Abstract
Purpose
To compare diagnostic performance for prediction of malignant potential in IPMNs between EUS, contrast-enhanced CT and MRI.
Materials and methods
76 patients with IPMN (benign = 37, malignant = 39) underwent EUS, contrast-enhanced CT, and MRI. EUS was analyzed based on formal reports and contrast-enhanced CT and MRI were retrospectively analyzed by two radiologists according to the consensus guidelines 2012. Diagnostic performance and imaging features of malignant IPMNs were analyzed using ROC analysis and multivariate analyses.
Results
Diagnostic performance of contrast-enhanced CT (AUC = 0.792 in R1, 0.830 in R2), MRI (AUC = 0.742 in R1, 0.776 in R2), and EUS (AUC = 0.733) for predicting malignant IPMNs were comparable without significant difference (p > 0.05). In multivariable analysis, enhancing solid component in contrast-enhanced CT and MRI and mural nodule in EUS (OR 1.8 in CT, 1.36 in MRI, 1.47 in EUS), MPD diameter ≥ 10 mm (OR 1.3 in CT, 1.4 in MRI, 1.66 in EUS), MPD diameter of 5–9 mm (OR 1.23 in CT, 1.31 in MRI), and thickened septa or wall (OR 1.3 in CT and MRI) were significant variables (p < 0.05). Interobserver agreement of thickened cyst septa or wall (k = 0.579–0.617) and abrupt caliber change of MPD (k = 0.689–0.788) was lower than other variables (k > 0.80).
Conclusion
Diagnostic performance of contrast-enhanced CT, MRI, and EUS for predicting malignant IPMNs was comparable with each modalities without significant difference.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Incidental pancreatic cysts are detected in approximately 13.5% to 19.6% of the patients undergoing imaging studies [1, 2], and more than half of the cysts are premalignant, i.e., mucinous cystic neoplasm (MCN) or intraductal papillary mucinous neoplasm (IPMN) [3]. Although the natural course of IPMNs is still not fully understood, there is no doubt that they are premalignant lesions [4]. Many clinical studies have reported the imaging criteria for discriminating malignant IPMNs from benign IPMNs [5,6,7,8]. In order to clarify and detail the management of IPMNs, the international consensus guidelines for the management of IPMN and MCN of the pancreas were established in 2006 and were revised in 2012 [9, 10]. According to the 2012 guidelines, cysts of ≥3 cm or with thickened, enhanced walls, an MPD size of 5–9 mm, non-enhanced mural nodules, abrupt change of the MPD caliber with distal pancreatic atrophy, and lymphadenopathy constitute “worrisome features” and further endoscopic ultrasound (EUS) examination is recommended. In cysts with obstructive jaundice, an obviously enhancing solid component or an MPD size of ≥10 mm is regarded as a “high-risk stigmata” and should undergo resection [10]. However, the consensus guidelines still comprise an intuitive and qualitative abstract based on previously published studies and clinical experts’ opinions. To our knowledge, there have only been a few reports attempting a more systematic and evidence-based analysis of the predictive findings of malignant IPMNs [11, 12], and therefore, a new effort is necessary in order to differently weigh the findings proposed in the consensus guidelines so as to allow accurate prediction of the malignant potential of IPMNs.
Several studies have indicated that EUS is superior to the other available imaging modalities due to its better spatial resolution for the evaluation of focal pancreatic solid or cystic lesions [13,14,15,16,17,18], and it is recommended as a problem-solving tool if a pancreatic cystic lesion has concerning features seen on CT or MR [10]. However, the diagnostic performance of CT and MRI has been improved due to progressed technology in recent years [11]. To the best of our knowledge, no study has yet been published comparing the diagnostic performance of EUS and contrast-enhanced CT or MRI for predicting the malignant potential of IPMN. Therefore, the purpose of our study is to compare the diagnostic performance and image features for predicting the malignant potential of IPMN of the pancreas, as seen on EUS, CT, and MRI, using the diagnostic criteria proposed in 2012.
Materials and methods
Patient population
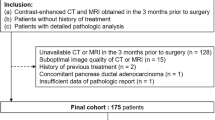
Our institutional review board approved this retrospective study and waived the requirement for informed consent. We reviewed our institution’s medical, pathology, and radiology databases from January 2010 through October 2014 and found that 112 patients with suspected IPMNs underwent preoperative EUS, CT, and MRI with magnetic resonance cholangiopancreatography (MRCP). The mean interval was 2.4 months between the radiologic examinations and their surgery (range, 3 days to 6 months), and the time interval between the EUS and CT or MRI was a maximum of 2 months. Figure 1 shows the flowchart of this study population. Among these patients, 23 were excluded for reasons concerning other pathological diagnoses. Finally, 76 patients were enrolled in our study (43 males and 33 females; mean age, 66.9 years; range, 49–85 years). Among these patients, 29 were previously included in our manuscript with a different research purpose [11]. All of these patients had undergone surgery, i.e., pylorus-preserving pancreaticoduodenectomy (n = 40), Whipple surgery (n = 1), distal pancreatectomy (n = 25), total pancreatectomy (n = 7), or median pancreatectomy (n = 3).
Imaging techniques
One of the following, commercially available, multidetector CT scanners was used: a 16-channel scanner (Sensation 16; Siemens, [n = 24]) or a 64-channel and 128-channel scanner (Brilliance 64, Philips Medical Systems, [n = 41]; Definition, Siemens Healthcare [n = 8]; or Aquilion, Toshiba Healthcare [n = 3]). A section thickness of 3 mm with a 3-mm reconstruction interval, a field of view of 300–370 mm, a gantry rotation time of 0.5 s, a tube current–time product of 150–200 mAs, and a peak voltage of 120 kVp were used for all four CT scanners. For the 16-, 64-, and 128-detector CT examinations, there were detector collimations of 0.75, 0.625 mm, and 0.5 mm, respectively. Table speeds of 13.5, 12.0, and 46.8 mm per rotation were used, retrospectively. A total of 1.5 mL of nonionic contrast material (iopromide 370, 370 mg of iodine per milliliter) per kilogram of body weight was injected at a rate of 2.0–3.0 mL/s using a power injector (Multilevel CT; Medrad, Indianola, PA, USA) with a 20-mL flush of normal saline following the contrast injection. The scanning delay for the pancreatic parenchymal phase was 22–24 s, after achieving enhancement of the descending aorta up to 100 HU. Hepatic venous phase scans were performed 70 s following the contrast material administration.
Each MR examination was performed using commercially available MR scanners, i.e., a 1.5-T MR unit (Signa Excite HDXT, GE Medical Systems, [n = 6]) with an 8-channel, phased-array torso coil or a 3.0-T MR unit (MagnetomVerio, Siemens Medical Solutions, [n = 58] or an Ingenia or Achieva, Philips Medical Systems [n = 12]) with a 12- or 32-channel phased-array torso coil. Baseline MRI included T1WI (repetition time, 4.0–6.6 ms; echo time, 1.3–4.4 ms; flip angle, 9°–15°; and 3–4.8-mm slice thickness) and T2WI (repetition time, 800–900 ms; echo time, 90–160 ms; flip angle, 90°–130°; and 7-mm slice thickness). Thick-slab MRCP images were acquired with six, oblique, coronal angles. The 3D respiratory-triggered T2WI MRCP (repetition time, 3000–5000 ms; echo time, 693–900 ms; flip angle, 130°–180°; and 0.9–1.4 slice thickness) with maximum intensity images for MRCP were generated. Dynamic images were obtained using T1-weighted, 3D gradient-echo sequences (repetition time, 3.2–5.2 ms; echo time, 1.2–2.5 ms; flip angle, 10°–12°; 4–6 slice thickness) after administration of 1.0-M gadobutrol (7.5 mL Gadovist; Bayer Healthcare, Berlin, Germany) at a dose of 0.1 mmol/kg with an injection rate of 2 mL/s. The arterial phase imaging was initiated at 8 s after contrast material arrival at the distal thoracic aorta. Portal-venous phase images at 60 s and delayed images at 2, 3, and 5 min were then obtained.
All patients underwent EUS examinations performed by clinically experienced gastroenterologists in order to evaluate the entire pancreas, cystic lesion, and MPD. Electronic radial sector scanning echoendoscopes (SSD-alpha 10 Ultrasound System; Aloka Co., Ltd., Tokyo, Japan; and GFUM-240 and GFUM2000, Olympus Optical Co, Tokyo, Japan) were used with frequencies of 7.5 or 12 MHz. The endoscope was introduced through the patient’s stomach and further down into the second part of the duodenum, after which the morphologic features of the cystic lesion and the MPD were assessed. The results were recorded according to the reporting format including the size, shape, location, mural nodule, thick septa, diameter of the MPD, communication between a cyst and an MPD, abrupt caliber change of an MPD, and lymphadenopathy.
Image interpretation
Two abdominal radiologists (H.Y.E. and M.H.Y., 17 and 9 years, respectively, of clinical experience in abdominal imaging) retrospectively and independently reviewed the CT and MR images. When multiple lesions were observed, the analysis was performed on the largest lesion. Each reviewer recorded the following parameters: morphologic type; size of the MPD; size of the cyst; the presence of a mural nodule/solid component with or without contrast enhancement; thick septa; the presence of an abrupt caliber change of the MPD; and lymphadenopathy. The MPD diameter was measured at the point of the maximally dilated duct of the pancreas. Mural nodules were defined as any solid nodule within the dilated MPD or cyst. Mural nodules were classified as either a nonenhancing mural nodule or an enhancing mural nodule. A thickened and enhanced cyst wall was defined as a cyst wall greater than 2 mm in width with enhancement, as seen on CT and MRI. On EUS, it was defined as a cyst wall greater than 2 mm in width. The total number of satisfied criteria of the consensus guidelines was summarized as “high-risk stigmata” and “worrisome features” [10]. The presumed malignant potential was then graded using a six-point scale in which 0 = no worrisome feature and high-risk stigmata, 1 = one worrisome features, 2 = two worrisome features, 3 = three worrisome features, 4 = four worrisome features, and 5 = at least one high-risk stigmata or five or more worrisome features. Retrospective review of each EUS examination was done by checking the presence or absence of each criterion referring to the EUS report and was performed by one radiologist (OOO with 6 years of clinical experience). If the necessary content was not written or uncertain in the report, an additional review of the images was performed by consensus with two radiologists.
Statistical analysis
Statistical differences in image findings between the benign and the malignant groups in each modality were evaluated using the Chi-square test, Fisher’s exact test, the Student’s t test, or the Wilcoxon rank-sum test, as appropriate. Interobserver agreement was calculated using the weighted kappa. And weighted κ values < 0 indicated no agreement, 0 < κ ≤ 0.2 slight, 0.2 < κ ≤ 0.4 fair, 0.4 < κ ≤ 0.6 moderate, 0.6 < κ ≤ 0.8 substantial, and 0.8 < κ ≤ 1 almost perfect agreement. Receiver-operating characteristic (ROC) analysis was conducted using the total score. As EUS was performed without contrast enhancement, the scoring without an “enhancing solid component” was also calculated. A two-tailed p < 0.05 was considered statistically significant. All of the statistical analyses were performed using SPSS 14.0 version for Windows and the R 3.1.3 version available without charge on the internet at http://cran.r-project.org/.
Results
Among the 76 patients with IPMN, 37 had benign IPMNs, i.e., 21 with low-grade dysplasia and 16 with intermediate grade dysplasia, and 39 had malignant IPMNs, i.e., 13 with high-grade dysplasia and 26 with invasive carcinoma.
The mean size of the cysts did not differ significantly between benign and malignant IPMNs (2.42 ± 0.92 vs. 2.34 ± 1.44 cm, p = 0.795). Table 1 summarizes each of the imaging findings in the benign and malignant IPMNs. On univariate analysis, all of the findings except for a cyst size of ≥3 cm and lymphadenopathy (p > 0.05) were significantly more common in malignant IPMNs. However, on multivariate analysis, an enhancing solid component (odds ratio (OR) 1.80 on CT; OR 1.36 on MR), an MPD size of 5–9 mm (OR 1.23 on CT; OR 1.40 on MR) and ≥10 mm (OR 1.30 on CT; OR 1.31 on MR) and thickened cyst walls (OR 1.30 on CT; OR 1.30 on MR) were significant variables (Fig. 2). Similarly, on EUS, an MPD with a size of >10 mm and non-enhanced mural nodules were the only significant findings (OR 1.66, p = 0.002 and OR 1.47, p < 0.001) (Fig. 3). Table 2 summarizes the predictors of the malignant potential of IPMNs. On CT and MR, most of the high-risk stigmata and worrisome features showed almost perfect interobserver agreement, except for the thickened, enhanced cyst walls (k = 0.579–0.617) and abrupt caliber change in the MPD (k = 0.689–0.788). The diagnostic performance for predicting a malignant IPMN is summarized in Table 3. The diagnostic performance of contrast-enhanced CT (AUC = 0.792 in R1, 0.830 in R2), MRI (AUC = 0.742 in R1, 0.776 in R2), and EUS (AUC = 0.733) for predicting malignant IPMNs was comparable and without a significant difference (p > 0.05) (Fig. 4). The AUC of CT and MRI was better when an enhancing solid component was included (CT; 0.753–0.830 and MRI; 0.636–0.776). If we defined a malignant IPMN as when the total scoring is 5, approximately 62%–64% of malignant IPMNs were identified with a specificity of 84%–89% (Table 4).
Intraductal papillary mucinous neoplasm associated with an invasive carcinoma in a 72-year-old man. A On venous phases contrast-enhanced CT, a 2.5-cm cystic mass of pancreatic head shows enhancing solid component (arrows score 5). B 3D MRCP image obtained with maximum intensity projection demonstrates pleomorphic cyst in the pancreatic head without duct dilatation (arrowheads). C Contrast-enhanced 3D axial gradient-echo MR image during venous phases also well depicts enhancing solid component (arrows score 5). D EUS with Doppler US well visualizes non-enhanced mural nodule (arrows) with thick septa (score 2)
Intraductal papillary mucinous neoplasm associated with an invasive carcinoma in a 75-year-old woman. A EUS shows 3.5 cm cyst in the pancreatic body. EUS demonstrates non-enhanced mural nodule (arrows) and communicate with 7-mm-sized-dilated main pancreatic duct (arrowheads score 3). B Contrast-enhanced CT during venous phase shows enhancing solid component (arrows score 5). C T2-weighted imaging with a HASTE sequence shows cyst communicate with dilated main pancreatic duct (arrowhead), thick septa, and solid component (arrows). D Contrast-enhanced 3D axial gradient-echo MR image during venous phases well depicts enhancing solid component (arrows score 5). E Cut section of specimen shows solid mural nodule (arrows) within the cysts. Dilated pancreatic duct also noted. It was confirmed to be intraductal papillary mucinous neoplasm associated with an invasive carcinoma
Discussion
In our study, the diagnostic performance of contrast-enhanced CT (AUC = 0.792, 0.830), MRI (AUC = 0.742, 0.776), and EUS (AUC = 0.733) for predicting malignant IPMNs was comparable and without a significant difference (p > 0.05). The diagnostic performance of CT and MRI was better when an enhancing solid component was included (CT, 0.753–0.792 and 0.804–0.830; MRI, 0.636–0.742 and 0.754–0.776). The enhanced solid component seen on CT and MRI and the mural nodule seen on EUS, an MPD diameter ≥10 mm, an MPD diameter of 5–9 mm, and thickened septa or wall were significant variables for the prediction of malignant IPMN (p < 0.05).
To the best of our knowledge, this study is the first attempt to compare the diagnostic performance of CT and MRI with EUS for predicting the malignant potential of pancreatic IPMNs. Although most of the previous studies reported the superiority of EUS to that of CT or MRI for evaluating the details of pancreatic cysts [16,17,18], our study demonstrated that contrast-enhanced CT, contrast-enhanced MR, and EUS were comparable for predicting the malignant potential of pancreatic IPMN without a significant difference (p > 0.05). In our study, if we defined a malignant IPMN when the total score was five or higher on CT and MR, sensitivity and specificity were best (62%–64% and 84%–89%, respectively). Considering that we defined the score as 5 when there was at least one high-risk stigmata or five or more worrisome features, it would be reasonable that pancreatic IPMNs with only worrisome features should be carefully considered and may be managed with careful follow-up, not prompt surgical resection, such as is used for pancreatic IPMNs with high-risk stigmata. All of the criteria of “high-risk stigmata” showed good diagnostic performance and were independent, significant variables in all three imaging modalities. In contrast, each criterion of “worrisome features” showed variable diagnostic performance for prediction of the malignant potential of pancreatic IPMNs, and these results are similar to those seen in previously published studies [12, 19,20,21,22,23]. Among the “worrisome features,” an MPD diameter of 5–9 mm seen on CT and MR, thickened septa or wall seen on CT and MR, and non-enhanced mural nodules seen on EUS were significant independent predictors for malignant IPMNs.
Corresponding to previous study results [11, 12, 19, 24], our results demonstrated that mural nodule enhancement or a solid component was a significant predictor of malignant IPMNs in all three imaging modalities. Yamashita et al. reported the good sensitivity, specificity, accuracy, PPV, and NPV (100%, 80%, 94%, 92% and 100%, respectively) of contrast-enhanced EUS for mural nodule detection and emphasized the role of contrast enhancement for evaluating pancreatic IPMNs [24], and our study results support the previous reports concerning the importance of contrast enhancement for diagnosing pancreatic IPMNs [24,25,26]. However, as contrast-enhanced EUS is not routinely performed in usual clinical practice, it is difficult to discriminate whether an observed mural nodule has a truly enhancing solid component or is a non-enhanced mural nodule when using conventional EUS. In addition, although EUS is currently widely used due to its good resolution and additional advantage of fine-needle aspiration cytology or biopsy, EUS is a more invasive technique than contrast-enhanced CT or contrast-enhanced MR. When considering the importance of recognizing the enhancement of mural nodules in pancreatic IPMNs, contrast-enhanced CT or contrast-enhanced MR would be better imaging tools for predicting the malignant potential of IPMNs than EUS.
Although there is controversy regarding how significant the MPD size is regarding the risk of malignancy [27], it is well-recognized that the malignant potential of pancreatic IPMNs is increased as the MPD diameter increases [6, 28]. Manfredi et al. showed that the MPD diameter in malignant IPMNs is significantly larger than that in benign borderline IPMNs (18 vs. 11 mm, respectively) [6]. Aso et al. reported that 64% of main-duct-type IPMNs without “high-risk stigmata” were malignant and suggested that all patients with main-duct-type IPMNs should undergo resection [22]. The MPD size varied significantly whether it was ≥10 or 5–9 mm.
Thickened septa or wall, abrupt caliber change of MPD, and lymphadenopathy were recently included as “worrisome features” in the 2012 guidelines [9, 10]. According to the meta-analysis of BD-IPMNs [12], thickened septa or wall, a cyst size greater than 3 cm and an MPD size of 5–9 mm showed a statistically significant association with malignancy; however, their absolute diagnostic value as indicators of malignancy was fairly weak, i.e., a pooled specificity of 60%–67%. Furthermore, owing to the newly included imaging findings in the 2012 guidelines, the specificity and PPV of malignant IPMNs were reduced, whereas there was increased sensitivity and NPV [29]. And as with these, the effectiveness of each criterion of the “worrisome features” has not yet been fully established.
Similar to previously published reports [9, 12, 19], in our study, thickened septa or wall was a significant, independent predictor on both CT and MRI [9, 12, 19]. However, interobserver agreement was lower than the other criteria and with moderate to substantial agreement (k = 0.579–0.617). In our study, an abrupt caliber change of MPD with distal atrophy, a cyst size of 3 cm or greater, and lymphadenopathy were not significant variables on multivariate analysis. Many studies have reported that an abrupt caliber change of MPD, a cyst size greater than 3 cm, and lymphadenopathy are not significant factors for malignant IPMNs [4, 9, 19, 23, 30, 31]. Because worrisome features showed variable diagnostic performance for the prediction of malignancy of pancreatic IPMNs, interpretation of the malignant potential of pancreatic IPMNs requires different weighting for each criterion.
Our study has several limitations. First, as it was retrospectively designed, there is an inevitable selection bias. And as pathologic specimens were also retrospectively correlated with imaging findings, there are limitations to the detailed correlation between pathology and imaging findings. Second, as we enrolled patients with surgically resected IPMNs, the enrolled patients did not reflect the entire spectrum of IPMN. Third, as EUS was performed without contrast enhancement, comparison of the diagnostic performance between CT or MRI and EUS in the same setting with contrast enhancement could not be evaluated. However, in order to overcome this problem, we also compared the diagnostic performance of CT, MR, and EUS under the same condition without an enhancing solid portion.
In conclusion, the diagnostic performance for prediction of malignant IPMN was comparable to that of contrast-enhanced CT, contrast-enhanced MR, and EUS and without a significant difference. All “high risk stigmata” and some “worrisome features,” including thickened cyst walls, an MPD size of 5–9 mm, and non-enhanced mural nodules, were independent, significant variables for the prediction of malignant IPMN.
Abbreviations
- IPMN:
-
Intraductal papillary mucinous neoplasm
- MPD:
-
Main pancreatic duct
- MRI:
-
Magnetic resonance imaging
- MRCP:
-
Magnetic resonance cholangiopancreatography
- CT:
-
Computed tomography
- EUS:
-
Endoscopic ultrasound
References
Lee KS, Sekhar A, Rofsky NM, Pedrosa I (2010) Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 105(9):2079–2084. doi:10.1038/ajg.2010.122
Zhang XM, Mitchell DG, Dohke M, et al. (2002) Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 223(2):547–553. doi:10.1148/radiol.2232010815
Kent TS Jr, Vollmer CM, Callery MP (2010) Intraductal papillary mucinous neoplasm and the pancreatic incidentaloma. World J Gastrointest Surg 2(10):319–323. doi:10.4240/wjgs.v2.i10.319
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, vol. 4. Geneva: World Health Organization
Ogawa H, Itoh S, Ikeda M, et al. (2008) Intraductal papillary mucinous neoplasm of the pancreas: assessment of the likelihood of invasiveness with multisection CT. Radiology 248(3):876–886. doi:10.1148/radiol.2482071578
Manfredi R, Graziani R, Motton M, et al. (2009) Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 253(1):106–115. doi:10.1148/radiol.2531080604
Nakajima Y, Yamada T, Sho M (2010) Malignant potential of intraductal papillary mucinous neoplasms of the pancreas. Surg Today 40(9):816–824. doi:10.1007/s00595-009-4227-y
Do RK, Katz SS, Gollub MJ, et al. (2014) Interobserver agreement for detection of malignant features of intraductal papillary mucinous neoplasms of the pancreas on MDCT. AJR Am J Roentgenol 203(5):973–979. doi:10.2214/AJR.13.11490
Tanaka M, Chari S, Adsay V, et al. (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6(1–2):17–32. doi:10.1159/000090023
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197. doi:10.1016/j.pan.2012.04.004
Kim SH, Lee JM, Lee ES, et al. (2015) Intraductal papillary mucinous neoplasms of the pancreas: evaluation of malignant potential and surgical resectability by using MR imaging with mr cholangiography. Radiology 274(3):723–733. doi:10.1148/radiol.14132960
Kim KW, Park SH, Pyo J, et al. (2014) Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg 259(1):72–81. doi:10.1097/SLA.0b013e31829385f7
Kamata K, Kitano M, Kudo M, et al. (2014) Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 46(1):22–29. doi:10.1055/s-0033-1353603
Koito K, Namieno T, Nagakawa T, et al. (1997) Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc 45(3):268–276
Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG (2001) Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas&quest. Am J Gastroenterol 96(12):3295–3300
Kubo H, Nakamura K, Itaba S, et al. (2009) Differential diagnosis of cystic tumors of the pancreas by endoscopic ultrasonography. Endoscopy 41(8):684–689. doi:10.1055/s-0029-1214952
Ohno E, Hirooka Y, Itoh A, et al. (2009) Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 249(4):628–634. doi:10.1097/SLA.0b013e3181a189a8
Tanaka M (2011) Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 8(1):56–60. doi:10.1038/nrgastro.2010.193
Seo N, Byun JH, Kim JH, et al. (2015) Validation of the 2012 international consensus guidelines using computed tomography and magnetic resonance imaging: branch duct and main duct intraductal papillary mucinous neoplasms of the pancreas. Ann Surg . doi:10.1097/SLA.0000000000001217
Kim JH, Eun HW, Kim KW, et al. (2013) Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR Am J Roentgenol 201(3):565–572. doi:10.2214/AJR.12.9511
Schmidt CM, White PB, Waters JA, et al. (2007) Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg 246(4):644–651 (discussion 651–644). doi:10.1097/SLA.0b013e318155a9e5
Aso T, Ohtsuka T, Matsunaga T, et al. (2014) “High-risk stigmata” of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas 43(8):1239–1243. doi:10.1097/MPA.0000000000000199
Jang JY, Park T, Lee S, et al. (2014) Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg 101(6):686–692. doi:10.1002/bjs.9491
Yamashita Y, Ueda K, Itonaga M, et al. (2013) Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med 32(1):61–68
Palmucci S, Trombatore C, Foti PV, et al. (2014) The utilization of imaging features in the management of intraductal papillary mucinous neoplasms. Gastroenterol Res Pract 2014:765451. doi:10.1155/2014/765451
Efthymiou A, Podas T, Zacharakis E (2014) Endoscopic ultrasound in the diagnosis of pancreatic intraductal papillary mucinous neoplasms. World J Gastroenterol 20(24):7785–7793. doi:10.3748/wjg.v20.i24.7785
Barron M, Roch A, Waters J, et al. (2014) Does preoperative cross-sectional imaging accurately predict main duct involvement in intraductal papillary mucinous neoplasm? J Gastrointest Surg 18(3):447–456
Sugiyama M, Izumisato Y, Abe N, et al. (2003) Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 90(10):1244–1249. doi:10.1002/bjs.4265
Nagai K, Doi R, Ito T, et al. (2009) Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg 16(3):353–358. doi:10.1007/s00534-009-0068-8
Augustin T, Vandermeer TJ (2010) Intraductal papillary mucinous neoplasm: a clinicopathologic review. Surg Clin N Am 90(2):377–398. doi:10.1016/j.suc.2009.12.008
Goh BK, Thng CH, Tan DM, et al. (2014) Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg 208(2):202–209. doi:10.1016/j.amjsurg.2013.09.031
Acknowledgements
We would like to thank Bonnie Hami, MA (USA) for her editorial assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Our institutional review board approved this retrospective study and waived the requirement for informed consent. For this type of study, formal consent is not required.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Choi, SY., Kim, J.H., Yu, M.H. et al. Diagnostic performance and imaging features for predicting the malignant potential of intraductal papillary mucinous neoplasm of the pancreas: a comparison of EUS, contrast-enhanced CT and MRI. Abdom Radiol 42, 1449–1458 (2017). https://doi.org/10.1007/s00261-017-1053-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1053-3