Abstract
Purpose
To evaluate diagnostic values of the liver imaging reporting and data system (LI-RADS) M (LR-M) category based on novel explicit criteria that accept both targetoid and nontargetoid LR-M features and the suggested reporting algorithm of LI-RADS v2018 to assess primary liver cancers (PLCs) on gadoxetic acid-enhanced MRI (Gd-EOB-MRI).
Methods
This retrospective study included 165 patients at high risk for hepatocellular carcinoma (HCC) with pathologically confirmed PLCs (HCC, n = 113; intrahepatic cholangiocarcinoma [iCCA], n = 23; and combined hepatocellular cholangiocarcinoma [cHCC-CCA], n = 29). Two radiologists independently analyzed Gd-EOB-MRI features and determined LI-RADS category for each tumor and categorized the likely etiology either as HCC or non-HCC malignancy if LR-M was assigned. Diagnostic performances for HCC or those for malignancy were compared according to imaging criteria.
Results
LR-M was assigned in 95.7%/91.3% of iCCAs; 55.2%/58.6% of cHCC-CCAs; and 21.2%/17.7% of HCCs in reviewers 1/2. Combination of LR-5 plus LR-M resulted in sensitivity of 95.2%/97.6% to diagnose PLCs as malignant, which were significantly higher than that of LR-5 plus “LR-M with ≥ 1 targetoid appearances” (84.8%/91.5%, Ps < 0.01). In comparison to LR-5, LR-5 plus “LR-M of HCC as likely etiology” resulted in significant increase in sensitivity (73.5%/79.6% versus 87.6%/92.9%, Ps < 0.001) but significant decrease in specificity (76.9%/75.0% versus 57.7%/50.0%, P = 0.002 and < 0.001) in the diagnosis of HCC.
Conclusion
The LR-M criteria v2018 are useful to differentiate non-HCC malignancies from HCCs and to accurately diagnose PLCs as a malignancy. Reporting the likely etiology in LR-M may facilitate a more sensitive detection of HCC, but along with a considerable decrease in specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The CT/MRI liver imaging reporting and data system (LI-RADS) diagnostic algorithm include a special category, i.e., LI-RADS M (LR-M), for observations that are probably or definitely malignant, but not necessarily hepatocellular carcinoma (HCC) [1]. This category primarily aims to maintain the specificity of LR-5 (definitely HCC) without the loss of sensitivity to detect malignancies including HCC with atypical imaging features, intrahepatic cholangiocarcinoma (iCCA), and combined hepatocellular cholangiocarcinoma (cHCC-CCA) [1, 2]. New explicit LR-M criteria have been introduced through the LI-RADS v2017 (same in v2018), which include targetoid appearances along with several nontargetoid imaging features [1].
Differential diagnosis between HCC and non-HCC primary liver cancer (PLC) on imaging is critical with regard to the treatment decision and prediction of prognosis [3]. However, it is often challenging as iCCA and cHCC-CCA share risk factors with HCC, and may present imaging features mimicking HCC such as arterial hypervascularity [4, 5]. To preserve the highest specificity of the LR-5 category, it would be essential to accurately categorize non-HCC PLCs as LR-M to prevent them from being assigned as LR-5. In this regard, LI-RADS has been reported to be efficient in general; however, there are substantial limitations to cHCC-CCA [6, 7].
Apart from avoiding false-positive diagnosis of non-HCC PLCs as definitely HCC (LR-5), it is imperative to accurately categorize both HCC and non-HCC PLCs as malignant for the timely management such as percutaneous biopsy or surgical excision [8]. Therefore, with regard to the PLCs that do not meet the LR-5 criteria, LR-M assignment would facilitate subsequent diagnostic work-up or treatment while LR-4 (probable HCC) or lower category assignment may further delay the process. In the LR-M assignment, targetoid appearances would play a major role as well-established imaging features favoring non-HCC malignancy, particularly iCCA [8]. Recently, nontargetoid LR-M features were incorporated in LR-M criteria, which include infiltrative appearance, marked diffusion restriction, necrosis or severe ischemia, and other features that in radiologist’s judgment suggests non-HCC malignancy [1]. Those features may be permitted to assign LR-M category in the observations that do not meet the LR-5 criteria [8]. Therefore, if nontargetoid LR-M features are present, PLCs without targetoid appearances and not meeting LR-5 criteria can be categorized as LR-M rather than LR-4 or a lower category. However, until now, little has been known about the diagnostic impact of new LR-M criteria including both targetoid and nontargetoid LR-M features for malignancy.
When assigning an observation as an LR-M category, the LI-RADS v2018 recommends reporting the most probable etiology in the radiological report [1]. Considering that LR-M is composed of a heterogeneous group of disease entities [9], imaging prediction of the likely etiology in LR-M observations may influence the management, including the need and urgency of biopsy. Therefore, it would be imperatively needed to assess the diagnostic value of the LR-M reporting algorithm containing the radiologist’s impression of whether the observation is HCC or non-HCC malignancy.
Therefore, this study aimed to evaluate the diagnostic values of LR-M criteria and reporting algorithm of the LI-RADS v2018 to assess PLCs on gadoxetic acid-enhanced MRI (Gd-EOB-MRI).
Materials and methods
Patients
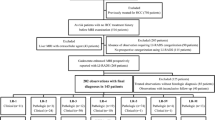
Using a computerized search of our institution’s pathology database, we identified patients with surgically confirmed HCC between Jan 2015 and Dec 2015 and patients with surgically or biopsy-confirmed iCCA or cHCC-CCA between Jan 2015 and Dec 2017 (Fig. 1). Biopsy-confirmed HCCs were not included to avoid the possibility of sampling errors in cHCC-CCA. Patient inclusion criteria were as follows: (i) patients at high-risk for HCC according to the LI-RADS definition [1] with pathologically confirmed PLC, (ii) available Gd-EOB-MRI before pathologic confirmation (≤ 2 months), (iii) no prior treatment for the target observation. We excluded patients with (i) Child–Pugh class C because hepatocyte uptake of gadoxetic acid is known to be affected by the liver function that may impact the image quality of Gd-EOB-MRI [10], (ii) MRI with severe motion artifact, (iii) no visible parenchymal mass on MRI matched to the pathologically diagnosed PLC. If patients presented with multiple tumors satisfying the inclusion criteria, one tumor was chosen as a representative according to the following principle: non-HCC malignancy was prioritized and the largest one was selected among those of same diagnosis. Two radiologists (M.Y.K. and I.J.) who were unblinded to the clinical information and pathologic diagnoses finalized the study population and annotated the selected tumors in HBP images for the image analysis. Final study population included 165 patients with either HCC (n = 113), iCCA (n = 23), or cHCC-CCA (n = 29) (Fig. 1). Patient characteristics are described in Table 1.
Gd-EOB-MRI acquisition
Gd-EOB-MRI examinations were performed using either 1.5T (n = 35) or 3 T (n = 130) MRI scanners. Routine liver MRI protocol in our institution included a fat-saturated T2-weighted fast spin-echo, a half-Fourier acquisition single-shot turbo spin-echo sequence (HASTE), a breath-hold three-dimensional in- and opposed-phase T1-weighted gradient echo sequence, free-breathing diffusion-weighted imaging (DWI) using b values of 0 and 800 s/mm2, and dynamic phase imaging with a fat-saturated T1-weighted gradient echo sequence. With regard to dynamic phase imaging, a standard dose (0.025 mmol/kg) of gadoxetic acid (Primovist®; Bayer Healthcare, Berlin, Germany) was intravenously administered via an antecubital vein catheter at a rate of 1.0 mL/s using a power injector (Spectris Solaris EP; Medrad, Warrendale, PA, USA) immediately followed by a 20 mL saline flush. Arterial phase (AP) images were acquired 7–8 s after contrast material reached at the distal thoracic aorta through an MR fluoroscopic monitoring system. Subsequently, portal venous phase (PVP), transitional phase (TP) and hepatobiliary phase (HBP) images were respectively obtained at approximately 50–60 s, 3 min, and 20 min after starting the intravenous contrast material administration.
Image analysis
Two board-certificated abdominal radiologists (H.J.K. and S.K.J. with 8 and 6 years of experiences in liver MRI, respectively) independently reviewed Gd-EOB-MRI scans. They were aware that the study population included patients who were at a high-risk for HCC with either HCC or non-HCC PLCs (iCCA or cHCC-CCA); however, they were blinded to the pathologic diagnosis of each tumor. The reviewers assessed the presence or absence of imaging features for each annotated observation, assigned an LI-RADS category, and determined the most probable etiology in LR-M observations. Furthermore, even for observations with definite tumor in vein (corresponding to the LR-TIV), the reviewers were asked to determine the LI-RADS category, either LR-M or LR-5, for the parenchymal mass which was used for a representative category for each PLC throughout this study.
Imaging features
The presence or absence of imaging features was assessed according to the definition of the LI-RADS [1]. The assessed imaging features included tumor size (mm), nonrim arterial phase hyperenhancement (APHE), and additional major HCC features (nonperipheral “washout” and enhancing “capsule”), targetoid appearances (rim APHE, peripheral “washout”, delayed central enhancement, targetoid diffusion restriction, and targetoid TP or HBP appearance), and nontargetoid LR-M features (infiltrative appearance, marked diffusion restriction, necrosis or severe ischemia, and other ancillary features suggesting non-HCC malignancy [presence of liver surface retraction and peritumoral bile duct dilatation]) [8]. Nonperipheral or peripheral “washout” was assessed only in PVP as suggested in the LI-RADS v2018 for Gd-EOB-MRI [1]. Threshold growth is one of the major HCC features; however, it was not assessed since only one MRI scan was evaluated per patient.
LI-RADS category assignment
LI-RADS category for each PLC was assigned either as LR-M, LR-5, LR-4, LR-3 (indeterminate probability of HCC), LR-2 (probably benign), or LR-1 (definitely benign) according to the LI-RADS v2018 algorithm with optional use of ancillary features and tie-breaking rules [1].
Likely etiology in LR-M observation
If an observation was assigned as LR-M category, reviewers were asked to report the likely etiology either as HCC or non-HCC PLC based on the reviewers’ judgment as suggested by the LI-RADS v2018 [1].
Statistical analysis
Imaging features were compared between LR-M versus non-LR-M observations and according to pathologic diagnoses (HCC versus non-HCC PLC) in LR-M observations using Fisher’s exact test. Inter-observer agreements for LR-M imaging features were assessed using κ statistics and were interpreted as follows: poor, κ < 0.20; fair, 0.20 ≤ κ < 0.40; moderate, 0.40 ≤ κ < 0.60; substantial, 0.60 ≤ κ < 0.80; and almost perfect, κ ≥ 0.80. Sensitivity, specificity, and accuracy for HCC diagnosis (HCC versus non-HCC PLC; or HCC versus iCCA) were compared among different diagnostic imaging criteria using the McNemar test. The additional role of nontargetoid LR-M features to targetoid appearance was investigated by comparing the sensitivity for the diagnosis of malignancy between LR-5 plus “LR-M with ≥ 1 targetoid appearances” and LR-5 plus LR-M v2018 (with or without targetoid appearances) using the McNemar test. Statistical analyses were performed with IBM SPSS version 23.0 for Windows (IBM Corp, Armonk, NY, USA). P value of less than 0.05 indicated statistical significance.
Results
LI-RADS categorization of primary liver cancers
Assigned LI-RADS categories according to the pathologic diagnoses are summarized in Table 2. HCCs were accurately assigned as LR-5 in 73.5% (83/113) and 79.6% (90/113) by reviewers 1 and 2, respectively, while approximately 20% of HCCs were assigned as LR-M and the remaining as LR-4. However, most iCCAs (95.7% [22/23] and 91.3% [21/23]) were accurately assigned as LR-M by both reviewers. Regarding cHCC-CCAs, approximately 60% were categorized as LR-M but a substantial proportion (37.9% [11/29] and 41.4% [12/29]) of them were false positively diagnosed as definitely HCC (LR-5). None of PLCs were assigned as LR-1 to LR-3 in both reviewers.
Imaging features
LR-M versus non-LR-M
Comparing the Gd-EOB-MRI imaging features between LR-M PLCs and non-LR-M PLCs is described in Online Resource Table 1. Among targetoid appearances in LR-M observations, rim APHE was most sensitive feature followed by targetoid HBP and delayed central enhancement in both reviewers (Fig. 2). None of the non-LR-M (LR-4 or LR-5) observations demonstrated any targetoid appearance. Nontargetoid LR-M features were detected in 90.3% (56/62) and 93.1% (54/58) of LR-M tumors, and also detected in 88.3% (91/103) and 83.2% (89/107) of non-LR-M tumors by reviewers 1 and 2, respectively, and the most frequent feature among them was a marked diffusion restriction (Online Resource Table 1).
A surgically-proven combined hepatocellular cholangiocarcinoma in a 58-year-old man with chronic hepatitis B. On gadoxetic acid-enhanced MRI, there is a 4 cm lobulated mass (arrows) in the left lateral segment of the liver, which shows targetoid appearances on the arterial phase (a) (rim arterial phase hyperenhancement), portal venous phase (b) (peripheral “washout”), transitional phase (c), and hepatobiliary phase (d). It demonstrates nontargetoid diffusion restriction on diffusion-weighted image of b = 800 s/mm2 (e) and intermediate hyperintensity on T2-weighted image (f). This observation was assigned as LR-M category by reviewers
In LR-M observations, at least one of the targetoid appearances were present in 72.6% (45/62) and 82.8% (48/58) by reviewers 1 and 2, respectively. Therefore, the remaining LR-M observations without any targetoid appearance (27.4% [17/62] and 17.2% [10/58]) were categorized according to the presence of nontargetoid LR-M features. The additional role of nontargetoid LR-M features to targetoid appearances for LR-M assignment was described in Online Resource Table 1. Marked diffusion restriction was the most common nontargetoid LR-M feature used in LR-M assignment for observations without any targetoid appearance (Fig. 3).
A surgically-proven hepatocellular carcinoma in a 69-year-old woman with chronic hepatitis B. On gadoxetic acid-enhanced MRI, a 1.5 cm nodular lesion (arrows) with nonrim hyperenhancement on the arterial phase (a) is seen in segment IV of the liver. It does not show “washout” on portal venous phase (b). It shows hypointensity on the transitional phase (c), hypointensity on the hepatobiliary phase (d), nontargetoid marked diffusion restriction on diffusion-weighted image (b = 800 s/mm2) (e), and intermediate hyperintensity on T2-weighted image (f). Based on the presence of marked diffusion restriction but not meeting LR-5 criteria, this observation was assigned as LR-M by reviewers
Inter-observer agreements for targetoid appearances were substantial for rim APHE and peripheral washout, moderate for delayed central enhancement, targetoid TP, and targetoid HBP, and poor for targetoid diffusion restriction (Online Resource Table 2). With regard to nontargetoid LR-M features, inter-observer agreements were fair for marked diffusion restriction and necrosis or severe ischemia and poor for infiltrative appearance.
HCC versus non-HCC primary liver cancer in LR-M observations
In LR-M observations, presence of at least one of the targetoid appearances was more frequent in non-HCC PLCs than HCCs while statistical significance was proven only in reviewer 1 (89.5% [34/38] versus 45.8% [11/24] and 89.5% [34/38] versus 70.0% [14/20], P < 0.001 and = 0.078 in reviewers 1 and 2, respectively) (Online Resource Table 3). LR-M assignment only based on nontargetoid LR-M features was more frequently performed in HCCs than non-HCC PLCs.
Performance of LI-RADS category and reporting algorithm
Diagnosis of HCC
Table 3 shows diagnostic performances of different imaging criteria of LI-RADS category and reporting algorithm to diagnose HCC: (i) LR-5, (ii) LR-4/5, (iii) LR-5 or “LR-M of likely etiology of HCC”, and (iv) LR-4/5 or “LR-M of likely etiology of HCC”.
Differentiation of HCC from non-HCC malignancy including iCCA and cHCC-CCA, among four criteria listed above, the highest sensitivity was observed in criteria (iv) followed by (iii), (ii), and (i), while the highest specificity in criteria (i) followed by (ii), (iii), and (iv). More specifically, criteria (iii) in comparison to (i), sensitivities were significantly higher (87.6% [99/113] versus 73.5% [83/113] and 92.9% [105/113] versus 79.6% [90/113] in reviewers 1 and 2, respectively) (Ps < 0.001); however, the specificities decreased significantly (P = 0.002 and < 0.001) (Table 3).
Differentiation between HCC and iCCA (i.e., excluding cHCC-CCA) revealed that the specificities of LR-5 or LR-4/5 for HCC diagnosis were greater than 95% or 90%, respectively, in both reviewers. However, imaging criteria of LR-5 with “LR-M of likely etiology of HCC” resulted in lower specificities of 73.9% (17/23) in both reviewers.
Diagnosis of malignancy
Sensitivities of LI-RADS categories (LR-5 plus LR-M) to detect PLCs as malignant are described in Table 4. For all PLCs, LR-5 plus LR-M v2018 (with or without targetoid appearances) demonstrated sensitivities of 95.2% (157/165) and 97.6% (161/165) in reviewers 1 and 2, respectively. These results were significantly higher than LR-5 plus “LR-M with ≥ 1 targetoid appearances” (84.8% [140/165] and 91.5% [151/165], P < 0.001 and P = 0.002, in reviewers 1 and 2, respectively). Among the pathologic diagnoses, HCC demonstrated statistically significant improvement in sensitivities using the imaging criteria of LR-5 plus LR-M v2018 in comparison to LR-5 plus “LR-M with ≥ 1 targetoid appearances” in both reviewers (Table 4).
Discussion
Our study demonstrated that the LI-RADS v2018 algorithm on Gd-EOB-MRI effectively distinguished non-HCC PLCs from HCC by assigning non-HCC PLCs as an LR-M category, despite the presence of substantial limitations in cHCC-CCA. Most PLCs including HCC, cHCC-CCA, and iCCA were categorized as LR-5 or LR-M which indicated the high sensitivity of the LI-RADS algorithm for the diagnosis of PLCs as a malignancy. Regarding LR-M imaging criteria, nontargetoid LR-M features, particularly the marked diffusion restriction, demonstrated additional values to the targetoid appearances to assign LR-M in PLCs. Additionally, adopting the likely etiology of LR-M observations reported by radiologists can ensure a more sensitive diagnosis of HCC despite the significant reduction of the specificity, as compared to only accepting the LR-5 or LR-4/5 categories.
In our study population, we assigned the LR-M category in more than 90% of iCCAs, approximately 60% of cHCC-CCAs, and approximately 20% of HCCs. This result was in good agreement with that of previous studies regarding LI-RADS performances [5, 6, 11]. The remaining PLCs were categorized as non-LR-M, and which were frequently categorized as LR-5 rather than LR-4. Therefore, LR-5 category demonstrated sensitivities of 73.5–79.6% and specificities of 75.0–76.9% for HCC diagnosis in our study. The relatively low specificity of LR-5 can be explained by the characteristics of our study population, which excluded benign observations and included only PLCs, since this study primarily focused on the role of LR-M and not on the LR-5 performance. Moreover, cHCC-CCAs, a well-known HCC mimicker, included half of the non-HCC malignancies in our study, and mainly contributed the low specificity by being assigned as LR-5 in about 40% of tumors. Exclusion of cHCC-CCAs was associated with high specificity greater than 95% with regard to the LR-5 in the differentiation of HCC and iCCA, similar to a prior study [12].
In the diagnosis of PLCs as a malignancy, our study showed that the combination of LR-5 and LR-M v2018 resulted in a sensitivity of 95.2–97.6%. Given that LR-M category is created to preserve the specificity of LR-5 category without loss of sensitivity to detect malignancy [1], high sensitivity of LR-5 plus LR-M categories for malignancy is essential. The LR-M category v2018 has explicit imaging criteria including both targetoid appearances and nontargetoid LR-M features [8], and our study results revealed the diagnostic value of nontargetoid LR-M features for the assignment of LR-M category even in PLCs without targetoid appearances. Specifically, in our study, LR-5 plus LR-M v2018 showed significantly higher sensitivity in the detection of malignancy than LR-5 plus LR-M with ≥ 1 targetoid appearances indicating the added role of nontargetoid LR-M features. This result indicated that PLCs that may possibly be assigned as LR-4 by the previous version LI-RADS, can be assigned as LR-M by the LI-RADS algorithm v2018. For non-HCC malignancies, LR-M assignment even for those without targetoid appearance would be particularly appropriate in avoiding inappropriate or delayed management. However, for HCCs, there is a need for further deliberation in assigning HCCs without targetoid appearance; however, not meeting the LR-5 as LR-4 or LR-M is more appropriate.
Our study results showed that among targetoid LR-M features on Gd-EOB-MRI, rim APHE was most frequently observed followed by HBP targetoid appearance, which was in good agreement with the previous studies [11, 13]. Among nontargetoid LR-M features, the marked diffusion restriction was the most frequent feature, showing the highest contribution in the assignment of LR-M in those tumors without any targetoid appearance. While targetoid diffusion restriction was one of the least frequent targetoid appearances, possibly due to the lower spatial resolution of DWI [14], nontargetoid marked diffusion restriction was frequently observed in PLCs of LR-M as well as non-LR-M. Considering that diffusion restriction is a suggestive feature of malignancy [14], the results that most PLCs presented diffusion restriction either targetoid or nontargetoid would be very expected.
Our results showed that in PLCs, the combination of “LR-M of likely etiology of HCC” with LR-5 or LR-4/5 categories, more sensitive diagnosis of HCC was achieved than LR-5 or LR-4/5 alone, respectively, while the specificity was significantly lower. Considering that a substantial proportion of LR-M observations include HCCs [9], reporting the likely etiology in LR-M observations either as HCC or not would help subcategorize LR-M tumors which may potentially affect the diagnostic work-up and treatment planning. However, as our study results showed, the specificity significantly decreased as a trade-off when adopting the likely etiology in LR-M observation for the HCC diagnosis so the clinical application of reported likely etiology should be made with caution.
There are some limitations in our study. We only included pathologically diagnosed PLCs. Considering that many HCCs with typical imaging features often treated with locoregional treatment without pathological diagnosis, our study population must be biased. However, as non-HCC malignancy can be false positively diagnosed as HCC based on imaging studies, we only accepted pathological diagnoses as reference standards to evaluate the performance of new diagnostic algorithm, i.e., LI-RADS algorithm v2018. In addition, our study only evaluated the performance of LI-RADS algorithm on Gd-EOB-MRI so the performances on other imaging modalities need to be further investigated.
In conclusion, the LR-M category of v2018 with updated explicit criteria on Gd-EOB-MRI is useful to differentiate non-HCC malignancies from HCCs as well as to accurately diagnose PLCs as a malignancy in combination with LR-5 category. Adopting the reported likely etiology of LR-M observations in the HCC diagnosis may facilitate a more sensitive detection of HCC, but along with a considerable loss of specificity in comparison to the LI-RADS category-based assessment.
Abbreviations
- APHE:
-
Arterial phase hyperenhancement
- cHCC-CCA:
-
Combined hepatocellular cholangiocarcinoma
- Gd-EOB-MRI:
-
Gadoxetic acid-enhanced magnetic resonance imaging
- HCC:
-
Hepatocellular carcinoma
- iCCA:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver imaging reporting and data system
- LR-5:
-
LI-RADS category 5 (definitely HCC)
- LR-M:
-
LI-RADS category M (probably or definitely malignant, but not necessarily HCC)
- PLC:
-
Primary liver cancer
References
American College of Radiology. CT/MRI LI-RADS v2018 core. [https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en]
Tang A, Bashir MR, Corwin MT et al (2018) Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 286:29-48
Joo I, Kim H, Lee JM (2015) Cancer stem cells in primary liver cancers: pathological concepts and imaging findings. Korean J Radiol 16:50-68
Kim SA, Lee JM, Lee KB et al (2011) Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern--correlation with clinicopathologic findings. Radiology 260:148-157
Jeon SK, Joo I, Lee DH et al (2019) Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 29:373-382
Choi SH, Lee SS, Park SH et al (2019) LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology 290:388-397
Ludwig DR, Fraum TJ, Cannella R et al (2019) Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol (NY) 44:2116-2132
Fowler KJ, Potretzke TA, Hope TA et al (2018) LI-RADS M (LR-M): definite or probable malignancy, not specific for hepatocellular carcinoma. Abdom Radiol (NY) 43:149-157
van der Pol CB, Lim CS, Sirlin CB et al (2019) Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology 156:976-986
American College of Radiology. CT/MRI LI-RADS v2018 manual.
Kim YY, Kim MJ, Kim EH et al (2019) Hepatocellular Carcinoma versus Other Hepatic Malignancy in Cirrhosis: Performance of LI-RADS Version 2018. Radiology 291:72-80
Joo I, Lee JM, Lee SM et al (2016) Diagnostic accuracy of liver imaging reporting and data system (LI-RADS) v2014 for intrahepatic mass-forming cholangiocarcinomas in patients with chronic liver disease on gadoxetic acid-enhanced MRI. J Magn Reson Imaging 44:1330-1338
Lee HS, Kim MJ, An C (2019) How to utilize LR-M features of the LI-RADS to improve the diagnosis of combined hepatocellular-cholangiocarcinoma on gadoxetate-enhanced MRI? Eur Radiol 29:2408-2416
Taouli B, Koh DM (2010) Diffusion-weighted MR imaging of the liver. Radiology 254:47-66
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by our institutional review board and the requirement for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, M.Y., Joo, I., Kang, H.J. et al. LI-RADS M (LR-M) criteria and reporting algorithm of v2018: diagnostic values in the assessment of primary liver cancers on gadoxetic acid-enhanced MRI. Abdom Radiol 45, 2440–2448 (2020). https://doi.org/10.1007/s00261-020-02545-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02545-z