Abstract
Objectives
The current LR-5 criteria of Liver Imaging Reporting and Data System (LI-RADS) determined by only major features provide high specificity, but unsatisfactorily low sensitivity for the noninvasive diagnosis of hepatocellular carcinoma (HCC). This study aimed to identify significant ancillary features (AFs) in LI-RADS version 2018 and develop the upgraded LR-5 criteria to improve diagnostic performance on gadoxetic acid–enhanced magnetic resonance imaging (MRI).
Methods
This retrospective study included 280 patients (366 observations including 281 HCCs) at high-risk for HCC who underwent gadoxetic acid–enhanced MRI between 2015 and 2017. Two readers evaluated major features and AFs for each observation and assigned a LI-RADS category. Independently significant AFs were identified through logistic regression analysis. Upgraded LR-5 criteria were developed by combining independently significant AFs with LR-4 assigned by major features alone. Sensitivities and specificities of the diagnostic criteria were compared using McNemar’s test.
Results
Two of the AFs favoring malignancy in general (mild-moderate T2 hyperintensity and hepatobiliary phase hypointensity) and two of the AFs favoring HCC in particular (nonenhancing “capsule” and mosaic architecture) were independently significant features for diagnosing HCC. By using the upgraded LR-5 criteria (LR-4 by major features alone + each aforementioned AF), sensitivities were significantly increased (69.4–76.9%) compared with the standard LR-5 (66.2%; all, p ≤ 0.004), whereas specificities (95.3–96.5%) were not significantly different (96.5%; all, p > 0.999).
Conclusions
Independently significant AFs may be used to upgrade from LR-4 to LR-5 to improve sensitivity without impairing specificity on gadoxetic acid–enhanced MRI.
Key Points
• Independently significant AFs for HCC on gadoxetic acid–enhanced MRI were mild-moderate T2 hyperintensity, hepatobiliary phase hypointensity, nonenhancing “capsule,” and mosaic architecture.
• When LR-4 criteria by major features alone in combination with significant AFs were upgraded to LR-5, sensitivities were higher than the standard LR-5, without impairing specificity.
• Independently significant ancillary features in Liver Imaging Reporting and Data System version 2018 may be used to upgrade from LR-4 to LR-5 to improve sensitivity without impairing specificity on gadoxetic acid–enhanced MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver Imaging Reporting and Data System (LI-RADS) is a comprehensive system for standardizing the terminology, technique, interpretation, reporting, and data collection of liver imaging [1]. LI-RADS has become an increasingly and widely used imaging criteria for the diagnosis of hepatocellular carcinoma (HCC) in high-risk patients [2]. LI-RADS enables noninvasive diagnosis of HCC with high specificity by using stringent criteria composed of only major features (LR-5, definitely HCC) [1]. However, the current LR-5 criteria may have a limitation of unsatisfactorily low sensitivity [3]. Recent studies have shown that low sensitivities of LR-5 criteria are more problematic for gadoxetic acid–enhanced MRI (EOB-MRI) than for extracellular contrast agent-enhanced MRI (ECA-MRI) [4,5,6]. This might be because LI-RADS was originally designed for ECA-MRI, and the use of EOB-MRI was incorporated later into its diagnostic algorithm [7].
LI-RADS is focused on specificity [1]. It has not been used in some area, especially East Asia because the sensitivity is important in this area; some parts of the treatment policy in East Asia are different from those in Western countries [2]. In Western countries, high specificity is emphasized to avoid false positive diagnosis of HCC, since patients with definite HCC may undergo liver transplantation for curative treatment based on imaging alone [8]. Meanwhile, in Asia, high sensitivity is preferred to detect early stage of HCC and to use locoregional ablative therapy for curative treatment [8]. In this reason, achieving high sensitivity as well as high specificity is important for the LI-RADS to be a global standard.
LI-RADS defines various ancillary features (AFs): AFs favoring malignancy (favoring malignancy in general and favoring HCC in particular) and AFs favoring benignity. AFs are intended to improve detection and characterization, increase confidence, or adjust LI-RADS categories, but not to upgrade to LR-5 using AFs favoring malignancy in general or HCC in particular without any weighted value on individual features [1, 9]. However, each AF favoring malignancy appears to vary in frequency and importance, and certain features may have greater emphasis or weight [10]. If certain significant AFs favoring malignancy could be given higher weighting than others, or used to upgrade from LR-4 to LR-5 on EOB-MRI, they may provide better sensitivity while maintaining high specificity.
Therefore, the purpose of our study was to identify significant AFs in LI-RADS version 2018 and develop the upgraded LR-5 criteria to improve diagnostic performance on EOB-MRI.
Materials and methods
Study subjects
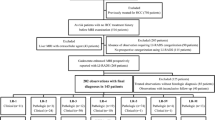
This retrospective study was approved by our institutional review board, which waived the requirement for informed consent. We searched our institution’s electronic medical records and identified 3795 patients at risk for developing HCC, who underwent liver dynamic MRI between 2015 and 2017. The inclusion criteria were as follows: (a) age ≥ 18 years; (b) patients at high-risk for HCC according to LI-RADS version 2018 (cirrhosis, chronic hepatitis B viral infection, or current or prior HCC); and (c) patients who underwent EOB-MRI. The exclusion criteria were as follows: (a) no observations other than hepatic cysts on MRI; (b) patients with insufficient final diagnosis such as unknown final diagnosis of malignancy as a result of immediate locoregional therapy or insufficient follow-up (< 2 years) for benign lesions to determine size stability; and (c) patients with cirrhosis due to congenital hepatic fibrosis or vascular disorders such as hereditary hemorrhagic telangiectasia, Budd-Chiari syndrome, chronic portal vein occlusion, cardiac congestion, or diffuse nodular regenerative hyperplasia. A total of 280 patients (mean age, 57.1 years; 199 men and 81 women) with 366 observations met the criteria and were included in this study. Of these, 164 patients with 218 observations had been reported in a prior study that compared the diagnostic performances of the European Association for the Study of the Liver 2018 and LI-RADS version 2018 on MRI for the noninvasive diagnosis of HCC in high-risk patients, in which AFs did not play major roles in HCC diagnosis [4].
Data collection
Clinical and laboratory data were collected for each patient from the electronic medical record. Data included sex, age, presence of cirrhosis, underlying cause of liver disease, Child-Pugh score, and model for end-stage liver disease (MELD) score. Pathologic diagnosis results were extracted from pathologic reports.
MRI examination
MRI was performed using 3.0-T systems (Magnetom Trio Tim, Siemens Healthineers; Intera Achieva, Ingenia, or Ingenia CX, Philips Healthcare; and Discovery MR 750w, GE Healthcare). The protocol included acquisition of dual-echo T1-weighted gradient-echo images (in-phase and opposed-phase), T1-weighted 3-dimensional gradient-echo images with dynamic contrast enhancement, navigator-triggered single- or multi-shot T2-weighted images, and diffusion-weighted images at b values of 0 or 50, 400, and 800 s/mm2. Dynamic T1-weighted imaging was performed before and after administering gadoxetic acid (Primovist, Bayer Pharma). The contrast agent was automatically administered intravenously using a power injector at a rate of 2 ml/s for a total dose of 0.025 mmol/kg body weight, followed by a 20 ml saline flush. Arterial phase scanning was initiated by using the test-bolus or the bolus-tracking technique, and images in the portal venous, transitional, and hepatobiliary phases were obtained approximately 60 s, 150 s, and 20 min after contrast agent injection, respectively. The detailed parameters of the MRI sequences are presented in Supplementary Table 1.
Observation registry
A radiologist (S.K., with 8 years of experience in abdominal radiology) marked individual observations (each ≥ 10 mm) to be reviewed on MRI, reported the observations based on segmental location, measured the size of the observations, and provided a list for review. The prior computed tomography (CT) or MRI examination was used to assess threshold or subthreshold growth, and ultrasound (US) was used to assess US visibility as a discrete nodule. When prior CT or MRI examinations or US examination were not available, they were considered as not applicable.
Image analysis
Two board-certified abdominal radiologists (M.-J.K. and S.L. with 26 years and 7 years of experience in liver imaging, respectively) independently reviewed the images using a Picture Archiving and Communication System (Centricity, GE Medical Systems). They were blinded to the final diagnosis of each observation, but were informed that the study population consisted of patients at high-risk for HCC. Each reader assessed the presence or absence of each LI-RADS version 2018 major feature and AF of the 366 observations marked on MRI, except for diameter, threshold growth, US visibility as discrete nodule, and subthreshold growth. When a feature could not be evaluated (e.g., fat sparing cannot be assessed in a patient without steatosis; iron sparing cannot be assessed in a patient without iron-overloaded liver), it was considered as not applicable. All assessed major features and AFs and their definitions based on LI-RADS version 2018 are summarized in Supplementary Table 2 [1]. Subsequently, each observation was assigned a category according to LI-RADS version 2018 as follows: LR-1 (definitely benign), LR-2 (probably benign), LR-3 (intermediate probability of malignancy), LR-4 (probably HCC), LR-5 (definitely HCC), LR-TIV (definite tumor in vein), and LR-M (probably or definitely malignant but not HCC specific) [1]. LI-RADS categorization was performed according to major imaging features alone and major features and AFs in combination, respectively. After independent image review, inter-reader agreement was evaluated. Discrepancies in major features and AFs between the readers were resolved by consensus discussion at least 2 weeks after the individual interpretation.
Reference standards
The diagnoses of HCCs and non-HCC malignancies were confirmed by pathology, including surgical resection (n = 299), explants for transplantation (n = 13), or core-needle biopsy (n = 4). The benign diagnoses were obtained by pathology (n = 7) or typical imaging features and stability at imaging for at least 2 years (n = 43). The mean interval between the MRI and pathologic diagnosis was 20.2 days (range, 0–90 days).
Statistical analysis
All analyses were performed on a per-observation basis. Per-observation estimates of diagnostic performances for each major feature and AF were calculated. To determine the major features and AFs predictive of HCC, logistic regression analysis was performed. Variables with a p value < 0.2 in the univariable analysis were entered into the multivariable analysis to identify independently significant major features and AFs for HCC diagnosis. For the multivariable analysis, a stepwise backward elimination method was used. Sensitivities and specificities of the diagnostic criteria were compared using McNemar’s test. The inter-reader agreement was evaluated using Cohen κ coefficient. Interpretation of κ values was as follows: poor, 0.00–0.20; fair, 0.21–0.40; moderate, 0.41–0.60; good, 0.61–0.80; and excellent, 0.81–1.00. A p value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 23.0 (IBM) and MedCalc version 16.2.1 (MedCalc Software).
Results
Characteristics of patients and observations
The characteristics of the 280 patients and 366 observations are presented in Table 1. Of the 280 patients, 162 (57.9%) had cirrhosis (134 caused by hepatitis B, eight by hepatitis C, 19 by alcohol, and one by non-alcoholic steatohepatitis), and the other 118 (42.1%) had chronic hepatitis B without cirrhosis. The majority of patients (98.2%) had Child-Pugh class A. The median MELD score was 7 (interquartile range, 6–8). A total of 366 observations included 281 (76.8%) HCCs, 35 (9.5%) non-HCC malignancies, and 50 (13.7%) benign lesions. Two hundred twenty patients had one lesion and 60 patients had two or more lesions.
Diagnostic performances of major features and AFs favoring malignancy
Table 2 shows the diagnostic performances of major features and AFs favoring malignancy in general or HCC in particular. Of the major features, sensitivity was the highest for nonrim arterial phase hyperenhancement (APHE) (82.2%) and specificity was the highest for enhancing “capsule” (88.2%). Among the AFs favoring malignancy in general, hepatobiliary phase hypointensity had the highest sensitivity (93.6%) followed by mild-moderate T2 hyperintensity (93.2%). AFs favoring HCC in particular showed low sensitivities (6.8–42.4%), but high specificities (95.3–98.8%).
Independently significant imaging features for diagnosing HCC
The univariable analyses demonstrated that all major features and AFs favoring malignancy except for fat sparing in solid mass were significant for predicting HCC (p ≤ 0.011; Table 2). According to the multivariable analyses, nonrim APHE (odds ratio [OR], 7.2; 95% confidence interval [CI], 3.2–16.1; p < 0.001), nonperipheral “washout” (OR, 4.0; 95% CI, 1.6–9.7; p = 0.002), and enhancing “capsule” (OR, 3.8; 95% CI, 1.4–10.3; p = 0.009) were independently significant in major features of LI-RADS version 2018. In addition, mild-moderate T2 hyperintensity (OR, 3.2; 95% CI, 1.3–7.7; p = 0.012) and hepatobiliary phase hypointensity (OR, 6.9; 95% CI, 2.5–18.6; p < 0.001) in AFs favoring malignancy in general, and nonenhancing “capsule” (OR, 10.8; 95% CI, 1.4–82.6; p = 0.022) and mosaic architecture (OR, 5.3; 95% CI, 1.1–25.2; p = 0.037) in AFs favoring HCC in particular, were independently significant MRI features for HCC diagnosis (Table 2).
Diagnostic performance of combined criteria using independently significant AFs
We developed the upgraded LR-5 criteria by combining LR-4 assigned by major features alone with independently significant AFs identified from the multivariable analysis (Table 3 and Supplementary Table 3). Observations that were assigned as LR-4 by major features alone and showed each independently significant AF were upgraded to LR-5 as follows: (a) upgraded LR-5A, LR-4 by major features alone with mild-moderate T2 hyperintensity; (b) upgraded LR-5B, LR-4 by major features alone with hepatobiliary phase hypointensity; (c) upgraded LR-5C, LR-4 by major features alone with nonenhancing “capsule”; and (d) upgraded LR-5D, LR-4 by major features alone with mosaic architecture (Figs. 1 and 2). By using the upgraded LR-5 criteria, sensitivities were significantly increased (69.4–76.9%) when compared with the standard LR-5 by major features alone (66.2%; all, p ≤ 0.004), whereas specificities (95.3–96.5%) were not significantly different (96.5%; all, p > 0.999).
Axial images obtained with gadoxetic acid–enhanced MRI in a 44-year-old man with chronic hepatitis B and surgically confirmed hepatocellular carcinoma (HCC). a Arterial phase, (b) portal venous phase, and (c) hepatobiliary phase of T1-weighted three-dimensional gradient-echo images show a 37-mm observation in segment V of the liver. The observation exhibits arterial phase hyperenhancement (a). The observation does not show “washout” in the portal venous phase (b), whereas it shows hepatobiliary phase hypointensity (c). Nonenhancing “capsule” is seen in T1-weighted images (a–c). T2-weighted image shows observation with mild-moderate T2 hyperintensity (d). This hepatic observation was assigned as LR-4 and could not be classified as LR-5 using Liver Imaging Reporting and Data System version 2018. In contrast, our upgraded LR-5 criteria or alternative criteria by utilizing independently significant ancillary features helped achieve the correct diagnosis of HCC
Axial images obtained with gadoxetic acid–enhanced MRI in a 65-year-old man with chronic hepatitis B and surgically confirmed hepatocellular carcinoma (HCC). a Arterial phase, (b) portal venous phase, and (c) hepatobiliary phase of T1-weighted three-dimensional gradient-echo images show a 79-mm observation in segment IV and VII of the liver. The observation exhibits arterial phase hyperenhancement (a). The observation does not show “washout” in the portal venous phase (b), whereas it shows hepatobiliary phase hypointensity (c). Nonenhancing “capsule” is seen in T1-weighted images (a–c). T2-weighted image shows observation with mild-moderate T2 hyperintensity (d). Mosaic architecture is seen in T1-weighted images and T2-weighted image (a–d). This hepatic observation was assigned as LR-4 and could not be classified as LR-5 using Liver Imaging Reporting and Data System version 2018. In contrast, our upgraded LR-5 criteria or alternative criteria by utilizing independently significant ancillary features helped achieve the correct diagnosis of HCC
We also calculated diagnostic performances of alternative diagnostic criteria by utilizing one or more of the independently significant AFs identified from the multivariable analysis (Table 4 and Supplementary Table 4). By using these alternative criteria, sensitivities significantly increased in varying degrees, depending on the number of independently significant AFs (≥ 1, 78.3%; ≥ 2, 76.9%; and ≥ 3, 70.5%) compared with the standard LR-5 (66.2%; all, p < 0.001), whereas specificities (95.3–96.5%) were not significantly different (96.5%; all, p > 0.999).
Inter-reader agreement
Supplementary Table 5 summarizes inter-reader agreement for major features and AFs favoring malignancy. Inter-reader agreements for major features and AFs favoring malignancy in general were good or excellent (κ = 0.78–0.93). Inter-reader agreements for AFs favoring HCC in particular were moderate or good (κ = 0.55–0.74).
Discussion
Our study demonstrated that two of the AFs favoring malignancy in general (mild-moderate T2 hyperintensity and hepatobiliary phase hypointensity) and two of the AFs favoring HCC in particular (nonenhancing “capsule” and mosaic architecture), in addition to three of the major features (nonrim APHE, nonperipheral “washout,” and enhancing “capsule”), were independently significant features for the noninvasive diagnosis of HCC on EOB-MRI. When LR-4 criteria by major features alone in combination with independently significant AFs were upgraded to LR-5, sensitivities were higher than the standard LR-5, without impairing specificity.
There have been several attempts to identify MRI features as independent predictors of HCC diagnosis [11,12,13]. However, previous studies used only limited number of major features or AFs for modifying diagnostic criteria [11,12,13]. In contrast, our study included all major features and AFs of LI-RADS version 2018 to determine independent features for predicting HCC on EOB-MRI and we found that four AFs (mild-moderate T2 hyperintensity, hepatobiliary phase hypointensity, nonenhancing “capsule,” and mosaic architecture) were independently significant features, along with major features (nonrim APHE, nonperipheral “washout,” and enhancing “capsule”). This implies that the aforementioned AFs may have strength and importance comparable with major features. Furthermore, these AFs may be weighted higher than other AFs or used to upgrade to LR-5.
In our study, among the AFs favoring malignancy in general, mild-moderate T2 hyperintensity and hepatobiliary phase hypointensity were highly sensitive (> 93%), but not specific for HCC. While mild-moderate T2 hyperintensity is highly suggestive of malignancy, it can be also seen in non-HCC malignancies [14]. Hepatobiliary phase hypointensity can occur in non-hepatocellular lesions such as hemangiomas or non-HCC malignancies due to lack of organic anion transporting polypeptide transporter expression in combination with strong enhancement of the hepatic parenchyma [9, 15]. Thus, the presence of mild-moderate T2 hyperintensity or hepatobiliary phase hypointensity alone may not be used to establish HCC diagnosis in the absence of major features. Instead, by combining mild-moderate T2 hyperintensity or hepatobiliary phase hypointensity with LR-4 criteria by major features and upgrading to LR-5, sensitivities were significantly improved without impairing specificities in our study, which is consistent with previous studies [16,17,18].
In our study, restricted diffusion was not an independently significant feature. Renzulli et al [13] reported that restricted diffusion is an independently significant feature for the diagnosis of HCC, in addition to APHE and hepatobiliary phase hypointensity on EOB-MRI. These inconsistent results may be attributed to differences in the definition of restricted diffusion. Renzulli et al [13] defined the presence of restricted diffusion as minimal perceptible hyperintensity to maximal hyperintensity similar to that of the spleen. Meanwhile, our study applied a strict definition for restricted diffusion according to LI-RADS version 2018, which is, intensity on diffusion-weighted imaging (DWI), not attributable solely to T2 shine-through, unequivocally higher than liver and/or apparent diffusion coefficient unequivocally lower than liver [1]. Since DWI depends on the scanner, field strength, and acquisition technique, different DWI techniques may also have led to discordant results [19]. In addition, DWI is prone to artifacts, which make reliable evaluation of hepatic lesions challenging, especially in the left liver [19].
Nonenhancing “capsule” and mosaic architecture, which are AFs favoring HCC in particular, showed high specificities. Capsule appearance, be it enhancing “capsule” or nonenhancing “capsule,” suggests hepatocellular origin and is one of the specific findings of HCC, because benign lesions and other malignancies such as cholangiocarcinoma and metastasis usually do not demonstrate capsule appearance [14]. Enhancing “capsule,” considered as one of the major features, may not be readily visible on EOB-MRI as on ECA-MRI; the enhancement of the “capsule” may be obscured by the relatively high enhancement of surrounding hepatic parenchyma in the portal venous or transitional phases when using EOB-MRI [5, 6]. Meanwhile, nonenhancing “capsule” may appear as a hypointense rim in the hepatobiliary phase image in addition to unenhanced T1- and T2-weighted images, thereby improving detection of tumor capsule and HCC diagnosis on EOB-MRI [20]. In our study, nonenhancing “capsule” in combination with LR-4 by major features provided better sensitivity with the same specificity. Mosaic architecture is unusual in non-HCC malignancies, and thus, highly specific for HCCs [21]. It is more frequently seen in HCCs > 3 cm [15]. Our study with a mean HCC size of nearly 3 cm revealed that sensitivity was highest for mosaic architecture among the AFs favoring HCC in particular. When mosaic architecture was combined with LR-4 by major features and upgraded to LR-5, sensitivity was improved without difference in specificity.
We also evaluated diagnostic performances of alternative diagnostic criteria by utilizing one or more of the independently significant AFs. When using these alternative criteria based on the number of independent AFs, sensitivities were significantly increased without significant loss of specificities, in the same manner as combining each independent AF.
This study has several limitations. First, the retrospective nature of the study at a single center may have introduced an inevitable selection bias. In our study, the majority of pathologic diagnosis was derived from surgical resection rather than biopsy. This study included a relatively large proportion of HCCs (76.8%), which may be due to selection bias where study populations underwent surgical resection. Second, the predominance of patients with chronic hepatitis B viral infection in our study may limit application in other populations with different major causes of HCC. Further prospective multicenter studies that include patients with various etiologies of liver disease are warranted to validate our results. Third, the final diagnoses of benign lesions were not based on pathologic diagnosis alone but on composite clinical reference standard. However, pathologic confirmation for highly suspected benign lesions is not recommended in clinical practice and application of a strict standard of reference (only pathology) for benign lesions may have resulted in confirmation bias. Fourth, the blinded readers had participated in imaging diagnosis in daily practice; thus, recall bias might have occurred. Finally, this study was performed only with EOB-MRI. Hepatobiliary phase hypointensity is a feature that can only be assessed on EOB-MRI. Therefore, there can be potential differences between ECA-MRI and EOB-MRI in categorization as a result of changing LR-5 criteria by using hepatobiliary phase hypointensity.
In conclusion, independently significant AFs may be used to upgrade from LR-4 to LR-5 to improve sensitivity without impairing specificity on gadoxetic acid–enhanced MRI.
Abbreviations
- AF:
-
Ancillary feature
- APHE:
-
Arterial phase hyperenhancement
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DWI:
-
Diffusion-weighted imaging
- ECA:
-
Extracellular contrast agent
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MELD:
-
Model for end-stage liver disease
- OR:
-
Odds ratio
- US:
-
Ultrasound
References
CT/MRI Liver Imaging Reporting and Data System version 2018. American College of Radiology Web site. https://www.acr.org/Clinical-Resources/Reporting-andData- Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed 12 Aug 2018
Kim TH, Kim SY, Tang A, Lee JM (2019) Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol 25:245–263
Lee S, Kim SS, Roh YH, Choi JY, Park MS, Kim MJ (2020) Diagnostic performance of CT/MRI liver imaging reporting and data system v2017 for hepatocellular carcinoma: a systematic review and meta-analysis. Liver Int. https://doi.org/10.1111/liv.14424
Lee S, Kim MJ, Kim SS et al (2020) Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol Int 14:70–79
Song JS, Choi EJ, Hwang SB, Hwang HP, Choi H (2019) LI-RADS v2014 categorization of hepatocellular carcinoma: intraindividual comparison between gadopentetate dimeglumine-enhanced MRI and gadoxetic acid-enhanced MRI. Eur Radiol 29:401–410
Min JH, Kim JM, Kim YK et al (2018) Prospective intraindividual comparison of magnetic resonance imaging with gadoxetic acid and extracellular contrast for diagnosis of hepatocellular carcinomas using the liver imaging reporting and data system. Hepatology 68:2254–2266
Hope TA, Fowler KJ, Sirlin CB et al (2015) Hepatobiliary agents and their role in LI-RADS. Abdom Imaging 40:613–625
Tang A, Cruite I, Mitchell DG, Sirlin CB (2018) Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ. Abdom Radiol (NY) 43:3–12
Cerny M, Chernyak V, Olivie D et al (2018) LI-RADS version 2018 ancillary features at MRI. Radiographics 38:1973–2001
Korean Society of Abdominal Radiology (2017) Diagnosis of hepatocellular carcinoma with gadoxetic acid-enhanced MRI: 2016 consensus recommendations of the Korean Society of Abdominal Radiology. Korean J Radiol 18:427–443
Rimola J, Forner A, Tremosini S et al (2012) Non-invasive diagnosis of hepatocellular carcinoma </= 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol 56:1317–1323
Choi SH, Byun JH, Lim YS et al (2016) Diagnostic criteria for hepatocellular carcinoma 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol 64:1099–1107
Renzulli M, Biselli M, Brocchi S et al (2018) New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut 67:1674–1682
Choi JY, Lee JM, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30–50
Chernyak V, Tang A, Flusberg M et al (2018) LI-RADS((R)) ancillary features on CT and MRI. Abdom Radiol (NY) 43:82–100
Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY (2010) Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 255:459–466
Chen N, Motosugi U, Morisaka H et al (2016) Added value of a gadoxetic acid-enhanced hepatocyte-phase image to the LI-RADS system for diagnosing hepatocellular carcinoma. Magn Reson Med Sci 15:49–59
Min JH, Kim YK, Sinn DH et al (2018) Adding ancillary features to enhancement patterns of hepatocellular carcinoma on gadoxetic acid-enhanced magnetic resonance imaging improves diagnostic performance. Abdom Radiol (NY) 43:2309–2320
Taouli B, Koh DM (2010) Diffusion-weighted MR imaging of the liver. Radiology 254:47–66
An C, Rhee H, Han K et al (2017) Added value of smooth hypointense rim in the hepatobiliary phase of gadoxetic acid-enhanced MRI in identifying tumour capsule and diagnosing hepatocellular carcinoma. Eur Radiol 27:2610–2618
Cannella R, Furlan A (2018) Mosaic architecture of hepatocellular carcinoma. Abdom Radiol (NY) 43:1847–1848
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health &Welfare, Korea (Grant No. 1520160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Myeong-Jin Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Lee et al [4]. One hundred sixty-four patients with 218 observations had been reported in a prior study that compared the diagnostic performances of the European Association for the Study of the Liver 2018 and LI-RADS version 2018 on MRI for the noninvasive diagnosis of HCC in high-risk patients, in which AFs did not play major roles in HCC diagnosis [4].
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Lee, S., Kim, Ss., Bae, H. et al. Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid–enhanced MRI. Eur Radiol 31, 855–863 (2021). https://doi.org/10.1007/s00330-020-07146-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07146-4