Abstract
Objectives
To assess information reflecting radiation dose and define diagnostic reference levels (DRL) on a European basis for four interventional radiology (IR) procedures considering clinical indication, anatomical region, and procedure.
Methods
A prospective European study was performed to provide data on the IR procedures percutaneous recanalization of iliac arteries, percutaneous recanalization of femoropopliteal arteries, transarterial chemoembolization of hepatocellular carcinoma, and percutaneous transhepatic biliary drainage. Hospitals were asked to complete a questionnaire giving information on procedure, equipment, and protocol. Patient size and weight, experience of the operator graded in number of procedures performed, and complexity level of each procedure were reported. Sixteen hospitals from 13 countries could be surveyed. The percentiles of the kerma-area product, fluoroscopy time, cumulative air kerma at the interventional reference point, and number of images were determined. The impact of equipment, year of installation, and complexity level of the procedure on dose were analyzed.
Results
DRLs based on clinical indication were defined. Dose values varied considerably within hospitals, between them, and within each subgroup of complexity level. The use of state-of-the-art equipment reduced dose significantly by 52%. Although dose also varied within each subgroup of complexity level, for transarterial chemoembolization of hepatocellular carcinoma and percutaneous transhepatic biliary drainage, dose significantly correlated with complexity.
Conclusions
This was the first study reporting exposure practice and defining DRLs based on clinical indication for four IR procedures on a European basis. These DRLs can serve as a baseline for comparison with local practice, the study as a guideline for future surveys.
Key Points
• The use of state-of-the-art angiographic equipment reduces dose significantly.
• A significant correlation between radiation dose and complexity level is found.
• Dose values vary considerably, both within and between individual hospitals, and within each complexity level of interventional radiology procedure.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The benefits of fluoroscopy-guided procedures in interventional radiology (IR procedures) are manifold. In contrast to open surgeries, IR procedures decrease risks and patient discomfort, and shorten both the length of time spent in the hospital and the total recovery period. However, IR procedures may also be associated with high radiation exposures for patients [1, 2]. There is a small but non-negligible risk that patients may experience harm by the radiation, such as induced skin injuries [3].
To promote optimization of radiation protection of patients, the European basic safety standards directive states that all European member states should establish, implement, and regularly review diagnostic reference levels (DRLs) for diagnostic examinations and, where appropriate, IR procedures (article 56 of [4]). According to the International Commission on Radiological Protection (ICRP), DRL is the Commission’s term for a form of investigation level used to aid in optimization of protection in the medical exposure of patients for diagnostics and interventional procedures [5]. Numerically, DRL is defined as the 75th percentile value of the distribution of median values of a dose quantity acquired at healthcare facilities. For IR procedures, multiple dose descriptors have been proposed as DRL quantity, such as the air kerma-area product (PKA), the air kerma at the patient entrance reference point (Ka,r), fluoroscopy time (T), and the number of frames (NI). The use of multiple quantities helps to identify the cause when radiation use is not optimized [6,7,8].
In contrast to DRLs for plain radiography or computed tomography (CT), the definition of DRLs for IR procedures is challenging because patient dose depends on a wide variety of factors in addition to procedure type and patient size (e.g., clinical indication, anatomical region, anatomical and procedural complexity, operator skills, access to target lesion, type of catheters, imaging technique, equipment, etc.). The definition is additionally complicated by the ambiguous nomenclature used for categorization of procedures.
There is a strong need for harmonization of DRL practice and nomenclature among institutions and different countries. Considering recommendations of the ICRP report 135, the Clinical task along with the Anatomical location and the IR Procedure (technique) should be specified (CAP approach) for an unequivocal selection of IR procedure and thus for unequivocal definition of DRLs. In this context, the term clinical DRL (DRLCI) was introduced to underline the definition of the corresponding DRL for a selected clinical task, thus for a specific clinical purpose or clinical indication (CI).
Against this background, the European Commission (EC) decided to launch a research project to establish DRLCI for Europe through a survey conducted in hospitals from various countries. The European Society for Radiology (ESR) completed the EC project entitled “European Study on Clinical Diagnostic Reference Levels for X-ray Medical Imaging” (EUCLID; [9]). The aim of this paper is to report the methods and results of the survey for IR procedures and to define DRLCI for Europe.
Materials and methods
List of surveyed IR procedures
After extensive discussions with stakeholder organizations such as the International Atomic Energy Agency, national radiological competent authorities, and professional radiological and medical physics associations, the following four procedures were selected: percutaneous recanalization of iliac arteries referred to as CI 1, transarterial chemoembolization of hepatocellular carcinoma (CI 2), percutaneous recanalization of femoropopliteal arteries (CI 3), and percutaneous transhepatic biliary drainage (CI 4). These IR procedures are performed in many institutions and associated with a wide dose range. For an unequivocal selection of IR procedures by the surveyed hospitals, clinical task, anatomical location, and IR procedure (technique) were clearly defined in advance (Table 1).
Prospective European multicenter survey
Hospitals from different European countries were contacted to provide relevant data on their angiographic equipment (hereafter referred to as “C-arm units”) and the parameter settings used for each of the abovementioned IR procedures. The hospitals contacted were part of the ESR’s “EuroSafe Imaging Stars” network and thus fulfilled various requirements for medical radiation protection, patient safety, and quality control. The main selection criterion for participation was sufficient workload to prospectively provide a representative sample of data sets for a minimum of 20 average-sized adult patients (body mass index, 18.5 to 25 kg/m2, or weight, 70 ± 15 kg; age, > 18 years) for each IR procedure within one year (June 2018–May 2019).
For each procedure included, the following information was requested:
-
1.
The year of installation, manufacturer, type, and detector type of the C-arm units used. Concerning quality control, participating hospitals were asked for the frequency of dose measurements performed to verify the displayed PKA.
-
2.
Special technical options and protocol parameters, i.e., the use of automatic exposure control, pulsed fluoroscopy mode, and the acquisition rate of cine mode.
-
3.
Patient weight and height, as well as dose-relevant parameters such as T, total PKA, and Ka,r of fluoroscopy and cine mode, and number of frames (NI) in cine mode (see [10] for the definition of parameters). Further personal data was not stored.
-
4.
Experience of the operator, measured in number of IR procedures performed, image quality, and complexity level of each IR procedure recorded. A physician was considered very experienced after the performance of 20 procedures of the corresponding kind, in total. The local teams at the participating hospitals were also asked for their subjective evaluation of the image quality achieved in the procedure, i.e., if it is (a) adequate or (b) inadequate, or (c) exceeds the quality required to successfully perform the intervention. In cases (b) and (c), follow-up questions were asked on reasons for this. The complexity indices introduced by Ruiz-Cruces et al [11] were used for definition of complexity levels to grade procedures as easy (numerical range of complexity level, 3–4 for CI 1-3 and 4–6 for CI 4), medium (5–7 for CI 1–3, 7–9 for CI 4), or highly difficult (8–9 for CI 1–3, 10–12 for CI 4). The criteria considered included anatomical characteristics, type and/or location of injury, and type of treatment.
Data collection and cleansing
Ethical board approval was the responsibility of each participating hospital. To collect data, a secure web application for research electronic data capture [12] was used. During the survey, all the data values were continuously reviewed to ensure that they were in the correct format and order of magnitude. For further data verification, participating hospitals were encouraged to provide anonymized DICOM radiation dose structured reports of one or more procedures. In cases where potential errors or missing information for DRL identification was identified in the submitted information, participants were contacted to verify, correct, or complete the data. Bi-weekly teleconferences between hospitals and research group members were organized for answering any questions arising. Moreover, emails were sent to all hospitals encouraging them to ask any questions related to data submission. After data cleansing, i.e., reviewing and correcting the data, representatives of the national radiological competent authorities received all the data from the hospitals from their countries to recheck any inconsistencies and errors.
Data analysis
Mean and median values as well as the 5th, 25th, 75th, and 95th percentiles of PKA, T, NI, and Ka,r were computed for each data sample, i.e., for each CI and hospital, separately. Percentiles were determined by linear interpolation between adjacent parameter values. Finally, for each CI, separately, the 75th and 50th percentiles of median values were computed. The 75th percentiles were referred to as the DRLCI. The statistical significance of differences between defined subgroups of parameters (e.g., impact of year of installation on dose) was tested using either the Mann-Whitney or the Kruskal-Wallis test, depending on how many subgroups were considered. All statistical tests were performed at a significance level of p < 0.05 with the program package IDL (Interactive Data Language, version 8.7.2, Harris Geospatial Solutions Inc., 2019).
Results
Reporting hospitals
A network of 16 large- or medium-size hospitals (one Austrian, one Belgian, one Dutch, one Finnish, one French, one German, one Greek, one Hungarian, one Irish, two Italian, one Polish, one Portuguese, one Swiss, two Spanish), with 25 C-arm units in total, was established (a table with the participating hospitals is published as supplementary material).
Not all participating hospitals could submit data samples with a minimum of 20 patients each. Data samples with less than 20 patients were not accepted. In summary, 61%, 64%, 81%, and 70% of the data requested for each of the four IR procedures (69%, on average) were included for further analysis. All but three data samples contained more than 30 patients.
Year of installation and its impact on dose
The C-arm units registered in the survey were installed between 2003 and 2018. On average, the systems had been in operation for seven years. Eleven units were installed after 2014, nine units before 2011.
For CI 1, CI 3, and CI 4, the PKA and Ka,r values reported for C-arm units produced between 2015 and 2018 were significantly (about 52%) lower than units produced between 2003 and 2010. The same relationship could not be found for CI 2. Two hospitals (FCB, GVA) with recently installed C-arm (2017 and 2018) units used relatively high doses for this procedure.
Equipment and its impact on dose
Two of the oldest units (installed in CHUC and UoC in 2003/2004) were equipped with an image intensifier, all other units with a digital flat panel detector. The PKA and Ka,r values received from the units with image intensifier were larger, though not significantly, than those for units equipped with digital detectors (Table 2).
Manufacturer and its impact on dose
More than three quarters (79%) of the C-arm units were produced by Siemens Healthineers, the other systems by Philips Healthcare. No relationship between the PKA and Ka,r values and the manufacturer was found.
Quality control
As part of regular quality control of the C-arm units, dose measurements were performed annually in 11, biannually in 2, quarterly in 1, and monthly in 1 of the participating hospitals. In one case, dose measurements were not performed at all.
In five hospitals, the dose display was not verified by measurements. For hospitals where the dose display was verified, the minimum, mean, and maximum percentage deviation of displayed PKA values from measured PKA values were 0.1%, 11%, and 25%, respectively.
Protocol parameters
In all but one participating hospital, the automatic exposure control was used. The pulsed fluoroscopy mode was constantly used in twelve hospitals and used for most patients in three hospitals. In one hospital, however, this mode was not used at all.
In cine mode, the mean frame rate was 4.2 frames per second (fps). While in 10 hospitals the mean frame rate was between 1 fps and 4 fps, 4 hospitals (APH, FCB, HRH, UMM) used a constant frame rate of 7.5 fps, and one hospital (GVA) even used a mean frame rate of 14 fps. During procedures, the cine mode settings were changed in 18% of all the procedures. In these cases, the frame rate decreased to 3.7 fps, on average. Apart from UOR and LUX, the frame rate was not varied between the four CIs.
Patient characteristics and dose quantities
The median weight and height of patients ranged from 62 to 85 kg and from 168 to 181 cm, respectively. For hospitals that submitted data samples with a minimum of 20 patients, the 5th, 25th, 75th, and 95th percentiles, as well as median and mean values, of PKA, T, Ka,r, and NI of the surveyed procedures are shown in Figs. 1, 2, 3, and 4a–d. The NIs of two participating hospitals (FCB, GVA) were excluded from data analysis because they did not provide NI but rather the total number of single fluoroscopic image frames. The DRLCI and the 50th percentiles of median dose values of PKA, T, Ka,r, and NI for CI 1–4 are listed in Table 3.
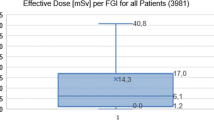
Semi-logarithmic box and whisker plots of (a) kerma-area product (PKA), (b) fluoroscopic time (T), (c) cumulative air kerma at patient reference point (Ka,r), and (d) number of images (NI), shown for the hospitals that submitted a data sample of 20 patients, at least, for the clinical indication 1 (CI 1). The boxes are drawn around the 25th and 75th percentiles and divided by the median. Dashed lines represent the arithmetic mean, and whiskers extend out the 5th and 95th percentiles
Semi-logarithmic box and whisker plots of (a) PKA, (b) T, (c) Ka,r, and (d) NI shown for the hospitals that submitted a data sample of 20 patients, at least, for CI 2. For further explanations, see Fig. 1
Semi-logarithmic box and whisker plots of (a) PKA, (b) T, (c) Ka,r, and (d) NI shown for the hospitals that submitted a data sample of 20 patients, at least, for CI 3. For further explanations, see Fig. 1
Semi-logarithmic box and whisker plots of (a) PKA, (b) T, (c) Ka,r, and (d) NI shown for the hospitals that submitted a data sample of 20 patients, at least, for CI 4. For further explanations, see Fig. 1
For most procedures and hospitals, mean dose values were substantially larger than median values, indicating a skewed dose distribution with a large tail of high values (see Figs. 1, 2, 3, and 4). The inter-percentile ranges between the 5th and 95th as well as 25th and 75th percentiles of PKA, which are shown by the lower and upper end of the whiskers and of the boxes in Figs. 1a, 2a, 3a and 4a, respectively, present the dose variations for each CI and hospital. The inter-percentile ratios of the 75th and 25th percentiles as well as the 95th and 5th percentiles of PKA are shown in Table 4 (left columns). These ratios show large intra-hospital dose variations for each CI. The median values of PKA of hospitals, represented by the solid dividing lines of the boxes in Figs. 1a, 2a, 3a and 4a, can strongly differ (right column of Table 4) showing large inter-hospital dose discrepancy.
Image quality and the impact of operator skills on dose
According to the evaluations of participants, image quality was adequate for answering the diagnostic question or for safely performing the procedure. Almost all procedures (98%) were performed by well-experienced physicians who had performed more than 20 procedures of the corresponding kind. Less-experienced physicians performed procedures at KUH, in particular, and at FCB, MUH, and UOR in a few cases.
Complexity level and its impact on dose
Averaged over the participating hospitals, the complexity levels were 5.1 ± 1.8, 4.4 ± 2.0, 4.5 ± 1.9, and 6.4 ± 2.5 for CI 1–4, respectively. Therefore, on average, IR procedures were medium difficult based on the criteria introduced by Ruiz-Cruces et al [11]. For CI 1–4, the mean number of highly difficult procedures were 2.4, 1.6, 1.2, and 1.6, respectively. Only a few hospitals performed more than 3 highly difficult procedures. For instance, hospital KUH performed 11 highly difficult procedures for CI 1. It is important to note that the highest dose values (highest 50th and 95th percentiles) of PKA of the corresponding CI were not necessarily found for the hospitals performing the most complex procedures.
In general, median PKA increased between easy and highly difficult procedures by 40%, 276%, 26%, and 232% for procedure types CI 1–4, respectively. However, this increase is statistically significant for CI 2 and CI 4, only. For each of the three complexity levels and procedures CI 1–4, the distribution of PKA and the corresponding 50th and 75th percentiles are shown in Fig. 5. There are highly difficult procedures with relatively low PKA and, vice versa, easy procedures with high doses. Independent of procedure type, the number of procedures with low doses decreases and the percentiles increase with increasing complexity while the variance of dose values increases.
Discussion
To the best of our knowledge, this study is the first survey on exposure practice and DRLCI based on dose data of IR procedures performed in hospitals of 13 different countries in all parts of Europe. Data were prospectively acquired within one year, and participating hospitals had to manually complete the online questionnaire to avoid the problem of a still incongruent, non-automatic categorization of IR procedures between different hospitals. The questionnaire included instructions, and bi-weekly teleconferences were organized for all participating hospitals to make clarifications and answer any questions. Despite extensive follow-up, some participating hospitals did not understand what was meant by a few questions of the questionnaire (e.g., when asking for the total number of images in cine mode, two hospitals erroneously provided the total number of fluoroscopic image frames).
One of the most important findings of this survey is the large intra- and inter-hospital dose variations. These dose variations cannot be traced back to:
-
the incorrect assignment of IR procedures to one of the IR procedures surveyed; considering clinical task, anatomical location, and procedure, the CAP approach precisely defined which IR procedures should have been included for CI 1–4
-
significantly different complexity levels, solely; in the results shown above, some hospitals which performed highly difficult procedures did not necessarily record the highest doses (e.g., median dose values of KUH are not the highest median dose values of CI 1 although this hospital classified 11 from 30 procedures as highly difficult)
-
equipment age, solely, as two hospitals (FCB, GVA) with relatively new equipment recorded relatively high patient doses. (FCB; GVA)
It can be presumed, therefore, that dose discrepancies also result from non-optimized IR procedures. Another important finding of this study was that dose subgroups of different complexity levels only differ significantly for CI 2 and CI 4. A reason could be the limited number of procedures, in particular of highly difficult procedures (Fig. 5), which could result in an ambiguous outcome. Furthermore, some of the complexity indices introduced by Ruiz-Cruces et al [11] do not allow an unambiguous, objective differentiation between complexity levels without further clarifications (e.g., it was asked in the questionnaire: “Is the biliary ductal dilatation very or moderately long”). This could lead to an increased variance of PKA values between the subgroups of complexity level. Considering the information provided by some hospitals that the assessment of complexity level for each individual procedure is quite laborious and cannot be performed automatically, for future surveys, complexity levels for which procedure and with which indices will be established must be discussed. If complexity levels will be established in future surveys, the provision of a guideline on how to unambiguously and objectively differentiate between the levels is recommended.
The current survey reveals considerable potential for further dose reduction by the performance of regular quality assurance and optimizing protocol parameters taking into account the following aspects:
C-arm units
The significant differences in PKA and Ka,r values between old and modern units may be due to technical developments of filters or detectors.
Operator experience
In our study, 98% of procedures were performed by well-experienced physicians. However, previous studies demonstrated a significant decrease of dose with increasing experience of physicians. For instance, Fetterly et al [13] found a significant decrease of dose for physicians who performed 100 procedures of the same kind, at least, within an investigation period of 14 months.
Quality control
The study showed deficiencies in some hospitals concerning the quality control of C-arm units, such as the verification of the dose display as specified in international technical standards, guidelines, and directives [4, 10, 14, 15]. For hospitals where the dose display was not verified by measurements (FCB, GVA, HRH, UoC, PSH), the uncertainty of registered dose values, PKA and Ka,r, could be large.
Protocol parameters
Basic safety and dose reduction features such as automatic exposure control and pulsed mode were available and used for most procedures CI 1–4. Compared to fluoroscopy mode, the dose in cine mode is many times larger [16, 17]. Therefore, the cine mode should be used with discernment and avoided when enough safety and accuracy of the procedure is achieved with fluoroscopy. Additionally, the pulse rate for cine (and fluoroscopy) mode should be reduced as far as possible. An important finding of this study is that six hospitals frequently used quite a low frame rate of 2 or even 1 fps in cine mode. In addition to protocol parameters, the beam size had to be collimated strictly to the region of interest of the body, but this parameter was not surveyed in this study.
Dose quantities
Whenever DRLCI are exceeded, the user should take into account patient size, operator skills, and complexity of the procedure to clarify the reasons for the excessive exposure. However, when applying good medical practice and using modern equipment, it should be possible to achieve the exposure level defined by the corresponding median value (Table 3) for standard-sized patients. Nevertheless, the dose must not be lowered to the point that the image quality is no longer sufficient for the intervention to succeed. The recording of parameters such as time T and number of frames NI (Table 3) can assist in understanding why individual doses might exceed DRLs. [5]. The dose parameter Ka,r is specified as another DRL quantity in light of the risk arising from patient skin dose [3]. In fact, for CI 2, there were individual procedures where Ka,r and PKA exceeded a critical threshold level of 3 Gy or 500 Gycm2 [18, 19], respectively, potentially resulting in skin damage. In conclusion, EUCLID’s DRLCI could serve users as a baseline for comparison with local exposure practice and for further optimization.
It is difficult to compare EUCLID’s DRLCI with pre-existing DRLs reported for other countries due to (i) the fact that these values were reported for IR procedures and not for clinical indications; (ii) inconsistencies in the reported description of IR procedures; and (iii) missing information on clinical task, anatomical location, and technical procedure. With these limitations in mind, EUCLID’s DRLCI are lower than those reported in studies within the last 5 years (Table 5, [11, 20,21,22]). One reason for the difference could be the time gap between the surveys and the evolution of technique within that period, in particular for the case of Ruiz-Cruces et al [11] who collected data between 2010 and 2013. Another reason could be that participating hospitals belong to the “EuroSafe Imaging Stars” initiative. This might have led to the selection of hospitals already engaged in an optimization program and, therefore, to lower doses.
This study has several limitations. Firstly, while only large- or medium-size hospitals were selected as it was initially assumed that these hospitals had sufficient workload, some of them ultimately failed to provide the data required by the survey (20 patients per CI) within the time period of 12 months. Secondly, the image quality achieved in the IR procedures was evaluated subjectively by the participants. In many previous studies where image quality was evaluated objectively (e.g., [16]), it was shown that image quality frequently exceeds the level required for confident diagnosis or procedure control. Thirdly, the values derived from 16 hospitals do not represent the IR practice of Europe.
Conclusion
This was the first study reporting information reflecting radiation dose of four frequently performed IR procedures in 16 hospitals of 13 European countries. Dose values varied considerably within hospitals, between them, and even within each subgroup of complexity level. Therefore, there is high potential for dose optimization, e.g., by using state-of-the-art angiographic equipment and/or adapting protocol parameters such as the pulse rate to the complexity of the procedure as well as clinical indication, anatomical region, and procedure used. In this context, EUCLID’s DRLCI can help in optimizing the dose in interventional procedures. Furthermore, the methods and results of this study can be used as a guideline for future Europe-wide dose surveys.
Abbreviations
- AEC:
-
Automatic exposure control
- CI:
-
Clinical indication
- DRL:
-
Diagnostic reference level
- ESR:
-
European Society of Radiology
- EUCLID:
-
European Study on Clinical DRLs
- fps:
-
Frames per second
- ICRP:
-
International Commission on Radiological Protection
- IR:
-
Interventional radiology
- K a,r :
-
Cumulative air kerma at the patient entrance reference
- NI :
-
Number of frames in cine mode
- pct:
-
Percentile
- P KA :
-
Kerma-area product
- PTA:
-
Percutaneous transluminal angioplasty
- PTBD:
-
Percutaneous transhepatic cholangiography and biliary drainage
- T :
-
Fluoroscopy time
- TACE:
-
Transarterial chemoembolization of hepatocellular carcinoma
References
European Commission (2014) Medical radiation exposure of the European population. Radiation Protection 180. European Commission, Brussels. Available via https://ec.europa.eu/energy/topics/nuclear-energy/radiation-protection/scientific-seminars-and-publications/radiation-protection-publications_en. Accessed 8 Jan 2021
United Nations Scientific Committee on the Effects of Atomic Radiation (2008) Sources and effects of ionizing radiation UNSCEAR Report to the General Assembly I:1-24. United Nations, New York. Available via https://www.unscear.org/unscear/en/publications/2008_1.html. Accessed 8 Jan 2021
Miller DL, Vañó E, Bartal G et al (2010) Occupational radiation protection in interventional radiology: a joint guideline of the cardiovascular and interventional radiology society of Europe and the society of interventional radiology. Cardiovasc Intervent Radiol 33:230–239
Council of the European Union (2014) 2013/59/Euratom on basic safety standards for protection against the dangers arising from exposure to ionising radiation and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Official Journal of the EU L 13:1-73. European Council, Brussels. Available via https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013L0059&qid=1614947445366. Accessed 8 Jan 2021
International Commission on Radiological Protection (2017) Diagnostic reference levels in medical imaging. ICRP Publication 135. Ann ICRP 46
Miller DL, Hilohi CM, Spelic DC (2012) Patient radiation doses in interventional cardiology in the U.S.: advisory data sets and possible initial values for U.S. reference levels. Med Phys 39:6276–6286
Miller DL, Kwon D, Bonavia GH (2009) Reference levels for patient radiation doses in interventional radiology: proposed initial values for U.S. practice. Radiology 253:753–764
Vañó E, Gonzalez L (2001) Approaches to establishing reference levels in interventional radiology. Radiat Prot Dosimetry 94:109–112
European Society of Radiology (2020) EUCLID – European study on clinical diagnostic reference levels for X-ray medical imaging. European Society of Radiology, Vienna. Available via http://www.eurosafeimaging.org/euclid/. Accessed 8 Jan 2021
International Electrotechnical Commission (2017) Medical electrical equipment - Part 2-43: particular requirements for basic safety and essential performance of X-ray equipment for interventional procedures IEC 60601-2-43+AMD1
Ruiz-Cruces R, Vañó E, Carrera-Magariño F et al (2016) Diagnostic reference levels and complexity indices in interventional radiology: a national programme. Eur Radiol 26:4268–4276
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Fetterly KA, Lennon RJ, Bell MR, Holmes DR, Rihal CS (2011) Clinical determinants of radiation dose in percutaneous coronary interventional procedures. JACC Cardiovasc Interv 4:336–343
European Commission (2012) Criteria for acceptability of medical radiological equipment used in diagnostic radiology, nuclear medicine and radiotherapy. Radiation Protection 162. European Commission, Brussels. Available via https://ec.europa.eu/energy/topics/nuclear-energy/radiation-protection/scientific-seminars-and-publications/radiation-protection-publications_en. Accessed 8 Jan 2021
International Atomic Energy Agency (2014) Radiation protection and safety of radiation sources: international basic safety standards. General safety requirements part 3. International Atomic Energy Agency, Vienna. Available via https://www.iaea.org/publications/8930/radiation-protection-and-safety-of-radiation-sources-international-basic-safety-standards. Accessed 8 Jan 2021
Vañó E, Gonzalez L, Fernandez JM, Prieto C, Guibelalde E (2006) Influence of patient thickness and operation modes on occupational and patient radiation doses in interventional cardiology. Radiat Prot Dosimetry 118:325–330
Pitton MB, Kloeckner R, Schneider J, Ruckes C, Bersch A, Düber C (2012) Radiation exposure in vascular angiographic procedures. J Vasc Interv Radiol 23:1487–1495
Balter S, Miller DL, Bushberg JT et al (2014) Outline of administrative policies for quality assurance and peer review of tissue reactions associated with fluoroscopically-guided interventions. National Council on Radiation Protection and Measurements (NCRP) Statement No. 11
Federal Republic of Germany (2018) Radiation protection ordinance. Federal law gazette Part 1 No. 41. Federal Republic of Germany, Bonn. Available at: https://www.bmu.de/en/law/radiation-protection-ordinance-1/. Accessed 8 Jan 2021
Etard C, Bigand E, Salvat C et al (2017) Patient dose in interventional radiology: a multicentre study of the most frequent procedures in France. Eur Radiol. https://doi.org/10.1007/s00330-017-4780-5
Schegerer A, Loose R, Heuser LJ, Brix G (2019) Diagnostic reference levels for diagnostic and interventional X-Ray procedures in Germany: update and handling. Rofo 191:739–751
Schmitz D, Vogl T, Nour-Eldin NA et al (2019) Patient radiation dose in percutaneous biliary interventions: recommendations for DRLs on the basis of a multicentre study. Eur Radiol 29:3390–3400
Acknowledgements
The authors are grateful to the participating hospitals for their contribution to this study. Furthermore, the support of the representatives of national authorities and professional societies is gratefully acknowledged. In particular, the authors thank Jonathan Clark, Ulrike Mayerhofer-Sebera, and Monika Hierath, ESR, for their untiring commitment in organizing and administrating this project. The EUCLID project was financially supported by the grant ENER/2017/NUCL/SI2.759174 of the European Commission.
Funding
This study has received funding from the European Commission, grant no. ENER/2017/NUCL/SI2.759174.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Guy Frija.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Boards.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• cross-sectional study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Schegerer, A.A., Frija, G., Paulo, G. et al. Radiation dose and diagnostic reference levels for four interventional radiology procedures: results of the prospective European multicenter survey EUCLID. Eur Radiol 31, 9346–9360 (2021). https://doi.org/10.1007/s00330-021-08029-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08029-y