted image) showed the placenta is completely covering the internal os. Abnormal placental tissue was abutting the bladder wall (arrow)
The criteria for the diagnosis of placenta accreta with placenta previa were the presence of at least one of the following sonographic findings: (a) obliteration of the normal hypoechoic or sub-placental zone, (b) extensive intra- or sub-placental lacunae (dilated venous spaces), (c) tortuous vessels or lacunae in the myometrium, and (d) disruption or thinning of the uterine serosa–bladder interface. Inclusion criteria were as follows: (1) diagnosed with placenta accreta based on ultrasonography and/or MRI and confirmed by intraoperative findings, (2) without hemorrhage and hemodynamically stable before surgery, (3) gestational age > 28 weeks, (4) a history of previous cesarean delivery, (5) placenta previa with the placenta located in the prior cesarean scar, (6) preoperative hemoglobin > 10 g/dL, and (7) a scheduled, not emergency, cesarean delivery.
The exclusion criteria included: (1) significant obstetrical complications such as severe hypertension, severe diabetes mellitus, uncontrolled infection, coagulation disorders, or hematological abnormalities, (2) use of anticoagulants such as heparin or aspirin, and (3) fetal anomaly, growth restriction, or distress.
Maternal Characteristics and Outcomes
Clinical characteristics reviewed included obstetrical history, maternal background, course of delivery, pathological findings regarding the degree of placental adhesion (accreta, increta, percreta), placental position, and previa totalis. Additional characteristics included patient age, body mass index, parity, number of previous cesarean deliveries, gestational age at cesarean delivery, Apgar score at 5 min after delivery, estimated blood loss, operation time, blood transfusion, postoperative hospital stay, and clinical findings. Operation time is defined as the duration of cesarean delivery and hysterectomy. Estimated blood loss was quantified using the volume of suction containers, weight of swabs, and visual estimation of vaginal blood loss. Complications and subsequent menstruation were followed up by telephone and Email. Access-related or balloon occlusion-related complications were evaluated daily by interventional radiologist and gynecologist until discharge, recorded and classified according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Maternal characteristics and outcomes were reviewed, and the safety and efficacy of IABO and IIABO were compared.
Interventional Procedure
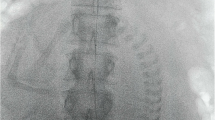
Prior to cesarean delivery, all patients and their families were informed of the risks of the procedure. Multidisciplinary treatment plan was made by interventional radiologist, anesthetist, and obstetrician in both groups. The decision to perform IABO or IIABO was made by consultation between obstetrician and patient. All prophylactic arterial balloon occlusion procedures were performed by interventional radiologist. On the day of cesarean delivery, aortic and internal iliac artery occlusion balloon catheters were placed in the angiography suite prior to cesarean delivery. We preformed procedure using an angiography imaging system (AXIOM Artis dTA, Siemens, Erlangen, Germany). In the IABO group, after local anesthesia, the right femoral artery was accessed using the Seldinger technique and a 7-French introducer sheath for placement of a 7-French aortic occlusion balloon catheter. In the IIABO group, bilateral femoral artery was accessed with 6-French sheaths for placement of a 6-French iliac artery occlusion balloon catheter. First, we performed aortography with a 4-French pigtail catheter (Terumo Clinical Supply Co. Ltd., Gifu, Japan) to identify and measure the internal iliac artery and infra-renal abdominal aorta; 20 ml of contrast agent (300 mg iodine/mL) was injected (flow rate; 10 ml/s, frame rate; 2 frames/s, exposure duration; 10 s). The diameter of the internal iliac artery and infra-renal abdominal aorta was measured from an image obtained during aortography, and the size of the balloon was adjusted accordingly. If MRI was performed before cesarean delivery, MRI was also used to estimate the diameter of the internal iliac artery or infra-renal abdominal aorta.
Next, in the IABO group, a 7-French occlusion balloon catheter (Rescue BalloonR, 12–14 mm diameter, Tokai Medical Product, Aichi, Japan) was positioned with its tip in the infra-renal abdominal aorta. In the IIABO group, a 6-French occlusion balloon catheter (OptimoR, 8–10 mm diameter, Tokai Medical Product, Aichi, Japan) was positioned with its tip in the anterior division of the contralateral internal iliac artery (Fig. 2). In order to confirm the anatomy of arteries, a contrast medium was injected from the injection-lumen of the balloon catheter. The proper positioning of the balloons and the effective vascular occlusion were angiographically confirmed during the insufflation of the balloons. And, we inflated the balloon and injected contrast medium distal to the balloon to document whether the balloon effectively occluded the artery. To minimize radiation exposure to the mother and fetus, images were obtained at 3 frames per second. Once catheter positioning was satisfactory, they were securely taped to the skin. Following the arterial interventional procedure, the patient was transferred to the surgical operating room for cesarean delivery under spinal anesthesia. Just before the cesarean delivery, we checked the catheter position with the mobile C-arm X-Ray machine in the surgical operating room. Fluoroscopy was utilized during the delivery, if necessary. Immediately after the infant was delivered and the umbilical cord clamped, the occlusion balloons were inflated by the interventional physicians. The obstetrician then surgically excised as much of the placenta as possible along with any myometrium and reconstructed the uterus under general anesthesia with tracheal intubation.
Prophylactic balloon occlusion of intra-abdominal aorta and internal iliac arteries in placenta accreta coexisting with placenta previa. A Digital subtraction abdominal aortogram was performed from right femoral approach prior to placement of an aortic occlusion balloon in a pregnant woman with combined placenta accreta and placenta previa. 20 ml of contrast agent (300 mg iodine/mL) was injected (flow rate; 10 ml/s, frame rate; 2 frames/s, exposure duration; 10 s). Developed uterine arteries were observed (arrows). The diameter measurement of the aorta was 13 mm. The diameter measurement of internal iliac arteries was 8 mm. B In the IABO group, the balloon was placed in the aorta just above the aortic bifurcation. We commonly used a 7-French occlusion balloon catheter (Rescue BalloonR, Tokai Medical Product, Aichi, Japan, diameter; 12–14 mm, length; 6 cm). Occlusion balloon was inflated manually. If inflated resistance of the syringe is felt, balloon inflation was stopped. C In the IIABO group, the balloons were placed in the anterior division of the bilateral internal iliac arteries. The diameter of the anterior division of the internal iliac artery and the balloon was 8 mm. We commonly used a 6-French occlusion balloon catheter (OptimoR, Tokai Medical Product, Aichi, Japan, diameter; 8–10 mm, length; 3 cm)
In the IABO group, the aortic balloon was alternately inflated for 30 min then deflated for 10 min. If hemorrhage persisted, the blockage was repeated again until the bleeding stopped. In the IIABO group, after the infant was delivered, the internal iliac balloons were inflated continuously until hemostasis was attained during the cesarean delivery. If it was difficult to manually remove the placenta, it was left in situ and hysterectomy was performed immediately in both groups. And, if it was difficult to control bleeding during the cesarean delivery, we also decided to perform a hysterectomy.
Patients were observed in the recovery room for 60 min following cesarean delivery completion. The balloon catheter was withdrawn following completion of the entire procedure. The arterial sheath was removed 6 h after completion of the procedure. Manual external compression was utilized for arterial access in the IIABO and IABO groups.
Fetal Radiation Dose
Radiation dose (in mGy) was determined at the end of the procedure. The entrance skin radiation dose in the area of the irradiated field was considered to be an approximation of the fetal radiation dose.
Statistics
Descriptive data are presented as mean ± standard deviation. Variables were compared using the Student t test and Mann–Whitney U test. The Fisher's exact test was used to compare potential risk factors for massive bleeding. We used SPSS v. 22 software (IBM Corp., Tokyo, Japan), and p values of < 0.05 were considered statistically significant.
Results
A total of 5012 deliveries were performed in our hospital between 2006 and 2019. Eighty-two patients with abnormalities of placentation were treated during this time, and 60 patients met inclusion criteria. A total of 28 cases underwent IABO, and 32 cases underwent IIABO. Table 1 shows the patients’ clinical characteristics. Maternal characteristics were similar in both groups. Table 2 notes the outcomes of the IABO and IIABO groups. Estimated blood loss, volume of blood transfusion, length of hospitalization, and rate of hysterectomy were not significantly different between the groups. The groups differed in operation time (p < 0.05), total time of balloon occlusion (p < 0.01), and fetal radiation dose (p < 0.001). The fetal radiation dose was higher with IIABO because of the longer time was needed to place the balloon. There was a trend toward hysterectomy in the IABO group. It may be because hysterectomy resulted a shorter operation time and balloon times. In 28 patients (47%), the uterus was preserved, and the patients resumed normal menstruation within a year. There were no access-related or balloon occlusion-related complications until discharge, and no damage to the adjacent pelvic organs during the operation was observed. There were no maternal or neonatal deaths. One patient in the IIABO group required transcatheter arterial embolization (TAE) 2 days following her hysterectomy because of continuous bleeding from adherent part of the placenta. Bleeding was immediately stopped after bilateral uterine artery embolization with gelatin sponge. There were no additional treatments (TAE or reoperation) following cesarean delivery in the IABO group. All mothers and babies were healthy at the time of discharge.
Discussion
Although our study is a non-randomized retrospective single-center study, the IABO and IIABO might be safe and effective in reducing post-cesarean delivery hemorrhage and decreasing the risk of hysterectomy in patients with combined placenta accreta and placenta previa. And, the average operation time, balloon occlusion time, and fetal radiation dose in patients with IABO were less than in patients with IIABO.
The incidence of a morbidly adherent placenta during a second pregnancy has increased significantly with the increase in cesarean delivery rate globally. A morbidly adherent placenta is a major cause of massive postpartum hemorrhage and is associated with significant maternal morbidity, mortality, and even perinatal death [10]. Previously, hysterectomy following cesarean delivery was the main therapeutic choice when life-threatening bleeding occurred in coexisting placenta accreta and placenta previa. Recently, interventional radiology procedures have been used in obstetrics to reduce intraoperative bleeding and decrease the rate of hysterectomy. These include uterine artery embolization, common iliac artery balloon occlusion, bilateral internal iliac balloon occlusion, and intra-aortic balloon occlusion [11]. The approach does not change just because of the presence of previa, but it may require more attention to bleeding.
Recently, the prophylactic use of IABO for patients with placenta accreta has garnered more attention. Because of the potential for major vascular complications to occur, IABO should be performed by a clinician trained in IABO with knowledge of the potential complications. A retrospective study of IABO reported postoperative complications of approximately 4.4%, including arterial thrombosis and femoral nerve ischemic injury [12]. We, however, had no severe complications related to the balloon occlusion procedure in our study. We believe this was due to the participation of an experienced interventional radiology team throughout the entire procedure.
We performed a literature review of available reports comparing IABO with IIABO for patients with placenta accreta through December 2019 using the key terms “aortic balloon occlusion,” “internal iliac artery balloon occlusion,” and “placenta accreta” from PubMed and Google Scholar. The inclusion criteria for the reports were as follows: (1) there was a description of the evaluation of estimated blood loss (EBL), blood transfusion, inflation and deflation time of the aortic balloon, size and length of the balloon, and timing of balloon inflation during the cesarean delivery; (2) publications were in the English language, and (3) studies included more than 50 patients. We did not include case reports. Table 3 shows the results of the literature review comparing IABO with IIABO for patients with placenta accreta. No severe complications related to the balloon occlusion procedures were observed in either group. IABO generally resulted in better outcomes. However, one study reported that the Apgar score was lower [6]. Inflation and deflation times of the aortic balloon and timing of balloon inflation varied. Some authors have reported significant reductions in blood loss and transfusion with IABO compared to IIABO [4, 5]. Others, including our study, found no decrease in blood loss or transfusion [6, 7, 11]. We found no significant difference in hysterectomy which might have been due to the small number of patients with placenta increta and percreta (8%). Differences between our study and prior reports may have resulted from patient or technique differences. Additional research is needed with a larger patient base.
The International Commission on Radiological Protection (ICRP) suggests that when the radiation dose is under 100 mGy, there should be no increased fetal teratogenic risk [13]. In our study, the fetal radiation dose was lower in the IABO group. The IIABO group required two sets of sheaths and balloons to reach the contralateral internal iliac arteries. The IABO group required only one sheath and balloon insertion, without crossing the abdominal bifurcation. Additionally, the aorta is thicker than the inner iliac artery, making it easier to place a balloon [7]. We did not find any radiation-related neonatal complications during the follow-up period in either group. However, the effects of radiation on the fetus should be followed up for a significant period of time.
There is no unified standard for balloon occlusion/inflation time and inflation timing. Balloon inflation time should be as short as possible to avoid reperfusion injury and thrombotic and embolic complications in the lower limbs. And, balloon deflation time should be adjusted to inflation time. Qasim et al. [14] reported no significant procedure-related complications if the aortic occlusion time was not over 40 min. Balloon deflation time has not been fully discussed in the past literature. We set inflation time to 30 min and deflation time to 10 min, because serious complications did not cause with deflation times of 10 min in the past literature [4,5,6,7]. However, there is room for further consideration. IABO prior to cesarean delivery results in longer occlusion times and may also effect blood flow to the fetus. Also, the timing of balloon inflation is controversial, because there are reports of lower Apgar scores with IABO prior to cesarean delivery [6, 12]. In our study, balloon inflation occurred after the fetus was delivered and before the placenta was removed. Thus, it had little influence on the blood supply to the fetus.
Complications associated with arterial balloon occlusion have been reported, including ischemic necrosis of the lower limbs, internal iliac arterial thrombosis, puncture point hematoma, reperfusion injury of tissues and organs, acute renal failure, and initial vessel injury [8, 9]. When an arterial sheath is removed, a larger femoral lesion may make compression and hemostasis more difficult, and a hematoma may form at the puncture site [15]. In our study, a smaller arterial sheath, compared to previous studies, was chosen based on the size of the balloon catheter, and no complications occurred in any patients. We believe that the choice of appropriate balloon size and smaller sheath are important to prevent unnecessary complications.
In our study, 32 patients (53%) underwent hysterectomy. Uterine artery embolization is considered as a treatment option for control of the bleeding before hysterectomy. However, bleeding with placenta accreta is sometimes difficult to perform TAE, because the uterus and placenta obtain extensive collateral supply from the uterine arteries as well as the aorta and the external iliac collaterals [16]. It may be difficult to perform TAE with the mobile C-arm X-Ray machine in our surgical operating room. Therefore, if it was difficult to control bleeding during the cesarean delivery, we decided to perform a hysterectomy.
There were several limitations to our study. First, this was a retrospective cohort study with a small sample size. Second, there was no control group, and the decision to perform IABO or IIABO was made by consultation between the doctor and patient. Therefore, there was selection bias, and the doctor’s experience could affect the decision of which procedure to use. Lastly, this was a non-randomized single-center study. A prospective study with a large number of patients should be performed to determine which procedure is better.
Conclusion
Although there were a limited numbers of patients, IABO and IIABO are safe and effective options for cesarean delivery in patients with combined placenta accreta and placenta previa. The average operation time, balloon occlusion time, and fetal radiation dose in patients with IABO are less than in patients with IIABO. There were no complications related to balloon occlusion of the aorta or internal iliac artery. However, prospective studies are needed to compare the efficacy and safety of these two different techniques.
References
Matalliotakis M, Velegrakis A, Goulielmos GN, Niraki E, Patelarou AE, Matalliotakis I. Association of Placenta Previa with a History of Previous Cesarean Deliveries and Indications for a Possible Role of a Genetic Component. Balkan J Med Genet. 2017;29, 20(2):5–10.
Fan Y, Gong X, Wang N, Mu K, Feng L, Qiao F, Chen S, Zeng W, Liu H, Wu Y, Zhou Q, Tian Y, Li Q, Yang M, Li F, He M, Beejadhursing R, Deng D. A prospective observational study evaluating the efficacy of prophylactic internal iliac artery balloon catheterization in the management of placenta previa-accreta: A STROBE compliant article. Medicine. 2017;96(45):e8276.
Goh WA, Zalud I. Placenta accreta: diagnosis, management and the molecular biology of the morbidly adherent placenta. J Matern Fetal Neonatal Med. 2016;29(11):1795–800.
Wang YL, Duan XH, Han XW, Wang L, Zhao XL, Chen ZM, Chu QJ, Zhang W. Comparison of temporary abdominal aortic occlusion with internal iliac artery occlusion for patients with placenta accreta—a non-randomised prospective study. Vasa. 2017;46(1):53–7.
Li K, Zou Y, Sun J, Wen H. Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: a retrospective cohort study. Eur Radiol. 2018;28(12):4959–67.
Wei Y, Luo J, Luo D. Comparison of Efficacy between Internal Iliac Artery and Abdominal Aorta Balloon Occlusions in Pernicious Placenta Previa Patients with Placenta Accrete. Gynecol Obstet Invest. 2019;84(4):343–9.
Mei Y, Zhao H, Zhou H, Jing H, Lin Y. Comparison of infrarenal aortic balloon occlusion with internal iliac artery balloon occlusion for patients with placenta accreta. BMC Pregnancy Childbirth. 2019;2, 19(1):147.
Xie L, Wang Y, Luo FY, Man YC, Zhao XL. Prophylactic use of an infrarenal abdominal aorta balloon catheter in pregnancies complicated by placenta accreta. J Obstet Gynaecol. 2017;37(5):557–61.
Sun W, Duan S, Xin G, Xiao J, Hong F, Hong H, Wu Y, Xu Y. Safety and efficacy of preoperative abdominal Aortic balloon occlusion in placenta increta and/or percreta. J Surg Res. 2018;222:75–84.
Zamzami TY. Maternal and perinatal outcome of massive postpartum hemorrhage: a review of 33 cases. Ann Saudi Med. 2003;23(3–4):135–9.
Chen L, Wang X, Wang H, Li Q, Shan N, Qi H. Clinical evaluation of prophylactic abdominal aortic balloon occlusion in patients with placenta accreta: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;15, 19(1):30.
Wei X, Zhang J, Chu Q, Du Y, Xing N, Xu X, Zhou Y, Zhang W. Prophylactic abdominal aorta balloon occlusion during caesarean delivery: a retrospective case series. Int J Obstet Anesth. 2016;27:3–8.
The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103[J]. Ann ICRP, 2007, 37(2-4): 1–332
Qasim Z, Brenner M, Menaker J, Scalea T. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation. 2015;96:275–9.
Peng ZH, Xiong Z, Zhao BS, Zhang GB, Song W, Tao LX, Zhang XZ. Prophylactic abdominal aortic balloon occlusion: An effective method of controlling hemorrhage in patients with placenta previa or accreta. Exp Ther Med. 2019;17(2):1492–6.
Buckley B. Interventional radiology in abnormal placentation. Obstet Gynecol Mag. 2010;12:57.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors provided clinical expertise and participated in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Written informed consent was obtained from all patients and residents participating in the study, as approved by the ethics committee of our hospital.
Consent for Publication
Written informed consent for publication was obtained from all patients and residents participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tokue, H., Tokue, A., Tsushima, Y. et al. Safety and Efficacy of Aortic Vs Internal Iliac Balloon Occlusion for Cesarean Delivery in Coexisting Placenta Accreta and Placenta Previa. Cardiovasc Intervent Radiol 43, 1277–1284 (2020). https://doi.org/10.1007/s00270-020-02548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-020-02548-9