Abstract
Objectives
Imaging assessment for the clinical management of femoroacetabular impingement (FAI) is controversial because of a paucity of evidence-based guidance and notable variability among practitioners. Hence, expert consensus is needed because standardised imaging assessment is critical for clinical practice and research. We aimed to establish expert-based statements on FAI imaging by using formal methods of consensus building.
Methods
The Delphi method was used to formally derive consensus among 30 panel members from 13 countries. Forty-four questions were agreed upon, and relevant seminal literature was circulated and classified in major topics to produce answering statements. The level of evidence was noted for all statements, and panel members were asked to score their level of agreement (0–10). This is the second part of a three-part consensus series and focuses on ‘General issues’ and ‘Parameters and reporting’.
Results
Forty-seven statements were generated and group consensus was reached for 45. Twenty-five statements pertaining to ‘General issues’ (9 addressing diagnosis, differential diagnosis, and postoperative imaging) and ‘Parameters and reporting’ (16 addressing femoral/acetabular parameters) were produced.
Conclusions
The available evidence was reviewed critically, recommended criteria for diagnostic imaging highlighted, and the roles/values of different imaging parameters assessed. Radiographic evaluation (AP pelvis and a Dunn 45° view) is the cornerstone of hip-imaging assessment and the minimum imaging study that should be performed when evaluating adult patients for FAI. In most cases, cross-sectional imaging is warranted because MRI is the ‘gold standard’ imaging modality for the comprehensive evaluation, differential diagnosis assessment, and FAI surgical planning.
Key Points
• Diagnostic imaging for FAI is not standardised due to scarce evidence-based guidance on which imaging modalities and diagnostic criteria/parameters should be used.
• Radiographic evaluation is the cornerstone of hip assessment and the minimum study that should be performed when assessing suspected FAI. Cross-sectional imaging is justified in most cases because MRI is the ‘gold standard’ modality for comprehensive FAI evaluation.
• For acetabular morphology, coverage (Wiberg’s angle and acetabular index) and version (crossover, posterior wall, and ischial spine signs) should be assessed routinely. On the femoral side, the head–neck junction morphology (α° and offset), neck morphology (NSA), and torsion should be assessed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Femoroacetabular impingement (FAI) is a motion-related clinical syndrome characterised by a triad of hip/groin pain, signs of limited motion, and typical imaging findings [1,2,3]. It results from a conflicting contact between the proximal femur and the acetabular rim [1, 4]. The recurring mechanical loading is suggested to contribute to acetabular labral and chondrolabral pathology, pain, and functional impairment and can, ultimately, lead to premature osteoarthritis of the hip [5]. However, it is imperative to understand that FAI is a dynamic phenomenon and not a static imaging diagnosis. Characteristic osseous morphologies (Cam/Pincer) are necessary to diagnose FAI [3], but many individuals with these morphologies are asymptomatic [4, 6], which warrants caution when interpreting imaging studies [7].
Hip pain and FAI remain disputed regarding incidence, diagnosis, prognosis, and management [2,3,4]. There is no consensus on how to define or classify hip disease although several research groups have put forth guidelines to better define FAI and to facilitate diagnosis and treatment [2, 3].
Imaging is a pivotal part of the diagnostic workup for FAI. The ultimate goals of imaging are diagnosing hip morphology, detecting osteoarthritis signs, appreciating associated soft-tissue damage, and considering differential diagnoses [3, 8, 9]. Accordingly, different imaging methods, along with a multitude of imaging parameters [8, 10], are used in clinical practice. Preceding research has supported the significance of radiographs on the initial assessment of FAI and recommended the use of cross-sectional imaging to further assess hip morphology and chondrolabral lesions [3, 8, 11].
With regard to imaging parameters, the alpha angle (α°) and femoral torsion (FT), two of the most commonly cited quantitative parameters of the femur [12], are controversial mainly owing to their proposed thresholds and methods of assessment [10, 13, 14]. On the acetabular side, evaluating version and coverage on radiographs has been reported to have limited reliability [15,16,17], with potential repercussions on patient care. Given the multitude of signs and evolving concepts used in hip-preservation imaging, choosing which imaging parameters and criteria to use can be challenging.
There is a lack of current best evidence on the diagnostic imaging pathway for FAI, partly due to the heterogeneity in the definition of hip-related pain and also due to no consensus among experts on which imaging modalities, diagnostic criteria, and parameters should be assessed routinely [8, 18]. The aim of this Delphi-based consensus is to establish evidence-based statements using formal methods of consensus building among an expert group. The current work, which belongs to a three-part series, presents recommendations on the imaging diagnosis, parameters, differential diagnosis, and postoperative imaging of FAI.
Methods
Institutional Review Board approval was not required as patients are not involved. This consensus is a part of a collaborative project aimed at establishing expert-based statements on FAI imaging. Following the first overview paper [19] (The Lisbon Agreement on femoroacetabular impingement imaging. Part 1: overview by Mascarenhas et al, published in European Radiology), two additional detailed manuscripts address and discuss all produced statements (Part 2 and Part 3). This manuscript corresponds to Part 2 of the consensus, focusing on ‘General issues, parameters, and reporting’.
Given the paucity of high-level evidence for assessment of FAI-related imaging, clinical consensus is lacking. Accordingly, collecting experts’ opinions using formal methods of consensus development, such as the Delphi method, is an acceptable way of creating practice recommendations [20, 21]. This method involves a sequence of discussion rounds to determine experts’ opinions on controversial topics, drafted on the basis of the existing literature to produce a final consensus agreement. Full details of the Delphi method, including (1) participants, (2) consensus method, (3) literature review and scoring evidence levels set by the Oxford Centre for Evidence-based Medicine [22], (4) drafting of statements, (5) final scoring and data analyses, and (6) paper drafting, are reported as Supplementary Material.
Results and discussion
Delphi process
Thirty panellists (21 musculoskeletal radiologists and 9 orthopaedic surgeons) comprised this consensus initiative. Nineteen (90.4%) of the radiologists had more than 10 years of experience in musculoskeletal imaging, and 18 had special dedication and/or expertise in hip imaging. All orthopaedic surgeons had more than 10 years of experience in hip-preservation surgery (HPS). Thirty, 28, 27, and 26 participants completed rounds 1, 2, 3, and 4, respectively (6 orthopaedic surgeons and 20 radiologists answered the final round). The overall dropout rate was 13% (33% among orthopaedic surgeons and 5% among radiologists).
Forty-seven statements were generated and distributed among the topics ‘General issues’ (9 statements), ‘Parameters and reporting’ (16 statements), ‘Radiographic assessment’ (8 statements), ‘Magnetic resonance imaging (MRI) evaluation’ (12 statements), and ‘Ultrasound’ (2 statements). At the end of the Delphi process, ‘group consensus’ was obtained for 45 statements. In the following paragraphs, we present the statements concerning Part 2, followed by a summary of the panel’s discussions.
Statements and recommendations
The pathway for the imaging management and assessment of suspected FAI (Fig. 1), along with statements on the diagnosis, differential diagnoses, and postoperative imaging of FAI (Table 1), was put forth by the panel.
-
1.
General issues
-
1a. Diagnosis and imaging
-
Statement: An anteroposterior radiograph of the pelvis and a lateral view of the hip are the minimum imaging studies that should be performed when assessing patients for FAI. |
Pathway for the imaging management and assessment of femoroacetabular impingement (FAI). An AP pelvis radiograph and a lateral femoral neck view of the symptomatic hip should be initially performed to assess pelvis and hip morphology (namely to identify Cam or Pincer morphologies). Frequently, if exclusion of other causes of hip pain or if further assessment of hip morphology and associated cartilage/labral lesions is warranted, cross-sectional imaging is appropriate to thoroughly appreciate differential diagnosis. In doubtful cases, diagnostic hip injections may be necessary to confirm the hip as the source of pain. AP, antero-posterior; CT, computed tomography; MRI, magnetic resonance; MRA, magnetic resonance arthrography; OA, osteoarthritis
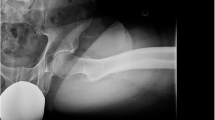
Radiographs should be used as the first-line assessment of suspected FAI, because they allow an overall evaluation of the pelvis and hips, as well as exclusion of other causes of symptoms [3, 8, 23]. Conjointly, radiographs and MRI are the preferred imaging modalities used to characterise hip pathomorphology and for treatment planning [8, 10, 23].
For an initial diagnostic approach, standard imaging should include two radiographs: an anteroposterior (AP) view of the pelvis and a lateral view of the hip [3, 8, 10]. The shape and orientation of the acetabulum may be assessed on the AP radiograph. The morphology of the proximal femur is best assessed on the orthogonal view of the femoral neck [3, 8, 11, 24] (Fig. 2). Femoral head–neck (FHN) asphericity in hips with FAI is most often localised in the anterosuperior region [6, 9, 25]. Although not accepted unanimously, these asphericities are usually best shown with a Dunn 45° view (hips in 45° of flexion and 20° of abduction) [26,27,28].
Parameters for the imaging assessment of the hip and pelvis in a patient with hip pain and suspected femoroacetabular impingement (FAI). Evaluating different features, namely (1) acetabular (coverage, version), (2) femoral (FHN junction, femoral neck-shaft angle, and femoral torsion), and (3) spinopelvic parameters (pelvic incidence, pelvic tilt, and sacral slope; see Supplementary Fig. 3 and Supplementary Table 8 for illustration and definitions), allows to define different pathomorphologies (bottom line). Ultimately, any combination of these morphologies may be present (4), reflecting impingement, instability, and/or microinstability. FHN, femoral head–neck
FAI is a dynamic phenomenon in which a structurally predisposed hip with minimal activity may become symptomatic and, conversely, a structurally normal hip may become symptomatic only with extreme activity/range of motion [2, 23]. Accordingly, in clinical practice, providing definite criteria to establish the diagnosis of FAI is challenging. However, in research and clinical trial settings, specific group classification using imaging criteria is important to establish homogeneous study groups. Therefore, based on published evidence and panel consensus, Pincer and Cam criteria are suggested (Table 2).
-
a)
Cam criteria
Statement: The main imaging criterion for defining Cam morphology is an alpha angle > 60° at any location around the anterosuperior FHN junction. Other measurements are used to a lesser extent, such as the head–neck offset and offset ratio. |
Statement: A threshold of 60° is recommended for the alpha angle because higher values are reported to be clinically more relevant. An anterior femoral offset < 8 mm may be regarded as ‘abnormal’. |
Since the original description of the α° [12], there has been much debate regarding its thresholds, though current evidence suggests that the α° should be defined according to the FHN location and sex [3, 6, 13, 25]. Based on cross-sectional studies (comparing asymptomatic individuals with Cam-type FAI patients) and on the natural course of FAI (short- and mid-term osteoarthritis progression in symptomatic hips with an α° > 60°), an α° threshold of 60° may be recommended (at any location) [6, 25, 29, 30]. Furthermore, recent research suggests that a Cam morphology with α° measurements above 57–60° at the 1:30- to 2-o’clock position is strongly associated with symptoms. Using this threshold would optimise discriminative power while favouring specificity [30, 31]. Nevertheless, caution is recommended because several patients with Cam morphology have signs of impingement with an α° < 60°, whereas others above that cut-off value will remain asymptomatic [4, 13, 31]. Considering that substantial overlap exists, additional clinical and imaging variables should be investigated, such as the combination with certain anatomical factors (e.g. decreased FT, acetabular morphology, spinopelvic parameters), sex, and athletic performance [4, 31, 32]. Other measurements are used to a lesser extent, such as the FHN offset (FO) and offset ratio [33,34,35,36]. Only limited data exist for the anterior FO, although a value of < 8 mm has been reported as ‘abnormal’ [33, 35, 37].
-
b)
Pincer criteria
Statement: Pincer morphology can be due to acetabular retroversion and/or overcoverage. Criteria for retroversion on imaging are a crossover sign, posterior wall sign, or ischial spine sign. Overcoverage is indicated by protrusio acetabuli, centre-edge angle of Wiberg (W-CEA) ≥ 40°, or acetabular index < 0°. |
Imaging signs of Pincer morphology include evidence of increased acetabular coverage and of abnormal acetabular version. Caution is warranted when interpreting radiographs, because pelvic tilt and rotation are known to affect some of these parameters (particularly AP coverage and retroversion) [38]. Although generally supported by the literature [39], further research is needed to define more precisely the value of radiographic signs in the diagnosis of Pincer morphologies, as well as the clinical relevance of cross-sectional imaging in this setting. Based on the above-mentioned rationale and the considerations below (see ‘acetabular parameters’), published evidence and panel consensus, Pincer criteria are suggested (Table 2).
Statement: The radiologist should not state that abnormal signs and parameters are indicative of FAI in an asymptomatic patient. |
Although FAI-associated morphologies may be mentioned in the radiological report, interpretation should be undertaken in conjunction with the clinical history and physical examination [4, 6, 40]. Similarly, deviations from currently accepted reference intervals (RefInts) in the morphological evaluation of the hip in asymptomatic individuals (or with unknown clinical status) should be handled with caution. Most imaging parameters classically related to FAI are present in a large proportion of asymptomatic individuals, suggesting that currently accepted thresholds may need to be redefined or cautiously interpreted/applied [4, 6, 25, 31].
-
1b. Differential diagnosis
Statement: Potential extra-articular sources of hip pain should always be sought clinically when assessing patients with FAI; in selected cases, cross-sectional imaging is warranted. |
Comprehensive imaging is crucial to assess intra- and extra-articular disease [31] as well as for surgical planning. Considering that several conditions can mimic and/or frequently coexist with FAI, if other potential causes of hip pain are not considered, patients may fail to improve after surgery or conservative treatment [41,42,43] (Table 3). Diagnostic injections may prove useful to confirm the hip as the source of symptoms [44] (Fig. 1).
-
1c. Postoperative imaging
Statement: Radiographs are the initial imaging modality for evaluating symptoms following FAI surgery. MRI, MRA, and, occasionally, CT (or CTA) should be used if the symptoms are not explained by the radiographs and/or if further anatomical information is desired. |
Radiographs (AP pelvis and a lateral hip view) are obtained routinely after HPS and as follow-up imaging. They are useful for assessment of bone morphology (residual deformities and/or bone over-resection) and to assess heterotopic ossification [45, 46]. In cases of persistent hip pain (i.e. 6 months after surgery), MRI or magnetic resonance arthrography (MRA) should be considered [45, 46] to evaluate the surgical repair and to exclude complications. Unilateral high-resolution (1.5 T or 3 T) MRI or MRA should be undertaken, and their data should be compared with preoperative examinations. CT is particularly useful to evaluate the following: (i) delayed or non-union after peri-acetabular or trochanteric osteotomy; (ii) osteochondroplasty (over- or under-resection); (iii) complications such as fractures or heterotopic ossification [46].
Although ultrasound is rarely used, it might be helpful to diagnose fluid collections, effusion, and deep venous thrombosis, as well as for image-guided aspiration [46].
Statement: Following FAI surgery, the underlying Cam-type and/or Pincer-type morphology, as well as the potential radiographic progression of hip osteoarthritis, should be assessed appropriately. |
Recommended imaging outcome measures to evaluate postoperative results are the following: (i) α° and FO for Cam correction; (ii) W-CEA, AI, and crossover sign for Pincer correction [47]; (iii) radiographic progression of osteoarthritis. Furthermore, clinical correlation with patient-reported outcome measures is suggested [3].
-
2.
Parameters and reporting
The recommended imaging parameters of the hip/pelvis to be assessed in a patient with suspected FAI (Fig. 2) and corresponding consensus statements were put forth by the panel (Tables 4 and 5).
-
2a. Femoral parameters
The most commonly described parameters to evaluate femoral morphology can be divided according to the main features that they assess: joint congruency, femoral head (FH) sphericity, and other important parameters, such as neck orientation in the coronal (neck-shaft angle (NSA)) and axial (torsion) planes (Fig. 3 and Supplementary Table 1).
-
a1) Neck-shaft angle
Statement: A standardised AP radiograph of the pelvis, or CT and/or MRI in the coronal femoral neck plane should be used for measuring the neck-shaft angle. |
Statement: The classic reference range for a ‘normal’ NSA derived from radiographs is 120–135°, for coxa vara is < 120°, and for coxa valga is > 135°. On CT, the reference NSA range is 120–140°. |
Imaging parameters to describe femoral morphology. See Supplementary Table 1 for definitions. a Offset and offset ratio, (b) osseous convexity of the femoral head neck junction, (c) alpha angle and omega angle, and (d) neck-shaft angle or centrum collum diaphyseal (CCD) angle. S, superior; a, anterior
Radiographs remain the clinical/research standard for evaluation of the NSA. However, a generally accepted measurement method is lacking [48] (Supplementary Fig. 1), because hip rotation and femoral torsion are known to influence NSA assessment. Accordingly, published RefInts vary widely depending on differences on the measurement methods and rotation correction used [48] (Supplementary Table 2) and, to a small extent, with age (values decrease with age) and sex (higher mean values have been observed in females) [48, 49].
CT coronal reformats of the proximal femur or coronal oblique MRI in the femoral neck plane allow a correct anatomical measurement of the NSA. In a recent series of 800 asymptomatic hips, Boese et al reported CT-based positionally corrected NSA mean values of 130° ± 5.9°. The difference between corrected/non-corrected measurements in that study was ~ 3° [49]. Mascarenhas et al reported a CT-based 95% RefInts of 120–141° in 1111 asymptomatic hips [6].
-
a2) Femoral head–neck junction
Statement: Radial MRI or CT are the most accurate imaging modalities for assessing the femoral head–neck junction. Radiographs, although less precise, may also be used to depict Cam morphology. |
Statement: The alpha angle is convenient to assess the FHN junction but has limited discriminatory power. Femoral offset is another useful parameter, but is less well established. |
Cam morphology corresponds to an asphericity of the FHN junction, most commonly at an anterosuperior location (1–2 o’clock on the clock face), and is usually assessed by measuring the α° [3, 12]. Importantly, Cam morphology has been detected in all populations, although few scholars have used the same case definitions, whereas others have used different definitions for males/females [4, 8], employed various imaging modalities, or measured the α° at different FHN positions [4, 6, 13, 30] (see also Cam criteria above).
The α° and FO describe different features of the FHN junction. The α° reflects the proximal aspect of the asphericity, whereas the FO describes the width of the femoral neck relative to the FH [8, 10, 35, 50] (Fig. 4 and Supplementary Fig. 2). Although these parameters are useful to quantify the morphology of the FHN junction, caution is warranted when using them in routine clinical practice. The α° is controversial due to its moderate reproducibility, moderate discriminative ability, and lack of conclusive data on ‘ideal’ threshold values [8, 18, 31] (Supplementary Tables 3 and 4).
-
a3) Femoral torsion
Statement: CT or MRI are the recommended imaging modalities for measurement of femoral torsion. |
a Right hip cross-table lateral view. To calculate the offset, three parallel lines are drawn: the first line passes through the centre of the long axis of the femoral neck; the second line, through the anterior aspect of the femoral neck; and the third line, through the anterior aspect of the femoral head. The head–neck offset is calculated by measuring the distance between the second and third lines. b Alpha angle measurement in a right hip MRI arthrogram, according to method 1 originally described by Nötzli in an arthro-MRI examination (also known as the ‘3-point method’): place a circle adjusted over the contour of the femoral head (dotted blue circle). The femoral neck axis (FNA) is defined as a line passing through the femoral head centre (FHC) and the centre of the neck (FNC) at its narrowest point (i.e. place a circle [not shown] with its corresponding diameter [dotted blue line] at the shortest possible distance between the anterior [ventral] and posterior [dorsal] outline of the femoral neck). Next, a line is drawn connecting the centre of these two circles. Then, a line is drawn connecting the FHC to the point where the contour of the femoral head or head–neck junction first exited the femoral head circle. The alpha angle is the angle formed by these two lines. A, anterior; P, posterior; M, medial; L, lateral
CT and MRI are the most commonly used modalities to assess FT, because they are validated and, in general, considered to provide accurate and reliable measurements [51, 52]. MRI may be preferred to avoid radiation exposure. Biplanar radiographs with three-dimensional (3D) modelling are an alternative to cross-sectional imaging and provide comparable results to those obtained with CT/MRI [53, 54]. Analysis of 3D datasets holds promise to become a more accurate method of FT quantification [55].
Statement: Several methods of measurement of femoral torsion have been reported, resulting in different reference intervals. There is insufficient evidence to support a single method over the others. |
The methods for assessing FT differ mainly in the definition of the centre of the neck and proximal femoral axis, which yields different values [52, 56, 57]. The method described by Murphy et al has been reported to be more accurate compared to true anatomic femoral torsion although no method can be recommended over another [58]. It is imperative to use the same method consistently if results are to be comparable (Fig. 5).
Statement: The normal femoral antetorsion in adults should be ~ 13° with a standard deviation of ~ 10° using the method of Reikeras et al (1983). |
Assessment of femoral torsion on cross-sectional imaging (selected methods represented; not comprehensive). On consecutive strict axial images over the proximal femur, determine the femoral head centre (FHC) (yellow circle and yellow line). Definition of the femoral neck axis (green line) can be obtained by several methods. Lee (red bar): A line is drawn on the first image on which the FHC can be connected with the most cephalic junction of the greater trochanter and the femoral neck; Reikeras (light-blue bar): A line connecting the FHC with the femoral neck centre is drawn on an image, where the anterior and posterior cortices run parallel to each other. Tomczak (dark-blue bar): The FHC is connected with the centre of the greater trochanter at the base of the femoral neck. Murphy (orange bar): the FHC is connected with the centre of the base of the femoral neck directly superior to the lesser trochanter. Then, over the distal femur, draw a tangent to the posterior aspect of the femoral condyles (blue line; choosing the slice where the condyles are more prominent). The angle between both lines represents the femoral torsion. Although some of these reference points are located on different adjacent slices, modern workstations should allow drawing and modifying a line across multiple images in one series or, alternatively, different slices can be superimposed on a single image with the help of postprocessing software
The reported RefInts of FT vary significantly. A multitude of reasons may explain such variation, but the measurement method employed accounts largely for the discrepant values. In a study comparing different measurement methods, mean values ranged from 11.4 ± 7.4 to 22.4 ± 6.8° [56]. Thus, distinct RefInts should be considered according to the method used. Sutter et al reported a mean value of 12.8 ± 10.1° in asymptomatic adults using MRI based on the method of Reikeras and colleagues [51]. Other authors have obtained similar results using an equivalent methodology (Supplementary Table 5).
-
2b. Acetabular parameters
Overall, the most commonly described parameters to assess acetabular morphology can be divided according to the main features that they measure: depth, coverage, or orientation (Fig. 6 and Supplementary Table 6).
-
b1) Coverage
Statement: An AP radiograph of the pelvis should be the first-line modality for assessing acetabular coverage. |
Statement: The centre-edge angle of Wiberg and the acetabular index should be assessed routinely in the evaluation of acetabular coverage. |
Imaging parameters to describe acetabular morphology. a Coxa profunda. b Protrusio acetabuli. c Centre-edge angle (CEA). The Wiberg-CEA (WCEA) is formed between a vertical line crossing the centre of the femoral head, perpendicular to the horizontal line uniting the inferior margins of both teardrops, and a line connecting the centre of the femoral head to the lateral margin of the acetabular bone dense area (known as the acetabular sourcil). The lateral CEA (L-CEA) is formed between the vertical line described and a line connecting the centre of the femoral head to the far lateral margin of the acetabulum. d Posterior wall sign, ischial spine sign, and crossover sign. e Anterior and posterior acetabular wall index. f Acetabular index. g The W-CEA should be distinguished from the L-CEA, as the most lateral point to consider would be the lateral end of the sourcil (W-CEA) rather than the most lateral rim of the acetabulum (L-CEA). Frequently, these two reference points might coincide. See Supplementary Table 6 for definition of the most relevant parameters. IIL, ilioischial line; AF, acetabular fossa; FH, femoral head; V, vertical; E, lateral edge of the sourcil; C, femoral head centre; I, medial edge of the sourcil; TD, tear drop; IS, ischial spine; PB, pelvic brim; PW, posterior wall; AW, anterior wall; a, neck axis
An AP radiograph of the pelvis should be the initial examination for assessing acetabular coverage. However, cross-sectional imaging may also be used because it provides similar measurements for most parameters [59, 60]. Conflicting reports exist regarding the effect of pelvic positioning on radiographic parameters of coverage, although tilt and rotation seem to mainly influence the radiographic signs of acetabular retroversion [38, 61].
The centre-edge angle of Wiberg (W-CEA) and acetabular index (AI) are the most often used parameters of superior–lateral coverage [62, 63]. Anterior and posterior acetabular coverage may be quantified using anterior wall and posterior wall indices [64]. Other parameters, such as the anterior centre-edge angle, extrusion index, or Sharp angle, are used less frequently [6, 25, 65]. Protrusio acetabuli should always be noted because it is a clinically relevant condition [47]. Coxa profunda should not be used to define acetabular overcoverage [66].
Statement: The centre-edge angle (CEA) on AP radiographs of the pelvis is measured using the centre of the femoral head and two distinct landmarks at the lateral acetabular roof: the outer edge of the acetabular sourcil, or the most lateral aspect of the bony acetabulum. The CEA values obtained using these two landmarks often are not identical and represent different areas of coverage in the 3D geometry of the acetabulum. |
On an AP radiograph of the pelvis, two types of CEA should be distinguished and stated clearly: the sourcil-edge CEA (W-CEA), which represents anterosuperior coverage, and the bone-edge or lateral CEA (L-CEA), which represents superolateral coverage [63, 67] (Fig. 6g). They are both useful and complementary for assessing acetabular coverage.
Statement: For the CEA of Wiberg, the classical radiographic-based reference intervals are < 20° for undercoverage, 20–25° for borderline undercoverage, 25–39° for normal coverage, and ≥ 40° for overcoverage. An acetabular index of < 0° on an AP radiograph of the pelvis is classically accepted as overcoverage, whereas a value > 13° represents undercoverage. |
The cut-off values for the W-CEA reported originally by Wiberg [68] have been considered the RefInt values and are recommended by this consensus group.
A recent large population-based study [69] and other studies (Supplementary Table 7) reported RefInts for the W-CEA and AI, which are significantly broader compared with the classical thresholds. Interestingly, if these updated RefInts were to be used, many hips considered ‘pathologic’ using the classical RefInt would now be classified as ‘normal’. This observation strongly suggests the need to update the thresholds used in the classification of lateral acetabular coverage based, for example, on the natural course of the disease.
Statement: There is insufficient evidence to recommend the routine reporting of posterior and anterior acetabular coverage in clinical practice, though they may be relevant in specific clinical situations, such as in specialised hip-preservation units and in research settings. |
On an AP radiograph of the pelvis, anterior and posterior acetabular coverage corresponds to the overlap of the anterior and posterior acetabular walls with the FH. It may be quantified by the percentage of the FH covered by each wall [65] or by anterior wall/posterior wall indices [64] (Supplementary Table 6).
-
b2) Version
Statement: An AP radiograph of the pelvis should be used for the initial assessment of acetabular version. However, CT or MRI should be considered if clinical and radiographic evaluations are suggestive of acetabular malversion. |
Statement: On an AP radiograph of the pelvis, the crossover sign should be assessed routinely. The posterior wall sign and the ischial spine sign should also be assessed. |
Statement: An isolated positive crossover sign is an indication of focal cranial retroversion, whereas a positive crossover sign combined with a posterior wall sign and ischial spine sign is an indication of global retroversion. |
Assessment of acetabular version with an AP radiograph of the pelvis [39] is paramount because surgical planning for correcting abnormal version is usually based on radiographic retroversion [70, 71]. Nevertheless, measurements of acetabular version on radiographs have been reported to be less reliable compared with those obtained using cross-sectional/3D imaging [16, 17], which may quantify individual acetabular morphologies more accurately. Limitations in radiographic evaluation are mainly inherent to the imaging modality and pelvic tilt [17].
The crossover sign, posterior wall sign, and ischial spine sign (Fig. 6) should be assessed because there is evidence that, depending on the presence of different signs and degree of acetabular retroversion, different surgical approaches may be considered. Accordingly, in contrast to cranial acetabular retroversion, patients with a global retroversion may benefit from pelvic reorientation instead of trimming of the acetabular rim [72].
Caution is warranted because of the following: (a) these radiographic signs are commonly present among asymptomatic individuals [73], (b) their accuracy per se is questionable [17, 39, 73]. Although the clinical added value of advanced imaging remains to be established, it should be considered when clinical and radiographic findings are consistent with Pincer FAI and retroversion.
Conclusions
Standardised plain radiographs, including AP pelvis and a Dunn 45° view, are the initial imaging diagnostic tools for assessment of hip pain and FAI. Thorough analyses of imaging parameters are paramount to identify osseous morphologies consistent with FAI and to exclude other structural disorders. MRI allows for further characterisation of hip morphology and chondrolabral disease, as well as differential diagnosis evaluation.
The first Delphi-based consensus for imaging of FAI was developed. The panel critically reviewed the available evidence, imaging parameters, classification criteria, and recommended pathways for diagnostic workup. The resulting consensus can serve as a tool to reduce variability in clinical practice and guide further research for FAI management.
Change history
21 January 2021
The DOI in reference 17 was incorrect.
Abbreviations
- 3D:
-
Three dimensional
- AF:
-
Acetabular fossa
- AI:
-
Acetabular index
- AP:
-
Anteroposterior
- CEA:
-
Centre-edge angle
- COS:
-
Crossover sign
- CT:
-
Computed tomography
- CTA:
-
CT arthrography
- dMRA:
-
Direct MR arthrography
- ESSR:
-
European Society of Musculoskeletal Radiology
- FAI:
-
Femoroacetabular impingement
- FAIS:
-
Femoroacetabular impingement syndrome
- FH:
-
Femoral head
- FHN:
-
Femoral head–neck
- FO:
-
Femoral head–neck offset
- FOV:
-
Field-of-view
- FT:
-
Femoral torsion
- HPS:
-
Hip preservation surgery
- ISS:
-
Ischial spine sign
- L-CEA:
-
Lateral centre-edge angle
- MRA:
-
Magnetic resonance arthrography
- MRI:
-
Magnetic resonance imaging
- NSA:
-
Neck-shaft angle
- OA:
-
Osteoarthritis
- PWS:
-
Posterior wall sign
- RefInts:
-
Reference intervals
- W-CEA:
-
Centre-edge angle of Wiberg
- α°:
-
Alpha angle
References
Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA (2003) Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res:112–120. https://doi.org/10.1097/01.blo.0000096804.78689.c2
Nepple JJ, Prather H, Trousdale RT et al (2013) Clinical diagnosis of femoroacetabular impingement. J Am Acad Orthop Surg 21(Suppl 1):S16–S19. https://doi.org/10.5435/JAAOS-21-07-S16
Griffin DR, Dickenson EJ, O'Donnell J et al (2016) The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med 50:1169–1176. https://doi.org/10.1136/bjsports-2016-096743
Mascarenhas VV, Rego PA, Dantas P et al (2016) Imaging prevalence of femoroacetabular impingement in symptomatic patients, athletes, and asymptomatic individuals: a systematic review. Eur J Radiol 85:73–95. https://doi.org/10.1016/j.ejrad.2015.10.016
Agricola R, Heijboer MP, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Waarsing JH (2013) Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis 72:918–923. https://doi.org/10.1136/annrheumdis-2012-201643
Mascarenhas VV, Rego PA, Dantas P et al (2018) Hip shape is symmetric, non-dependent on limb dominance and gender-specific: implications for femoroacetabular impingement. A 3D CT analysis in asymptomatic subjects. Eur Radiol 28:1609–1624. https://doi.org/10.1007/s00330-017-5072-9
Palmer WE (2010) Femoroacetabular impingement: caution is warranted in making imaging-based assumptions and diagnoses. Radiology 257:4–7. https://doi.org/10.1148/radiol.10091298
Mascarenhas VV, Ayeni OR, Egund N et al (2019) Imaging methodology for hip preservation: techniques, parameters, and thresholds. Semin Musculoskelet Radiol 23:197–226. https://doi.org/10.1055/s-0039-1688714
Pfirrmann CWA, Mengiardi B, Dora C, Kalberer F, Zanetti M, Hodler J (2006) Cam and Pincer femoroacetabular impingement: characteristic MR arthrographic findings in 50 patients. Radiology 240:778–785. https://doi.org/10.1148/radiol.2403050767
Tannast M, Siebenrock KA, Anderson SE (2007) Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol 188:1540–1552. https://doi.org/10.2214/AJR.06.0921
Dudda M, Albers C, Mamisch TC, Werlen S, Beck M (2008) Do normal radiographs exclude asphericity of the femoral head-neck junction? Clin Orthop Relat Res 467:651–659. https://doi.org/10.1007/s11999-008-0617-5
Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J (2002) The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br Vol 84:556–560
Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CWA (2012) How useful is the alpha angle for discriminating between symptomatic patients with cam-type femoroacetabular impingement and asymptomatic volunteers? Radiology 264:514–521. https://doi.org/10.1148/radiol.12112479
Lerch TD, Todorski IAS, Steppacher SD et al (2018) Prevalence of femoral and acetabular version abnormalities in patients with symptomatic hip disease: a controlled study of 538 hips. Am J Sports Med 46:122–134. https://doi.org/10.1177/0363546517726983
Zaltz I, Kelly BT, Hetsroni I, Bedi A (2012) The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res 471:2463–2470. https://doi.org/10.1007/s11999-012-2689-5
Larson CM, Moreau-Gaudry A, Kelly BT et al (2014) Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res 473:1247–1254. https://doi.org/10.1007/s11999-014-4055-2
Wassilew GI, Heller MO, Diederichs G, Janz V, Wenzl M, Perka C (2012) Standardized AP radiographs do not provide reliable diagnostic measures for the assessment of acetabular retroversion. J Orthop Res 30:1369–1376. https://doi.org/10.1002/jor.22086
Kassarjian A (2019) Hip hype: FAI syndrome, Amara’s law, and the hype cycle. Semin Musculoskelet Radiol 23(3):252–256. https://doi.org/10.1055/s-0039-1677695
Mascarenhas VV, Castro MO, Rego PA et al (2020) The Lisbon agreement on femoroacetabular impingement imaging-part 1: overview. Eur Radiol 30:5281–5297. https://doi.org/10.1007/s00330-020-06822-9
Steurer J (2011) The Delphi method: an efficient procedure to generate knowledge. Skeletal Radiol 40:959–961. https://doi.org/10.1007/s00256-011-1145-z
McMillan SS, King M, Tully MP (2016) How to use the nominal group and Delphi techniques. Int J Clin Pharmacol 38:655–662. https://doi.org/10.1007/s11096-016-0257-x
OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653
Sutter R, Pfirrmann CWA (2017) Update on femoroacetabular impingement: what is new, and how should we assess it? Semin Musculoskelet Radiol 21:518–528. https://doi.org/10.1055/s-0037-1606141
Atkins PR, Shin Y, Agrawal P et al (2018) Which two-dimensional radiographic measurements of cam femoroacetabular impingement best describe the three-dimensional shape of the proximal femur? Clin Orthop Relat Res 477(1):242–253. https://doi.org/10.1097/CORR.0000000000000462
Mascarenhas VV, Rego P, Dantas P, Gaspar A, Soldado F, Consciência JG (2017) Cam deformity and the omega angle, a novel quantitative measurement of femoral head-neck morphology: a 3D CT gender analysis in asymptomatic subjects. Eur Radiol 27:2011–2023. https://doi.org/10.1007/s00330-016-4530-0
Domayer SE, Ziebarth K, Chan J, Bixby S, Mamisch TC, Kim YJ (2011) Femoroacetabular cam-type impingement: diagnostic sensitivity and specificity of radiographic views compared to radial MRI. Eur J Radiol 80:805–810. https://doi.org/10.1016/j.ejrad.2010.10.016
Meyer DC, Beck M, Ellis T, Ganz R, Leunig M (2006) Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res 445:181–185. https://doi.org/10.1097/01.blo.0000201168.72388.24
Saito M, Tsukada S, Yoshida K, Okada Y, Tasaki A (2016) Correlation of alpha angle between various radiographic projections and radial magnetic resonance imaging for cam deformity in femoral head–neck junction. Knee Surg Sports Traumatol Arthrosc:1–7. https://doi.org/10.1007/s00167-016-4046-9
Agricola R, Waarsing JH, Thomas GE et al (2014) Cam impingement: defining the presence of a cam deformity by the alpha angle: data from the CHECK cohort and Chingford cohort. Osteoarthritis Cartilage 22:218–225. https://doi.org/10.1016/j.joca.2013.11.007
Golfam M, Di Primio LA, Beaulé PE, Hack K, Schweitzer ME (2017) Alpha angle measurements in healthy adult volunteers vary depending on the MRI plane acquisition used. Am J Sports Med 45:620–626. https://doi.org/10.2214/AJR.06.0921
Mascarenhas VV, Rego PA, Dantas P et al (2018) Can we discriminate symptomatic hip patients from asymptomatic volunteers based on anatomic predictors? A 3-dimensional magnetic resonance study on cam, Pincer, and spinopelvic parameters. Am J Sports Med 46:3097–3110. https://doi.org/10.1177/0363546518800825
Beaulé PE, Grammatopoulos G, Speirs A et al (2018) Unravelling the hip pistol grip / CAM deformity: origins to joint degeneration. J Orthop Res 36(12):3125–3135. https://doi.org/10.1002/jor.24137
Eijer H, Leunig M, Mahomed N, Ganz R (2001) Cross-table lateral radiographs for screening of anterior femoral head-neck offset in patients with femoro-acetabular impingement. Hip Int 11:37–41
Fraitzl CR, Kappe T, Pennekamp F, Reichel H, Billich C (2012) Femoral head-neck offset measurements in 339 subjects: distribution and implications for femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc 21:1212–1217. https://doi.org/10.1007/s00167-012-2042-2
Ehrmann C, Rosskopf AB, Pfirrmann CWA, Sutter R (2015) Beyond the alpha angle: alternative measurements for quantifying cam-type deformities in femoroacetabular impingement. J Magn Reson Imaging 42(4):1024–1031. https://doi.org/10.1002/jmri.24861
Pollard TCB, Villar RN, Norton MR et al (2010) Femoroacetabular impingement and classification of the cam deformity: the reference interval in normal hips. Acta Orthop 81:134–141. https://doi.org/10.3109/17453671003619011
Kang ACL, Gooding AJ, Coates MH, Goh TD, Armour P, Rietveld J (2010) Computed tomography assessment of hip joints in asymptomatic individuals in relation to femoroacetabular impingement. Am J Sports Med 38:1160–1165. https://doi.org/10.1177/0363546509358320
Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD (2014) Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res 473(4):1255–1266. https://doi.org/10.1007/s11999-014-3936-8
Rhee C, Le Francois T, Byrd JWT, Glazebrook M, Wong I (2017) Radiographic diagnosis of Pincer-type femoroacetabular impingement: a systematic review. Orthop J Sports Med 5:232596711770830. https://doi.org/10.2106/00004623-200301000-00001
Frank JM, Harris JD, Erickson BJ et al (2015) Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy 31:1199–1204. https://doi.org/10.1016/j.arthro.2014.11.042
Krishnamoorthy VP, Beck EC, Kunze KN et al (2019) Radiographic prevalence of sacroiliac joint abnormalities and clinical outcomes in patients with femoroacetabular impingement syndrome. Arthroscopy 35:2598–2605.e1. https://doi.org/10.1016/j.arthro.2019.03.030
Krishnamoorthy VP, Kunze KN, Beck EC et al (2019) Radiographic prevalence of symphysis pubis abnormalities and clinical outcomes in patients with femoroacetabular impingement syndrome. Am J Sports Med 47:1467–1472. https://doi.org/10.1177/0363546519837203
Tibor LM, Sekiya JK (2008) Differential diagnosis of pain around the hip joint. Arthroscopy 24:1407–1421. https://doi.org/10.1016/j.arthro.2008.06.019
Khan W, Khan M, Alradwan H, Williams R, Simunovic N, Ayeni OR (2015) Utility of intra-articular hip injections for femoroacetabular impingement: a systematic review. Orthop J Sports Med 3(9):2325967115601030. https://doi.org/10.1177/2325967115601030
Li AE, Jawetz ST, Greditzer HG IV, Burge AJ, Nawabi DH, Potter HG (2016) MRI evaluation of femoroacetabular impingement after hip preservation surgery. AJR Am J Roentgenol 207:392–400. https://doi.org/10.2214/AJR.15.15427
Dietrich TJ, Grob K, Kim C-HO (2017) Postoperative imaging after impingement surgery. Semin Musculoskelet Radiol 21:529–538. https://doi.org/10.1055/s-0037-1606131
Hanke MS, Steppacher SD, Anwander H, Werlen S, Siebenrock KA, Tannast M (2016) What MRI findings predict failure 10 years after surgery for femoroacetabular impingement? Clin Orthop Relat Res:1–16. https://doi.org/10.1007/s11999-016-5040-8
Boese CK, Dargel J, Oppermann J et al (2015) The femoral neck-shaft angle on plain radiographs: a systematic review. Skeletal Radiol 45:19–28. https://doi.org/10.1007/s00256-015-2236-z
Boese CK, Jostmeier J, Oppermann J et al (2015) The neck shaft angle: CT reference values of 800 adult hips. Skeletal Radiol 45:455–463. https://doi.org/10.1007/s00256-015-2314-2
Ganz R, Leunig M, Leunig-Ganz K, Harris WH (2008) The etiology of osteoarthritis of the hip. Clin Orthop Relat Res 466:264–272. https://doi.org/10.1007/s11999-007-0060-z
Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CWA (2012) Femoral antetorsion: comparing asymptomatic volunteers and patients with femoroacetabular impingement. Radiology 263:475–483. https://doi.org/10.1148/radiol.12111903
Tomczak RJ, Guenther KP, Rieber A, Mergo P, Ros PR, Brambs HJ (1997) MR imaging measurement of the femoral antetorsional angle as a new technique: comparison with CT in children and adults. AJR Am J Roentgenol 168:791–794. https://doi.org/10.2214/ajr.168.3.9057536
Rosskopf AB, Ramseier LE, Sutter R, Pfirrmann CWA, Buck FM (2014) Femoral and tibial torsion measurement in children and adolescents: comparison of 3D models based on low-dose biplanar radiography and low-dose CT. AJR Am J Roentgenol 202:W285–W291. https://doi.org/10.2214/AJR.13.11103
Rosskopf AB, Buck FM, Pfirrmann CWA, Ramseier LE (2017) Femoral and tibial torsion measurements in children and adolescents: comparison of MRI and 3D models based on low-dose biplanar radiographs. Skeletal Radiol 46:469–476. https://doi.org/10.1007/s00256-017-2569-x
Hartel MJ, Petersik A, Schmidt A et al (2016) Determination of femoral neck angle and torsion angle utilizing a novel three-dimensional modeling and analytical technology based on CT datasets. PLoS One 11:e0149480. https://doi.org/10.1371/journal.pone.0149480.s002
Kaiser P, Attal R, Kammerer M et al (2016) Significant differences in femoral torsion values depending on the CT measurement technique. Arch Orthop Trauma Surg 136:1259–1264. https://doi.org/10.1007/s00402-016-2536-3
Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CWA (2015) Assessment of femoral antetorsion with MRI: comparison of oblique measurements to standard transverse measurements. AJR Am J Roentgenol 205:130–135. https://doi.org/10.2214/AJR.14.13617
Schmaranzer F, Lerch TD, Siebenrock KA, Tannast M, Steppacher SD (2019) Differences in femoral torsion among various measurement methods increase in hips with excessive femoral torsion. Clin Orthop Relat Res 477(5):1073–1083. https://doi.org/10.1097/CORR.0000000000000610
Stelzeneder D, Hingsammer A, Bixby SD, Kim Y-J (2012) Can radiographic morphometric parameters for the hip be assessed on MRI? Clin Orthop Relat Res 471:989–999. https://doi.org/10.1007/s11999-012-2654-3
Air ME, Harrison JR, Nguyen JT, Kelly BT, Bogner EA, Moley PJ (2019) Correlation of measurements of the prearthritic hip between plain radiography and computed tomography. PM R 11:158–166. https://doi.org/10.1016/j.pmrj.2018.06.001
Jackson TJ, Estess AA, Adamson GJ (2016) Supine and standing AP pelvis radiographs in the evaluation of Pincer femoroacetabular impingement. Clin Orthop Relat Res 474:1692–1696. https://doi.org/10.1007/s11999-016-4766-7
Werner CML, Ramseier LE, Ruckstuhl T et al (2012) Normal values of Wiberg’s lateral center-edge angle and Lequesne’s acetabular index–a coxometric update. Skeletal Radiol 41(10):1273–1278. https://doi.org/10.1007/s00256-012-1420-7
Wylie JD, Kapron AL, Peters CL, Aoki SK, Maak TG (2017) Relationship between the lateral center-edge angle and 3-dimensional acetabular coverage. Orthop J Sports Med 5:2325967117700589. https://doi.org/10.1177/2325967117700589
Siebenrock KA, Kistler L, Schwab JM, Büchler L, Tannast M (2012) The acetabular wall index for assessing anteroposterior femoral head coverage in symptomatic patients. Clin Orthop Relat Res 470:3355–3360. https://doi.org/10.1007/s11999-012-2477-2
Tannast M, Hanke MS, Zheng G, Steppacher SD, Siebenrock KA (2015) What are the radiographic reference values for acetabular under- and overcoverage? Clin Orthop Relat Res 473:1234–1246. https://doi.org/10.1007/s11999-014-4038-3
Nepple JJ (2013) Coxa profunda is not a useful radiographic parameter for diagnosing Pincer-type femoroacetabular impingement. J Bone Joint Surg Am 95:417. https://doi.org/10.2106/JBJS.K.01664
Mittal A, Bomar JD, Jeffords ME, Huang M-T, Wenger DR, Upasani VV (2016) Defining the lateral edge of the femoroacetabular articulation: correlation analysis between radiographs and computed tomography. J Child Orthop 10:365–370. https://doi.org/10.1007/s11832-016-0768-y
Wiberg G (1939) Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteo-arthritis. Karolinska Institutet, Orthopedic Clinic
Laborie LB, Engesæter IØ, Lehmann TG et al (2013) Radiographic measurements of hip dysplasia at skeletal maturity—new reference intervals based on 2,038 19-year-old Norwegians. Skeletal Radiol 42:925–935. https://doi.org/10.1111/j.1651-2227.2002.tb02857.x
Siebenrock KA, Schaller C, Tannast M, Keel M, Büchler L (2014) Anteverting periacetabular osteotomy for symptomatic acetabular retroversion: results at ten years. J Bone Joint Surg Am 96:1785–1792. https://doi.org/10.2106/JBJS.M.00842
Hartigan DE, Perets I, Walsh JP, Close MR, Domb BG (2016) Clinical outcomes of hip arthroscopy in radiographically diagnosed retroverted acetabula. Am J Sports Med 44:2531–2536. https://doi.org/10.1177/0363546516652615
Wall PDH, Brown JS, Parsons N, Buchbinder R, Costa ML, Griffin D (2014) Surgery for treating hip impingement (femoroacetabular impingement) (review). Cochrane Database Syst Rev 9:CD010796. https://doi.org/10.1002/14651858.CD010796.pub2
Bensler S, Dietrich TJ, Zubler V, Pfirrmann CWA, Sutter R (2019) Pincer-type MRI morphology seen in over a third of asymptomatic healthy volunteers without femoroacetabular impingement. J Magn Reson Imaging 49:1296–1303. https://doi.org/10.1002/jmri.26297
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dr. Vasco Vogado Mascarenhas.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Several authors have significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it did not directly involve patients.
Ethical approval
Institutional Review Board approval was not required because the study did not directly involve patients or any intervention.
Study subjects or cohorts overlap
This is the second part of a 3-part consensus series. ‘Lisbon agreement on FAI imaging-part 1: overview’ has been published in ER.
Regarding the consensus, forty-seven statements were generated in total (in Part 1, we only addressed 17 of these 47 statements).
Part 2: ‘The Lisbon Agreement on femoroacetabular impingement imaging—part 2: general issues, parameters, and reporting’. Twenty-five statements specifically pertaining to ‘General issues’ (nine addressing diagnosis, differential diagnosis, and postoperative imaging) and ‘Parameters and reporting’ (16 addressing femoral/acetabular parameters) were produced and developed.
Part 3: ‘The Lisbon Agreement on femoroacetabular impingement imaging-part 3: imaging techniques’. Twenty-two statements specifically pertaining to “Imaging techniques” were generated and developed. Eight statements on “Radiographic assessment” and 12 statements on “MRI evaluation” gained consensus.
Methodology
• Delphi-based consensus study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 8398 kb)
Rights and permissions
About this article
Cite this article
Mascarenhas, V.V., Castro, M.O., Afonso, P.D. et al. The Lisbon Agreement on femoroacetabular impingement imaging—part 2: general issues, parameters, and reporting. Eur Radiol 31, 4634–4651 (2021). https://doi.org/10.1007/s00330-020-07432-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07432-1