Abstract
Objectives
To evaluate the influence of the urinary bladder volume on the detectability of urolithiasis at the ureterovesical junction (UVJ) using a low-dose CT (LD-CT) with iterative reconstruction (IR) and a standard-dose CT (SD-CT) without IR in a large cohort.
Methods
Four hundred patients (278 males (69.5%), mean 44.6 ± 14.7 years) with urolithiasis at the UVJ were investigated either by an LD-CT with IR (n = 289, 72%) or an SD-CT without IR (n = 111, 28%) protocol. The detectability of distal urolithiasis was assessed by a dichotomous assessment (definite or questionable) by two radiologists in consensus and by a quantitative analysis of the signal density distribution across a line drawn parallel to the distal ureter. Based on the resulting graph, minimum/maximum density values and mean/maximum upslopes and downslopes were derived and calculated automatically. In all patients, the total bladder volume was calculated by a slice-by-slice approach on axial CT images.
Results
Patients with definite stones showed significantly higher urinary bladder volumes compared to patients with questionable stones in both LD-CT and SD-CT (p < 0.01). These results were independent of stones’ length and patients’ BMI values. Using cutoffs of 92 ml for LD-CT and 69 ml for SD-CT, high positive predictive values/accuracy rates of 96%/85% (LD-CT) and 98%/86% (SD-CT) were observed to identify definite urinary stones.
Conclusions
Urinary bladder volume has a significant impact on the detectability of distal urolithiasis. Moderate bladder filling by pre-CT hydration with subsequent CT scan at the time of high urge to void increases the detectability of urinary stones at the UVJ in clinical routine.
Key Points
• Urinary bladder volume significantly affects the detectability of distal urolithiasis
• Higher bladder volumes are associated with improved detectability of distal urinary stones
• Oral pre-CT hydration for urolithiasis is easily applicable and cost-effective
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is the most frequent cause of acute flank pain. It shows an increasing incidence and prevalence around the world [1] and is associated with rising healthcare costs [2, 3]. Among the variable locations where ureteral stones may lodge, the ureterovesical junction (UVJ) is one of the most common, ranging from 36 to 61% [4, 5]. Compared with ultrasound, non-enhanced CT (NECT) provides a higher diagnostic yield in the detection of urinary stones, particularly in the distal ureter [6, 7], and has become the modality of choice for evaluation of urolithiasis [8,9,10]. Moreover, in patients with a body mass index (BMI) < 30 kg/m2, a low-dose CT (LD-CT) with a mean dose of < 3 mSv was proposed [11,12,13] and was implemented into current international guidelines to reduce the radiation risk [14, 15] with a reported pooled sensitivity of 0.93 and a specificity of 0.97 in a recent meta-analysis [16]. Additionally, a LD-CT with iterative reconstruction (IR) was shown to provide excellent diagnostic performance and interobserver agreement, while significantly reducing the radiation exposure compared to filtered back projection [17, 18].
Despite all the advantages of an LD-CT, large cross-sectional studies in the USA demonstrated only a small clinical implementation rate of LD-CT for evaluation of renal stones ranging from 2% between 2011 and 2013 [19] to 7.6% during 2015–2016, indicating a slight increase of 5.6% compared to CT examinations in 2011–2012 [20]. Moreover, the detection rate of small ureteral stones < 3 mm was considerably lower using LD-CT compared to a standard-dose CT (SD-CT) with a sensitivity of 86% versus 100% [11]. Recently, a lower interobserver agreement of emergency urology residents and radiologists was reported in interpreting CT studies of patients with renal colic with kappa values of 0.93 for SD-CT and only 0.73 for LD-CT [21]. In another prospective study, where emergency patients with renal colic received both an SD-CT and an LD-CT, all false-positive stones (8/27, 30%) were reported to be inside the ureter on LD-CT and turned out to be phleboliths on SD-CT [22].
As the stones’ diameter gets smaller the closer to the UVJ they are located [5, 23], a precise detection of distal urolithiasis becomes increasingly difficult and the potential error rate increases [24]. Data about a routinely applicable and cost-effective method for increasing the detectability of distal urolithiasis using different CT protocols are yet missing. In this study, we investigated the influence of the volumetric urinary bladder filling on the detectability of stones at the UVJ using LD-CT with IR and SD-CT without IR in a large cohort.
Materials and methods
Study population
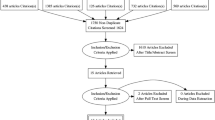
This retrospective study was HIPAA compliant and was approved by our institutional review board (IRB). Written informed consent was waived by our local IRB. From 2098 consecutive adult patients with clinical symptoms of acute urolithiasis, who underwent NECT between January 2010 and December 2017, 400 patients (278 males (69.5%) and 122 females (30.5%); mean 44.6 ± 14.7 years; age range, 18–90 years) revealed urolithiasis at the ureterovesical junction (Fig. 1). The inclusion criterion was a single ureteral stone in the axial, coronal, and sagittal plane. Clinical and radiological data were taken from the electronic and radiologic database of our institution. The median body mass index (BMI) was 26 kg/m2 (IQR 23–29 kg/m2) and was documented in 214 out of 400 patients (54%). All patients underwent the diagnostic workup that is routinely applied to all patients with suspected urolithiasis at our institution. This includes a detailed medical history, a physical examination, a biochemical workup, and diagnostic imaging with ultrasound and unenhanced CT. All CT examinations were reviewed by one radiological resident/consultant (1–6 years of experience in CT imaging) and one senior consultant (7–16 years of experience in CT imaging) to agree on a final diagnosis in consensus.
CT acquisition
CT was performed in all patients in supine position using a 256-detector row CT scanner (Brilliance iCT; Philips Medical Systems). All patients received a non-enhanced CT (NECT), where the scan region covered the area from the diaphragm to the pubic symphysis. In 289 patients (72%), a low-dose CT in a supine position with advanced hybrid iterative reconstruction (iDose4, Philips Healthcare) was used. Technical parameters of the low-dose (LD) protocol included the following: tube voltage 120 kV, reference tube current time product 40 mAs, collimation 2 mm × 128 mm × 0.625 mm, pitch 0.922, and gantry rotation time 0.4 s. In the remaining 111 patients (28%), who were investigated before the introduction of iterative reconstruction techniques in August 2012 in our department, a standard-dose CT without iterative reconstruction was performed using the following protocol: tube voltage 120 kV, variable mAs settings determined by the automatic exposure control system (ACS and z-axis dose modulation (Z-DOM)), with an average exposure of 93mAs (effective), collimation 2 mm × 128 mm × 0.625 mm, pitch 0.993, and gantry rotation time 0.5 s. All CT exams were reconstructed in axial, coronal, and sagittal orientations from isotropic voxels with a 3 mm slice thickness and 50% overlap, a soft-tissue kernel, and a 512 × 512 matrix from thin-sliced source images.
Radiation dose measurement
The dose-length product (DLP) was recorded from the automatically generated dose report for each CT. The effective dose was calculated by multiplying the dose-length product (mGy × cm) by a conversion factor (0.0155 mSv × mGy − 1 × cm − 1) [25]. In 289 patients, where low-dose CT was applied, a median effective dose of 1.9 mSv (IQR 1.5–2.7 mSv) was measured, whereas in the remaining 111 patients with standard-dose CT, a median effective dose of 3.9 mSv (IQR 3.5–4.1 mSv) was noted.
Qualitative assessment of the reader confidence to diagnose distal urolithiasis
Based on the final CT reports, a dichotomization of the reader confidence regarding the diagnosis of urolithiasis at the UVJ was applied to all investigated CT scans:
-
1.
Definite stone
-
2.
Questionable stone
Questionable stones included “probable” or “uncertain” stones, according to the CT reports.
Quantitative assessment of the detectability of distal urolithiasis
The quantification of the urolithiasis detectability of at the UVJ was made using an in-house programmed Plug In of ImageJ, an open source imaging software [26, 27]. First, a line was manually drawn parallel to the course of the distal ureter, which contained the calculus. Second, the algorithm automatically measured all signal densities across the line in Hounsfield units (HU). Third, all signal densities across the drawn line were plotted on a graph obtaining a parabolic shape (Fig. 2). Finally, based on the graph, minimal and maximal density values, as well as mean and maximum slopes left to the maximum density (upslope) and right to the maximum density (downslope), were derived and calculated automatically (Fig. 3).
Comparison of two patients with a definite stone (a) and a questionable stone (b) of 3 mm diameter in each case (upper row, white arrows) with corresponding quantitative analysis graphs (lower row), demonstrating the length of the drawn line in mm (x-axis) and the corresponding signal densities (HU) across the line (y-axis). Vertical blue lines of the graphs represent mean up- and downslopes within the automatically defined slope limits (vertical brown lines). Note the decrease of maximum signal densities between a (356HU) and b (101HU)
Volumetry of the urinary bladder
In all CT data sets, the area of the urinary bladder was manually traced by an experienced radiologist slice-by-slice on every axial slice, where the bladder could be depicted using the integrated volumetry option of the RIS/PACS system (Centricity™ 5.1, GE Healthcare). The total bladder volume was automatically calculated in cubic centimeter according to the sum of all traced areas in axial CT slices (Fig. 4).
Statistics
Continuous variables are presented as mean ± standard deviation (SD) in normally distributed data or as median and interquartile range (IQR) otherwise. The Shapiro-Wilk test assessed normality of continuous data. Pairwise comparisons were performed using a t test for normally distributed data or Mann-Whitney U test otherwise. Proportions from two independent samples were compared by chi-squared test. Receiver operating characteristic (ROC) curve analysis was performed to analyze the diagnostic accuracy of the urinary bladder volume to discriminate between patients with definite and questionable stones. The diagnostic performance was expressed as the area under the curve (AUC), cutoff values, sensitivity, specificity, positive and negative predictive values (PPV and NPV), and accuracy with corresponding 95% confidence intervals (95% CI) using the modified Wald method. The Youden index obtained optimal cutoff values. Statistical significance was defined as p < 0.05. Data collection and statistical calculations were performed using MedCalc 15.8 (MedCalc Software) and SPSS Statistics 22 (IBM SPSS) software.
Results
Patient characteristics
The basic characteristics of the study population are presented in Table 1. The majority of the patients were males (69.5%, mean age 44.6 ± 14.7 years) without any significant difference among both CT protocols. Also, the stones’ length in any orientation, the clinical confidence, the bladder volume, and the quantitative parameters like left and right mean and maximum slopes and the minimum and maximum density values were comparable for both CT protocols. Only the DLP and the effective doses were significantly lower in patients who received an LD-CT with IR compared to those who had an SD-CT without IR (DLP 125.4 mGy cm (LD-CT) vs. 250.5 mGy cm (SD-CT), p < 0.001; effective dose: 1.9 mSv (LD-CT) vs. 3.9 mSv (SD-CT), p < 0.001).
Qualitative and quantitative detectability of distal urinary stones
When comparing the qualitative clinical confidence assessment with the quantitative results, larger maximum/mean stones’ length, higher maximum density values, increased bladder volumes, and higher values of the corresponding left and right slopes of the density distribution curves were associated with a higher clinical confidence of a stone (e.g., definite stone, p < 0.001 for all, Table 2). Only minimum density values of the stones were not significantly altered between both groups using both CT protocols (p > 0.05 for both). Moreover, all quantitative results and comparisons were comparable among patients with LD-CT with IR and those with SD-CT without IR.
Urinary bladder volume and detectability of urinary stones
After dichotomization of all stones into “definite stone” or “questionable stone,” the corresponding bladder volumes of patients with different stone lengths ranging from ≤ 3 up to ≥ 6 mm were compared with each other using both a LD-CT with IR and a SD-CT without IR. Using LD-CT with IR, the median bladder volumes in patients with definite stones ranged from 95 to 126 cm3 and were significantly higher than the median bladder volumes of patients with questionable stones, which ranged between 51 and 65 cm3 (p < 0.01 for all comparisons, Table 3). Comparable results were observed in patients, who received an SD-CT without IR, where median bladder volumes of patients with definite stones (84 to 174 cm3) differed significantly from median bladder volumes in patients with questionable stones (31 to 51 cm3, p < 0.01). These results were for both CT protocols independent of stones’ length (Table 3, Fig. 5).
Diagnostic performance of urinary bladder volume to discriminate patients with definite and questionable stones
In patients who received an LD-CT, a urinary bladder volume of 92 cm3 led to an AUC of 0.877, a sensitivity of 84%, a specificity of 89%, a PPV and NPV of 96% and 65%, and an accuracy of 85% to discriminate patients with definite and questionable stones. A slightly better diagnostic performance was observed in patients with an SD-CT and a lower cutoff of the urinary bladder volume of 69 cm3. Using this threshold, an AUC of 0.886, a sensitivity of 86%, a specificity of 90%, a PPV of 98%, a NPV of 57%, and an accuracy of 86% could be achieved (Table 4, Fig. 6).
Urinary bladder volumes in patients with different BMI and CT protocol
In a subgroup of 214 patients with documented BMI, urinary bladder volumes were compared between patients with a BMI < 30 kg/m2 and those with a BMI ≥ 30 kg/m2, who received either an LD-CT or an SD-CT. Using any of the two CT protocols, obese patients (BMI ≥ 30 kg/m2) with definite stones had significantly higher urinary bladder volumes compared to patients with questionable stones (LD-CT 106 cm3 vs. 65 cm3, p = 0.004; SD-CT 144 cm3 vs. 50 cm3, p = 0.03, Table 5). Patients with a BMI ≥ 30 kg/m2 and < 30 kg/m2 and definite or questionable stones failed to show a significant difference in urinary bladder volumes (p > 0.05 for all comparisons, respectively, Table 5).
Discussion
The present study examined the effect of volumetric filling of the urinary bladder concerning the detectability of distal ureteral stones at the UVJ in 400 patients who received either a non-enhanced LD-CT with IR or an SD-CT without IR. Our results demonstrated significantly higher urinary bladder volumes in patients with definite stones compared to patients with questionable stones. The automatic quantitative stone assessment also supported this finding in terms of steeper density curve slopes and higher maximum and lower minimum stone density values in the “definite stone” group compared with the “questionable stone” group. Moreover, this result was irrespective of the stones’ length or the applied CT protocol, as both CT protocols revealed comparable results. Furthermore, we calculated optimal cutoff values of urinary bladder volumes for a proper distinction of definite stones of 92 ml for patients receiving an LD-CT with IR and of 69 ml for patients receiving an SD-CT without IR. Thereby, we obtained high PPVs/accuracy rates of 96%/85% (LD-CT) and 98%/86% (SD-CT), respectively. These results are of high clinical importance, as they indicate the benefit of pre-CT oral hydration in patients with clinical suspicion of urolithiasis in order to achieve a better detectability of distal urinary stones. This is yet to be mentioned in the current recommendations and workup guidelines for urolithiasis [10, 14, 15].
Current international guidelines recommend the use of LD-CT as the method of choice for patients with suspected urolithiasis [10, 14, 15]. However, in obese patients with a BMI > 30 kg/m2, an SD-CT is preferred due to the lower sensitivity and specificity of LD-CT for detecting ureteral stones [10, 14, 15]. In contrast, our results showed a significant difference of urinary bladder volumes in obese patients (BMI ≥ 30 kg/m2) between definite and questionable stones using either LD-CT with IR or SD-CT without IR with a lower p value for LD-CT (p = 0.008 vs. p = 0.03). These interesting findings may be caused by the IR algorithm, which allows to significantly reduce the noise compared with the filtered back projection (FBP) while maintaining the same image quality [28]. This finding is also supported by a recent study, which demonstrated significantly better image quality and diagnostic confidence in overweight and obese patients (BMI ≥ 25 kg/m2) compared to those with a BMI < 25 kg/m2 using an LD-CT protocol with statistic IR technique [29]. Hence, our findings support a possible universal pre-CT patient hydration with consecutive increased bladder volume in clinical routine independent of the applied CT protocol and the BMI values of the patients. Recent publications demonstrated an additional value of dual-source CT (DECT) to conventional CT for determining stone composition, especially for differentiating uric acid stones from non-uric acid stones, as the former may be treated pharmacologically rather than invasively [30, 31]. A recent study demonstrated that stone detection and characterization by DECT was also feasible in patients with large body habitus of > 90 kg of weight [32]. The drawbacks of DECT (inconsistent characterization of small stones < 3 mm and stone mimics [33]) might be reduced by a simple and cost-effective oral hydration, especially in case of small stones ≤ 3 mm at the UVJ [5, 23]. Pre-CT oral hydration may help discriminate among different stone mimics such as pelvic phleboliths or incidental transitional cell carcinomas (TCC) of the bladder [34] or of the distal ureter, which are found in up to 75% of cases of ureteral TCC [35]. Previous studies suggested different CT-based signs for this purpose like the rim sign, indicating a ureteral wall edema [36, 37] and the comet tail sign as a hallmark of a dilated vein leading to the phlebolith [37, 38] as well as prone CT scanning [39]. However, they either turned to be not clinically helpful [40] or required an additional radiation exposure for prone scanning after an initial supine position [39]. A recent study discriminated urinary stones from phleboliths on LD-CT using radiomics and machine learning, which resulted in an overall accuracy of 85%, an AUC of 0.902, and positive and negative predictive values of 81.5% and 90%, respectively [41]. The combination of these promising techniques and an appropriate bladder filling might further increase the diagnostic yield of distal urolithiasis in the future and requires further investigation.
Our calculated volume cutoffs represent only small amounts of a healthy functional bladder capacity in adults of nearly 350 ml [42, 43], ranging from 20% (69/350 ml) in SD-CT to 26% (92/350 ml) in LD-CT. Thus, a pre-CT hydration with at least 250 ml of water, which represents a commonly used saline solution volume for intravenous hydration and slightly more than one plastic cup for oral hydration, should generally lead to the desired increase in bladder volume. Thereafter, the CT scan should only be initiated when an urge to void becomes noticeable. This approach probably would not affect the patients’ compliance, as micturition is normally prompted near complete bladder volume capacity [44]. Moreover, moderate bladder filling was already implemented for ultrasound investigations in distal urolithiasis [45]. Additionally, intravenous hydration is commonly applied routinely to patients with uncomplicated renal colic and low urine volume, due to reduced intake or increased loss of water, which leads to an increased production of concentrated solutes and to recurrent stone formation [46, 47].
Our study had several limitations. Firstly, it was limited by the retrospective study design. It requires further external prospective validation with different CT protocols before clinical application. Secondly, despite the observed difference of bladder volumes in obese patients with definite and questionable stones, the sample size in both CT protocols was rather small, so that we were unable to provide a discrete cutoff value of the bladder filling for these patients to prevent a sampling bias with resulting low accuracy of the results [48, 49]. Especially, these findings require further verification. Thirdly, the implemented dichotomization for qualitative assessment of the detectability of distal urolithiasis into “definite stone” and “questionable stone” is subjective and may be debatable. However, this assessment was performed by two radiologists (including one senior consultant) in consensus and was adapted from other publications dealing with urolithiasis [50, 51]. Moreover, our subjective arrangement of the reader detectability was also supported by the presented objective automatic quantitative stone assessment.
Conclusions
Urinary bladder volume has a significant impact on the detectability of distal urolithiasis, indicating that moderate oral or intravenous pre-CT hydration and subsequent CT scan at the time of high urge to void allows for an easily applicable and cost-effective method to increase the detectability of stones at the UVJ in clinical routine.
Abbreviations
- BMI:
-
Body mass index
- CT:
-
Computed tomography
- DECT:
-
Dual-source CT
- DLP:
-
Dose-length product
- HU:
-
Hounsfield units
- IQR:
-
Interquartile range
- IR:
-
Iterative reconstruction
- LD-CT:
-
Low-dose CT
- NECT:
-
Non-enhanced CT
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating curve analysis
- SD-CT:
-
Standard-dose CT
- TCC:
-
Transitional cell carcinoma
- UVJ:
-
Ureterovesical junction
References
Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD (2017) Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus 3:18–26
Scales CD, Saigal CS, Hanley JM et al (2014) The impact of unplanned postprocedure visits in the management of patients with urinary stones. Surgery 155:769–775
Saigal CS, Joyse G, Timilsina AR (2005) Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 68:1808–1814
Moon YJ, Kim HW, Kim JB, Kim HJ, Chang YS (2015) Distribution of ureteral stones and factors affecting their location and expulsion in patients with renal colic. Korean J Urol 56:717–721
Eisner BH, Reese A, Sheth S, Stoller ML (2009) Ureteral stone location at emergency room presentation with colic. J Urol 182:165–168
Ray AA, Ghiculete D, Pace KT, Honey RJ (2010) Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology 76:295–300
Thoeny HC, Hoppe H (2003) Unenhanced spiral CT in urolithiasis: indication, performance and interpretation. Rofo 175(7):904–910
Smith R, Rosenfield A, Choe K et al (1995) Acute flank pain: comparison of non-contrast-enhanced CT and intravenous urography. Radiology 194:789–794
Teichman JM (2004) Acute renal colic from ureteral calculus. N Engl J Med 350:684–693
Ziemba JB, Matlaga BR (2015) Guideline of guidelines: kidney stones. BJU Int 116:184–189
Poletti PA, Platon A, Rutschmann OT, Schmidlin FR, Iselin CE, Becker CD (2007) Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am J Roentgenol 188:927–933
Jin DH, Lamberton GR, Broome DR et al (2010) Effect of reduced radiation CT protocols on the detection of renal calculi. Radiology 255:100–107
Niemann T, Kollmann T, Bongartz G (2008) Diagnostic performance of low-dose CT for the detection of urolithiasis: a meta-analysis. AJR Am J Roentgenol 191:396–401
Türk C, Petrik A, Sarica K et al (2016) EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol 69:468–474
Fulgham PF, Assimos DG, Pearle MS, Preminger GM (2013) Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol 189:1203–1213
Xiang H, Chan M, Brown V, Huo YR, Chan L, Ridley L (2017) Systematic review and meta-analysis of the diagnostic accuracy of low-dose computed tomography of the kidneys, ureters and bladder for urolithiasis. J Med Imaging Radiat Oncol 61:582–590
Kwon JK, Chang IH, Moon YT, Lee JB, Park HJ, Park SB (2015) Usefulness of low-dose nonenhanced computed tomography with iterative reconstruction for evaluation of urolithiasis: diagnostic performance and agreement between the urologist and the radiologist. Urology 85:531–538
Park SH, Do KK, Moon YT et al (2014) Pilot study of low-dose nonenhanced computed tomography with iterative reconstruction for diagnosis of urinary stones. Korean J Urol 55:581–586
Lukasiewicz A, Bhargavan-Chatfield M, Coombs L et al (2014) Radiation dose index of renal colic protocol CT studies in the United States : a report from the American College of Radiology National Radiology Data Registry. Radiology 271:445–451
Weisenthal K, Kathrik P, Shaw M et al (2017) Evaluation of kidney stones with reduced–radiation dose CT: progress from 2011−2012 to 2015−2016—not there yet. Radiology 286:581–589
Hong JY, Lee DH, Chang IH, Park SB, Kim CW, Chi BH (2017) Inter-observer agreement between urologists and radiologists in interpreting the computed tomography images of emergency patients with renal colic. Urol J 15:6–9
Malkawi IM, Han E, Atalla CS, Santucci RA, O'Neil B, Wynberg JB (2016) Low-dose (10%) computed tomography may be inferior to standard-dose CT in the evaluation of acute renal colic in the emergency room setting. J Endourol 30:493–496
Song H, Cho ST, Kim KK (2010) Urolithiasis investigation of the location of the ureteral stone and diameter of the ureter in patients with renal colic. Korean J Urol 51:198–201
Jellison FC, Smith JC, Heldt JP et al (2009) Effect of low dose radiation computerized tomography protocols on distal ureteral calculus detection. J Urol 182:2762–2767
Deak PD, Smal Y, Kalender WA (2010) Multisection CT protocols: sex- and age-specific conversion factors used to determine effective dose from dose-length product. Radiology 257:158–166
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schindelin J, Rueden CT, Hiner MC, Eliceiri KW (2015) The ImageJ ecosystem : an open platform for biomedical image analysis. Mol Reprod Dev 529:518–529
Kulkarni N, Uppot RN, Eisner BH, Sahani DV (2012) Radiation dose reduction at multidetector CT with adaptive statistical iterative reconstruction for evaluation of urolithiasis: how low can we go? Radiology 265:158–166
Gervaise A, Naulet P, Beuret F et al (2014) Low-dose CT with automatic tube current modulation, adaptive statistical iterative reconstruction, and low tube voltage for the diagnosis of renal colic: impact of body mass index. AJR Am J Roentgenol 202:553–560
Graser A, Johnson TR, Bader M et al (2008) Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest Radiol 43:112–119
Wisenbaugh ES, Paden RG, Silva AC, Humphreys MR (2014) Dual-energy vs conventional computed tomography in determining stone composition. Urology 83:1243–1247
Kordbacheh H, Baliyan V, Uppot RN, Eisner BH, Sahani DV, Kambadakone AR (2019) Dual-source dual-energy CT in detection and characterization of urinary stones in patients with large body habitus: observations in a large cohort. AJR Am J Roentgenol 212:796–801
Jepperson M, Cernigliaro J, Sella D et al (2013) Dual-energy CT for the evaluation of urinary calculi: image interpretation , pitfalls and stone mimics. Clin Radiol 68:e707–e714
Kundra V, Silverman PM (2003) Imaging in the diagnosis , staging , and follow-up of cancer of the urinary bladder. AJR Am J Roentgenol 180:1045–1054
Browne RF, Meehan CP, Colville J, Power R, Torreggiani WC (2005) Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics 25:1609–1627
Heneghan JP, Dalrymple NC, Verga M, Rosenfield AT, Smith RC (1997) Soft-tissue “ Rim” sign in the diagnosis of ureteral calculi helical CT. Radiology 202:709–711
Rucker CM, Menias CO, Bhalla S (2004) Mimics of renal colic: alternative diagnoses at unenhanced helical CT. Radiographics 24:11–28
Bell TV, Fenlon HM, Davison BD, Ahari HK, Hussain S (1998) Unenhanced helical CT criteria to differentiate distal ureteral calculi from pelvic phleboliths. Radiology 207:363–367
Levine J, Neitlich J, Smith RC (1999) The value of prone scanning to distinguish ureterovesical junction stones from ureteral stones that have passed into the bladder: leave no stone unturned. AJR Am J Roentgenol 172:977–981
Guest AR, Cohan RH, Korobkin M et al (2001) Assessment of the clinical utility of the rim and comet-tail signs in differentiating ureteral stones from phleboliths. AJR Am J Roentgenol 177:1285–1291
De Perrot T, Hofmeister J, Burgermeister S et al (2019) Differentiating kidney stones from phleboliths in unenhanced low-dose computed tomography using radiomics and machine learning. Eur Radiol. https://doi.org/10.1007/s00330-019-6004-7
Fitzgerald MP, Stablein U, Brubaker L (2002) Urinary habits among asymptomatic women. Am J Obstet Gynecol 187:1384–1388
Latini JM, Mueller E, Lux MM, Fitzgerald MP, Kreder KJ (2004) Voiding frequency in a sample of asymptomatic American men. J Urol 172:980–984
Lukacz ES, Sampselle C, Gray M et al (2011) A healthy bladder : a consensus statement. Int J Clin Pract 65:1026–1036
Moesbergen TC, de Ryke RJ, Dunbar S, Wells JE, Anderson NG (2011) Distal ureteral calculi: US follow-up. Radiology 260:575–580
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367:333–344
Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A (1996) Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 155:839–843
Hanczar B, Hua J, Sima C, Weinstein J, Bittner M, Dougherty ER (2010) Small-sample precision of ROC-related estimates. Bioinformatics 26:822–830
Bean WT, Stafford R, Brashares JS (2012) The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 35:250–258
Schmidt-Holtz J, Laqmani A, Butscheidt S et al (2018) Iterative model reconstruction (IMR) in MDCT below 2 mSv for the detection of urinary calculi: diagnostic accuracy and image quality in comparison to filtered back-projection and 4th generation iterative reconstruction (iDose4). Rofo 190(07):630–636
Remer EM, Herts BR, Primak A et al (2014) Detection of urolithiasis: comparison of 100% tube exposure images reconstructed with filtered back projection and 50% tube exposure images reconstructed with sinogram-affirmed iterative reconstruction. Radiology 272:749–756
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Maxim Avanesov.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avanesov, M., Togmat, J., Solmaz, M. et al. Increased urinary bladder volume improves the detectability of urinary stones at the ureterovesical junction in non-enhanced computed tomography (NECT). Eur Radiol 29, 6953–6964 (2019). https://doi.org/10.1007/s00330-019-06279-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06279-5