Abstract
Objectives
To develop a diagnostic algorithm for positron emission tomography (PET)–detected incidental breast lesions using both breast imaging reporting and data system (BI-RADS) and maximum standardized uptake value (SUVmax) criteria.
Methods
Fifty-six PET-detected incidental breast lesions from 51 patients, which were subsequently investigated by breast ultrasound within 1 month of the PET study, constituted the study cohort and they were finally verified by tissue diagnosis or a 2-year follow-up. Based on the maximum specificity with sensitivity > 60.0% and maximum sensitivity with specificity > 60.0%, two SUVmax cutoff values were calculated at 2 and 3.7. BI-RADS ≥ 4 was considered as highly suspicious for malignancy. The diagnostic accuracies were estimated for SUVmax levels above or below the cutoff points combined with the BI-RADS suspicion level.
Results
Overall, 46 benign and 10 malignant lesions were studied. The diagnostic characteristics of SUVmax ≥ 2, SUVmax ≥ 3.7, and BI-RADS ≥ 4 were 80.0%, 60.0%, and 80.0% for sensitivity, 73.9%, 95.7%, and 92.7% for specificity, and 75.0%, 89.3%, and 90.2% for accuracy, respectively. When the SUVmax threshold was set at 2, combined with BI-RADS suspicion level, the sensitivity, specificity, and accuracy were 100.0%, 69.6%, and 75.0%, respectively. The results for SUVmax threshold set at 3.7 combined with BI-RADS were 90.0%, 91.3%, and 91.1% for the sensitivity, specificity, and accuracy, respectively. A diagnostic algorithm was accordingly generated.
Conclusion
The need for biopsy should be justified in low BI-RADS lesions presenting with high SUVmax at 3.7 or higher. The biopsy of patients with high B-IRADS and low SUVmax could be preserved.
Key Points
• A diagnostic algorithm was developed for PET-detected incidental breast lesions using both BI-RADS and SUVmax criteria.
• Diagnostic performance was calculated separately and conjunctively for SUVmax ≥ 2, SUVmax ≥ 3.7, and BI-RADS ≥ 4.
• The need for biopsy can be justified in BI-RADS < 4 lesions with SUVmax ≥ 3.7. Lesions with BI-RADS < 4 and indeterminate SUVmax (2 < SUVmax < 3.7) benefit from a short-interval follow-up. BI-RADS < 4 lesions with SUVmax < 2 may confidently be scheduled for routine screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Incidental breast lesions are occasionally encountered in F-18 fluorodeoxyglucose positron emission tomography/computed tomography (F-18 FDG PET/CT) and may represent a second primary cancer in up to 57.0% of cases [1]. Due to the lack of a standardized PET-based guideline for characterizing lesions as benign, indeterminate, or highly suspicious for malignancy, there is a need for second-look breast ultrasonography for the optimal management of incidental breast lesions. The breast imaging reporting and data system (BI-RADS) lexicon has been developed as a standard and reliable method for harmonizing recommendations and determining the risk of malignancy for breast imaging [2, 3] with a good interobserver agreement and a high level of confidence [4]. However, as the first-line imaging method, it has shown a relatively low positive predictive value for biopsy recommendation, a high false-positive rate, and a wide range of malignancy likelihood in BI-RADS 4 (2.0–95.0%), resulting in a significant number of time-consuming and unnecessary invasive procedures [5,6,7,8]. Moreover, there is still a 0.8–2.0% risk of malignancy in lesions, which has been categorized as putatively benign (BI-RADS 1 to 3, respectively), which may result in a delay in urgent treatment [6].
F-18 FDG PET/CT has emerged as a highly sensitive imaging tool in clinical oncology providing valuable information about the biological aggressiveness and histological grading of most tumoral lesions by depicting the glucose metabolic rate. It is extensively applied for differentiating benign from malignant solitary pulmonary nodules [9] and low grade from high-grade brain tumors [10] in the current practice. However, considering the major metabolic overlap of malignant and benign breast lesions, this clinical setting is discouraged [11, 12]. In spite of the higher uptake of F-18 FDG in malignant breast lesions [11], current guidelines do not recommend the routine use of F-18 FDG PET/CT scan as a reliable diagnostic tool for the differentiation of benign and malignant breast lesions [12, 13].
To the best of our knowledge, this is the first attempt to explore the complementary role of metabolic parameters and BI-RADS in stratifying incidental breast lesions. We sought to examine the diagnostic performance of metabolic criteria derived from F-18 FDG PET/CT in combination with BI-RADS ultrasound categorization to characterize incidental breast lesions. The aim of this study was to develop an algorithm to be used in the case of incidental breast lesions to avoid missed breast cancers and unnecessary biopsies.
Methods and materials
Patients
This retrospective study included 5029 patients without a known history of breast cancer, undergoing F-18 FDG PET/CT scan at Masih Daneshvari Hospital from May 2012 to Sep 2015; in total, 66 incidental breast lesions were identified. Fifteen patients did not refer for further breast ultrasound investigation due to their advanced primary disease or the data was not available. Finally, 51 patients with a total of 56 lesions subsequently underwent breast US examination within 1 month from PET scan constituted the study cohort. Age, sex, and primary cancer type were recorded for each patient.
Imaging acquisition
F-18 FDG PET/CT acquisition protocol
An integrated PET/CT device (D-690, General Electric Medical Systems) was used. The fasting period was maintained for at least 8 h. The level of blood glucose at the time of radiotracer injection was < 150 mg/dl. Sixty minutes (± 10.0%) after 4.6 MBq/kg IV administration of F-18 FDG (0.12 mCi/kg), CT acquisition was craniocaudally initiated from the vertex to the mid-thigh (or to toe as indicated) in the supine position with a multidetector CT scanner under the following parameters: 50–120 auto mAs tube current, 120 kV, noise factor 19, 2.5-mm thickness, and tidal breathing. Thirty minutes before imaging acquisition, 40 ml meglumine 76.0% (containing 370 mg iodine/ml) in 1500 water was administered as oral contrast. The PET data were then collected in the reverse direction at 3 min per bed position immediately after CT acquisition. Corrections were made in the raw data in terms of attenuation, dead time, and random and scatter coincidence; subsequently, images were reconstructed by an iterative method and HD (high definition) technique.
Breast ultrasound examination
A qualified breast imaging specialist performed breast sonography examinations outside the clinic after reviewing the results of PET/CT, using high-resolution instruments with compact linear (8–15 MHz) or linear (5–12 MHz) transducers.
Diagnostic criteria
Metabolic criteria
The PET (AC, non-AC), CT, and fused PET/CT images of the eligible cohort were retrieved and reviewed at a workstation (Advantage Window, 4.5, Volumeshare software, GE 690) by a team comprising an experienced radiologist and a nuclear physician. Increased F-18 FDG uptake higher than the background activity of surrounding normal breast tissue was the focus for detecting abnormalities on AC and non-AC PET images with or without corresponding CT abnormalities, including ill-defined soft tissue density, nodule, or skin thickening, which were considered incidental breast lesions.
Based on the visual assessment, a qualitative metabolic dichotomous criterion was defined as negative for malignancy if the F-18FDG uptake was equal to or less than the value in the liver and positive for malignancy if the lesion activity was more than that of the liver (severe F-18 FDG uptake). The SUVmax, as the most validated PET-derived metabolic parameter, was determined after drawing a circular three-dimensional region of interest surrounding the metabolically active breast lesion. The receiver operating characteristic (ROC) curve was then generated for the SUVmax and tested for diagnostic performance. Two different SUVmax cutoff values were calculated. For a sensitive threshold, a value of SUVmax was selected with the highest sensitivity accompanying specificity above 60.0%. For a specific threshold, a SUVmax was selected with the highest specificity and sensitivity above 60.0%. A SUVmax equal to or above the cutoff values was considered as positive for malignancy (PET positive).
Morphologic criteria
Using the BI-RADS scoring system, outside-clinic breast imaging specialists interpreted the US results. For descriptive purposes, the breast lesions were categorized into BI-RADS 1 to 5 with respect to their orientation, shape, echo pattern, boundary, margin, and posterior acoustic characteristics [5, 14,15,16]. To define a dichotomous diagnostic test, the BI-RADS categories were further reclassified into BI-RADS < 4 (BI-RADS negative) and BI-RADS ≥ 4 (BI-RADS positive), which are representatives of benignity and malignancy, respectively.
High interobserver agreement for the BI-RADS lexicon obviates the need for single observer interpretation and centralized breast assessment.
Combined criteria
Regarding both metabolic and morphologic features, the incidental breast lesions were classified as double positive (PET positive plus BI-RADS positive), PET-only positive, BI-RADS-only positive, and double negative (PET negative plus BI-RADS negative). As defined in the metabolic criteria section, two thresholds were employed for SUVmax values with maximum sensitivity and specificity. Correspondingly, two combined criteria were defined with two different SUVmax thresholds. The combined criteria were further reclassified as a dichotomous diagnostic test into benign (both PET negative and BI-RADS negative) and malignant (either PET positive or BI-RADS positive) groups. Finally, a diagnostic algorithm was proposed to evaluate the incidental breast lesions using morphological criteria together with two SUVmax thresholds.
Standard of reference
All incidental breast lesions were finally verified as benign or malignant according to the histopathologic results or oncologist decision making based on a 2-year clinical formal follow-up including routine clinical examination and serial breast US as well as follow-up PET/CT whenever available. Tissue biopsy was obtained in 20 patients immediately after baseline breast imaging (43.1%) (needle biopsy n = 12, excisional biopsy n = 8), and the 36 remaining (56.9%) underwent clinical formal follow-up (median follow-up duration = 24.18 months). Two patients developed histologically proven malignant lesions 9 and 6 months after the benign baseline US examinations categorized as BI-RDAS I and BI-RADS III, respectively. The corresponding SUVmax was 2.9 (Fig. 1) and 3.8, respectively. Thirty-four lesions revealed no further evidence of malignancy by the end of the study and were thus considered benign lesions in 29 patients.
Statistical analysis
Parametric and nonparametric tests were applied to analyze variables with normal and abnormal distributions, respectively. Continuous (age and SUVmax) and categorical (sex, BI-RADS, lesion intensity, and primary cancer type) variables are respectively described as mean ± SD and frequency. For comparisons between the benign and malignant groups, Fisher’s exact test with linear-by-linear association and Mann-Whitney U test were performed. The ROC curves were generated to investigate the diagnostic characteristics of SUVmax for the identification of malignancy. ROC curve evaluation was performed using AUC. For the statistical analyses, SPSS version 23 was used, and the significance level was set at 0.5.
Results
Patients
In total, 51 out of 5029 non-breast cancer patients were confirmed to have incidental breast lesions with 56 lesions (1.0%), 48 females (94.1%), and 3 males (5.9%) and aged 49.18 ± 14.31 (age range 17–71) years. The most common primary cancer types in patients with incidental breast lesion were lymphoma (HD and NHL) (n = 15, [29.4%]), genitourinary cancer (n = 15, [29.4%]), gastrointestinal cancer (esophageal, gastric, and colon cancer) (n = 9, [17.6%]), and lung cancer (n = 5, [9.8%]).
Overall, 46 out of 56 (82.1%) lesions from 41 patients were of a benign origin. Twelve (26.1%) were histopathologically confirmed (fibrocystic change [n = 5], fibroadenoma [n = 4], intraductal papilloma [n = 3]), and the remaining 34 (73.9%) were confirmed as benign by lesion stability over a mean serial imaging period of 24.18 months. Among 10 (17.9%) histologically verified malignant lesions, three subtypes were identified including lymphoma (n = 1, 10.0%), invasive lobular carcinoma (n = 3, 30.0%), and invasive ductal carcinoma (n = 6, 60.0%).
The benign and malignant groups showed no significant difference regarding age (49.25 years [17–77] vs. 48.88 years [30–66]; p = 0.9) and gender (benign lesions: female [n = 39, 95.1%] vs. male [n = 2, 4.8%]; malignant lesion: female [n = 9, 90.0%] vs. male [n = 1, 10.0%]; p = 0.53). The most common primary cancer type was genitourinary cancer (n = 6/10, [60.0%]) in the malignant group, which was significantly more frequent than the benign group (9/41, 22.0%; p = 0.039).
Morphological criteria
In the benign group, 44 incidental breast lesions were classified as BI-RADS I-III (95.7%), and the 2 remaining as BI-RADS IV (diagnosed with fibroadenoma). Eight out of 10 (80.0%) malignant lesions were classified as BI-RADS IV (n = 6 [60.0%]) and BI-RADS V (n = 2 [20.0%]), which prompted tissue diagnosis. Two benign-appearing malignant lesions, categorized as BI-RADS I and III at the baseline ultrasound, showed interval changes at 9- and 6-month follow-ups and were finally verified to be invasive ductal carcinoma (Table 1). The malignant and benign groups were significantly different regarding BI-RADS categories (p = 0.000). Using BI-RADS ≥ 4 as a dichotomous diagnostic test, sensitivity, specificity, and accuracy were calculated as 80.0%, 92.7%, and 90.2%, respectively.
CT corresponding features showed significant difference between study groups. Nodule was demonstrated as the unique associated morphological abnormality of malignant lesions (n = 10, 100.0%) which was not significantly more prevalent compared to the benign group (n = 23, 60.5%) (p = 0.057). Other structural abnormalities in the benign group were ill-defined soft tissue density (n = 12, 31.6%) and skin thickening (n = 3, 7.9%). There was a trend toward a greater mean diameter of malignant nodules; however, due to small sample size, the difference did not approach a statistical significance (14.4 vs. 19.6 mm, p = 0.060). There was no significant correlation between lesion size and metabolic activity in this cohort (p = 0.53).
Metabolic criteria
Severe F-18 FDG uptake was observed in 6 of 10 malignant lesions (60.0%) but only in 2 of 46 benign lesions (4.3%) (p = 0.000). The frequency of sizeable lesions (> 7 mm) with mild to moderate metabolic activity (less than liver activity) were 95.7% in benign lesions (n = 44) and 40.0% in pathologically diagnosed malignant lesions (n = 4). The diagnostic performance of the visually interpreted metabolic criteria was determined as 60.0%, 95.7%, and 89.3% for sensitivity, specificity, and accuracy, respectively.
Malignant lesions showed a significantly higher mean SUVmax in comparison with benign lesions (6.32 ± 3.9) (2.64 ± 1.6; p = 0.003). Figure 1 demonstrates the area under the ROC of SUVmax for detecting malignancy (0.80; CI 0.62–0.97, p = 0.003). The SUVmax threshold of 2 had sensitivity = 80.0%, specificity = 73.9%, and accuracy = 75.0%. The specificity and accuracy reached 95.7% and 89.3%, respectively, by applying the SUVmax cutoff of 3.7, as the specific threshold, but the sensitivity decreased (60.0%).
Based on the histopathological subtypes, 5/6 invasive ductal carcinoma (83.3%) demonstrated severe FDG uptake with a mean SUVmax of 6.67 (± 3.94) (Fig. 2). All 3 invasive lobular carcinomas had mild to moderate F-18 FDG uptake (SUVmax = 1.86 ± 1.1) (Fig. 3). Breast lymphoma demonstrated intense F-18 FDG uptake with mean SUVmax = 8.8. Histopathologically proven benign lesions including fibrocystic change (n = 5), intraductal papilloma (n = 3) in addition to non-otherwise specified benign lesions showed mild metabolic activity with a mean SUVmax of 1.12 (± 0.98), 1.23 (± 1.01), and 1.33 (± 1.13), respectively. Among the 4 biopsy-proven fibroadenomas, 2 had intense F-18 FDG uptake with a mean SUVmax = 4.47(± 2.78) (Fig. 4). Table 1 outlines the metabolic and morphological characteristics of PET-detected incidental breast lesion, regarding the histopathological subtype, based on descriptive classification.
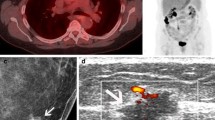
Invasive ductal carcinoma incidentally detected in a 66-year-old woman with endometrial cancer being worked up for subsequent treatment strategy. MIP (a), PET, NECT, and fused PET/CT in axial view (b–d) demonstrated a small focus of intense F-18 FDG uptake (arrow) corresponding with a small-sized irregular isodense nodule in the left breast (arrow). Corresponding mammographic (e) and sonographic (f) revealed no abnormal finding. The lesion was PET positive BI-RADS negative which needed a histologic examination
Invasive lobular carcinoma incidentally detected in a 57-year-old woman with colon cancer being worked up for subsequent treatment strategy. MIP (a), PET, NECT, and fused PET/CT in axial view (b–d) demonstrated a partially calcified irregular mass in the right breast (arrow) with no discernible metabolic activity. Corresponding mammographic (e) and sonographic (f) findings demonstrated lesion containing microcalcifications (arrow). The lesion was BI-RADS 4 (positive) and PET negative which needed a histologic examination
Fibroadenoma incidentally detected in a 68-year-old woman with ovarian cancer being worked up for subsequent treatment strategy. MIP (a), PET, NECT, and fused PET/CT in axial view (b–d) demonstrated focal intense F-18 FDG uptake (arrow) corresponding with a calcified irregular isodense soft tissue density in the right breast (arrow). Corresponding sonographic image (e) demonstrated an echogenic oval-shaped nodule with circumscribed margin (arrow), compatible with BI-RADS 2. The lesion was PET positive BI-RADS negative
Combined criteria
Regarding combined criteria, the dominant features for benign lesions was double negative (32/46, 69.6%), followed by PET-only positive feature (10/46, 21.7%). Two (4.3%) benign lesions presented as BI-RADS-only positive and 2 (4.3%) as double positive. Moreover, all malignant lesions featured at least one positive criterion; 2 (20.0%) lesions presented as PET-only positive, 2 (20.0%) as BI-RADS-only positive and 6 (60.0%) as double positive.
Combined criteria by SUVmax threshold at 3.7 classified benign lesions as follows: 42 (91.3%) double negative, 1 (2.2%) PET-only positive, 2 (4.3%) BI-RADS-only positive, and 1 (2.2%) double positive. In the malignant group, 5 lesions (50.0%) were double positive. Three lesions (30.0%) were detected only by the BI-RADS component and 1 (10.0%) only by the PET component. The higher threshold SUVmax resulted in 1 false negative finding in the malignant group (double negativity).
Table 2 summarizes the descriptive classification of combined criteria in malignant and benign lesions.
The diagnostic performance was calculated as sensitivity = 100.0%, specificity = 69.6%, and accuracy = 75.0% for combined criteria with SUVmax threshold at 2 and sensitivity = 90.0%, specificity = 91.3%, and accuracy of 91.1% for combined criteria with SUVmax threshold at 3.7.
Comparison of BI-RADS, PET, and two combined criteria in terms of diagnostic performance is shown in Table 3.
Using sensitive SUVmax, specific SUVmax, and BI-RADS, a systematic approach was proposed to maximize the diagnostic yield of morphological and metabolic criteria (Fig. 5).
Discussion
The present study provided new insights into the complementary role of PET-derived metabolic information to improve the diagnostic yield of BI-RADS for incidental breast lesions. Based on the current results, the highest sensitivity would be achieved by a combination of morphological and metabolic criteria with a consistent specificity and accuracy. BI-RADS recommendations would be best modified by applying a two-threshold SUVmax diagnostic approach. The algorithm with simple classification methodology to stratify incidental breast lesions helps determine the next steps. In addition to BI-RADS 4 and 5 US lesions, metabolically active lesions with SUVmax ≥ 3.7 need a histologic evaluation. For BI-RADS 2 and 3, US lesions with intermediate (i.e., SUVmax between 2 and 3.7) and low (i.e., SUVmax < 2) metabolic activity, short-term, and routine follow-ups would be sufficient, respectively.
There are conflicting results for the performance characteristics of BI-RADS in PET-detected incidental breast lesions. A wide range of sensitivity (75.0%, 96.8%, and 100.0%) and specificity (51.3%, 61.8%, and 89.2%) has been reported for BI-RADS to stratify incidental breast lesions [14, 17, 18]. The current study revealed a sensitivity = 80.0% and specificity = 92.7% for BI-RADS in this clinical setting. The results may be applied to decide before proceeding to the histological evaluation; however, other imaging modalities including breast MRI, mammography, and dedicated PET mammography may provide further insight into complicated cases [19].
The morphological heterogeneity of the lesions comprised the study cohorts, different predictive values of sonographic features, including margin (circumscribed vs. speculated), orientation (parallel vs. nonparallel), and shape (oval vs. irregular) [15], and a significant overlap of benign and malignant lesions in BI-RADS 3 and 4 (approximately benign to malignant ratio of 1 [16]) may contribute to the diversity of the results. Moreover, they included a relatively small sample size within each BI-RADS category, which may impose confounding effects on the performance characteristics of BI-RADS in incidental breast lesions and somewhat explain the various diagnostic performances observed for BI-RADS in different studies.
Evidence of the potential value of SUVmax, as the most validated and conveniently measured semiquantitative metabolic parameter, in differentiating benign and malignant incidental breast lesions is scarce and discouraging. Based on previous studies, malignant incidental breast lesions demonstrated a borderline, significantly higher level of SUVmax than did benign lesions (4.2 vs. 2.3, p = 0.001 [14]; 2.02 vs. 3.71, p = 0.0001 [18]; 3.9 vs. 1.9, p = 0.005 [20]; 2.4 vs. 1.5 [21]; 3.13 vs. 1.85, p = 0.054 [1]). Two studies have proposed the optimal cutoff for SUV max to discriminate malignant from the benign incidental breast lesion with a sensitivity of 66.7% for SUVmax = 2 [14] and sensitivity of 61.3% and specificity of 76.3% for SUVmax = 2.3 [18] in incidental breast lesions.
The present study revealed a better sensitivity for SUVmax = 2 to detect malignant incidental breast lesions (80.0%). According to previous research, the intensity of F-18 FDG uptake in breast lesions may be influenced by several factors including histological subtype [22,23,24], diameter, tumor grading [23, 25, 26], tumor proliferation index (Ki67) [22, 23, 27, 28], steroid hormone receptor expression [24, 28,29,30], and p53 status [23, 31, 32]. The potential correlation between SUVmax and tumor biological characteristics may explain the relatively small differences in SUVmax diagnostic performance in the incidental breast lesions observed in these reports.
The present study revealed an excellent sensitivity (90.0%) for combined criteria with SUVmax threshold at 3.7 which outperformed each morphologic and metabolic criterion alone. Indeed, a high level of glucose uptake, as the dominant metabolic feature of invasive ductal carcinoma [11, 12], allowed the PET component to detect the accelerated metabolic activity before the morphological changes developed, and hence contributed to the improvement of BI-RADS sensitivity in incidental breast lesion. Moreover, invasive lobular carcinoma, as a low cell density tumor with an infiltrative growth pattern, would be best identified by BI-RADS component, which significantly compensates for PET sensitivity loss in such low FDG-avid malignant lesions [11, 12]. However, there is still a major concern in the diagnosis of invasive lobular carcinoma by applying combined criteria with SUVmax threshold at 3.7. Most studies demonstrated that US-BI-RADS has a relatively lower sensitivity for invasive lobular carcinoma in comparison with invasive ductal carcinoma [33] and imposes a significant risk of missed diagnosis. This study revealed that SUVmax cutoff at 2 correctly identified one invasive lobular carcinoma with no suspicious feature based on US examination and thus improved test sensitivity up to 100.0%.
Despite a marked improvement in sensitivity, the present study revealed that metabolic parameters did not have a significantly negative influence on the test specificity and overall accuracy. Based on previous studies, the most causes of false-positive results in the breast lesions are infectious processes, lactation, radiation, and surgery which were all considered exclusion criteria in the present study and most other similar reports [34]. Furthermore, fibroadenoma as the most common benign breast mass with high F-18 FDG uptake usually presents as a low FDG-avid lesion and tends to occur at a lower age (below 40 years [35]) compared with other PET-detected incidental breast lesion (above 40 years [14, 17, 36]). Thus, the most common potential source of false-positive lesions contributes little in the ultimate performance characteristics of combined criteria.
The present study has some major limitations. A lack of histopathological confirmation for most benign lesions provides limited evidence for detailed metabolic features of any individual entities; however, regarding the aggressive behavior of most breast cancers, a 2-year period of clinical formal follow-up reliably serves as definitive diagnostic criteria to exclude malignancy. Therefore, the final results on the overall diagnostic performance have not been negatively influenced by this limitation. The small sample size, particularly in the malignant breast lesion group, precludes addressing the potential impact of metabolic information on individual categories. This issue is particularly important in BI-RADS 3 and 4, which are the most challenging classes. Moreover, breast cancer may coincide with certain genitourinary cancers including ovarian cancer, for which subgroup analyses may indicate particular associations [37]. Breast sonography was performed by different breast imaging specialists. Based on previous studies, there is an excellent interobserver agreement for BI-RADS lexicon providing harmonized and accurate consensus for different observers [4], which may rule out any potential biasness. Certain interfering variables were not collected and studied including breast density, size, location, and morphology of the tumor, and subcategories of pathology. Furthermore, in a patient collective probably high risk for breast cancer (e.g., BRCA mutation carriers), ultrasound BI-RADS categories might differ because the baseline risk for breast cancer is probably high. These possible confounders should be further studied in the future. In addition, further studies are needed, with preferably larger sample sizes, to confirm and validate the results in a new set of cases. Finally, considering the strength of MRI for assessment of breast lesions, we suggest integrating MRI with metabolic activity in future studies.
In conclusion, the diagnostic performance of BI-RADS could be maximized by applying a 2-threshold SUVmax diagnostic approach. Lesions with either BI-RADS ≥ 4 or SUVmax ≥ 3.7 should prompt a histopathological exam. BI-RADS-negative lesions with an indeterminate SUVmax (2 ≤ SUVmax < 3.7) do not sufficiently meet an indication to justify a recommendation for biopsy to exclude malignancy, and probably most people benefit from a follow-up imaging at short intervals. BI-RADS-negative lesions with SUVmax < 2 can be confidently considered for a routine follow-up care.
Abbreviations
- BI-RADS:
-
Breast imaging reporting and data system
- F-18 FDG PET/CT:
-
F-18 fluorodeoxyglucose positron emission tomography/computed tomography
- HD:
-
High definition
- PET:
-
Positron emission tomography
- ROC:
-
Receiver operating characteristic
- SUV:
-
Standardized uptake volume
References
Litmanovich D, Gourevich K, Israel O, Gallimidi Z (2009) Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: incidence and clinical significance. Eur J Nucl Med Mol Imaging 36:1558–1564
D’orsi C, Bassett L, Berg W, Feig S, Jackson V, Kopans DJ (2003) Breast imaging reporting and data system: ACR BI-RADSmammography. 4th edition. American College of Radiology
Mendelson EB, Berg WA, Merritt CR (2001) Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound seminars in roentgenology. Semin Roentgenol 36:217–225
Lee HJ, Kim EK, Kim MJ et al (2008) Observer variability of breast imaging reporting and data system (BI-RADS) for breast ultrasound. Eur J Radiol 65:293–298
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175
Hille H, Vetter M, Hackelöer BJ (2012) The accuracy of BI-RADS classification of breast ultrasound as a first-line imaging method. Ultraschall Med 33:160–163
Kim EK, Ko KH, Oh KK et al (2008) Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 190:1209–1215
Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RLJR (2008) BI-RADS 3, 4, and 5 lesions: value of US in management—follow-up and outcome. Radiology 248:773–781
Naidich DP, Bankier AA, MacMahon H et al (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481
Lim HS, Yoon W, Chung TW et al (2007) FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics 27:S197–S213
Rosen EL, Eubank WB, Mankoff DA (2007) FDG PET, PET/CT, and breast cancer imaging. Radiographics 27:S215–S229
Samson DJ, Flamm CR, Pisano ED, Aronson NJ (2002) Should FDG PET be used to decide whether a patient with an abnormal mammogram or breast finding at physical examination should undergo biopsy? Acad Radiol 9:773–783
Kang BJ, Lee JH, Yoo IeR et al (2011) Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am J Roentgenol 197:341–347
Hong AS, Rosen EL, Soo MS, Baker JA (2005) BI-RADS for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 184:1260–1265
Costantini M, Belli P, Ierardi C, Franceschini G, La Torre G, Bonomo L (2007) Solid breast mass characterisation: use of the sonographic BI-RADS classification. Radiol Med 112:877–894
Shin KM, Kim HJ, Jung SJ et al (2015) Incidental breast lesions identified by 18F-FDG PET/CT: which clinical variables differentiate between benign and malignant breast lesions? J Breast Cancer 18:73–79
Chae EY, Cha JH, Kim HH et al (2012) Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: clinical significance and differential diagnosis. Acta Radiol 53:530–535
Naseri M, Farzanehfar S, Ranjbar S, Parvizi M, Abbasi MJABC (2017) An overview on positron emission mammography in breast cancer detection and follow up: particular concerns in Iran as a developing country. Archives of Breast Cancer 4:39–41
Beatty JS, Williams HT, Gucwa AL et al (2009) The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am J Surg 198:495–499
Benveniste AP, Yang W, Benveniste MF, Mawlawi OR, Marom EM (2014) Benign breast lesions detected by positron emission tomography-computed tomography. Eur J Radiol 83:919–929
Bos R, van Der Hoeven JJ, van Der Wall E et al (2002) Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20:379–387
Gil-Rendo A, Martínez-Regueira F, Zornoza G et al (2009) Association between [18F] fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 96:166–170
Avril N, Rosé CA, Schelling M et al (2000) Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 18:3495–3502
Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A (2006) Clinicopathologic factors associated with false negative FDG–PET in primary breast cancer. Breast Cancer Res Treat 98:267–274
Ueda S, Tsuda H, Asakawa H et al (2008) Utility of 18 F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18 F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer 8:165
Buck A, Schirrmeister H, Kühn T et al (2002) FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 29:1317–1323
Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa MJ (2007) The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 14:260–268
Buck AK, Schirrmeister H, Mattfeldt T, Reske SN (2004) Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging 31:S80–S87
Avril N, Menzel M, Dose J et al (2001) Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med 42:9–16
Crippa F, Seregni E, Agresti R et al (1998) Association between [18 F] fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med 25:1429–1434
Ikenaga N, Otomo N, Toyofuku A et al (2007) Standardized uptake values for breast carcinomas assessed by fluorodeoxyglucose-positron emission tomography correlate with prognostic factors. Am Surg 73:1151–1157
Kim SH, Cha ES, Park CS et al (2011) Imaging features of invasive lobular carcinoma: comparison with invasive ductal carcinoma. Jpn J Radiol 29:475
Adejolu M, Huo L, Rohren E, Santiago L, Yang WT (2012) False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol 198:W304–W314
Lee M, Soltanian HT (2015) Breast fibroadenomas in adolescents: current perspectives. Adolesc Health Med Ther 6:159
Korn RL, Yost AM, May CC et al (2006) Unexpected focal hypermetabolic activity in the breast: significance in patients undergoing 18F-FDG PET/CT. AJR Am J Roentgenol 187:81–85
Yoneda A, Lendorf ME, Couchman JR, Multhaupt HA (2012) Breast and ovarian cancers: a survey and possible roles for the cell surface heparan sulfate proteoglycans. J Histochem Cytochem 60:9–21
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mehrdad Bakhshayeshkaram.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because the Review Board of Shahid Beheshti University of Medical Sciences waived the need for an informed consent.
Ethical approval
Institutional Review Board of Shahid Beheshti University of Medical Sciences approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakhshayeshkaram, M., Salehi, Y., Abbasi, M. et al. A preliminary study to propose a diagnostic algorithm for PET/CT-detected incidental breast lesions: application of BI-RADS lexicon for US in combination with SUVmax. Eur Radiol 29, 5507–5516 (2019). https://doi.org/10.1007/s00330-019-06106-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06106-x