Abstract
Objectives
Although diffusion-weighted imaging (DWI) is reported to be accurate in detecting bowel inflammation in Crohn’s disease (CD), its ability to assess bowel fibrosis remains unclear. This study assessed the role of DWI in the characterization of bowel fibrosis using surgical histopathology as the reference standard.
Methods
Abdominal DWI was performed before elective surgery in 30 consecutive patients with CD. The apparent diffusion coefficients (ADCs) in pathologic bowel walls were calculated. Region-by-region correlations between DWI and the surgical specimens were performed to determine the histologic degrees of bowel fibrosis and inflammation.
Results
ADCs correlated negatively with bowel inflammation (r = − 0.499, p < 0.001) and fibrosis (r = − 0.464, p < 0.001) in 90 specimens; the ADCs in regions of nonfibrosis and mild fibrosis were significantly higher than those in regions of moderate–severe fibrosis (p = 0.008). However, there was a significant correlation between the ADCs and bowel fibrosis (r = − 0.641, p = 0.001) in mildly inflamed segments but not in moderately (r = − 0.274, p = 0.255) or severely (r = − 0.225, p = 0.120) inflamed segments. In the mildly inflamed segments, the ADCs had good accuracy with an area under the receiver-operating characteristic curve of 0.867 (p = 0.004) for distinguishing nonfibrosis and mild fibrosis from moderate–severe fibrosis.
Conclusions
ADC can be used to assess bowel inflammation in patients with CD. However, it only enables the accurate detection of the degree of bowel fibrosis in mildly inflamed bowel walls. Therefore, caution is advised when using ADC to predict the degree of intestinal fibrosis.
Key Points
• Diffusion-weighted imaging was used to assess bowel inflammation in patients with Crohn’s disease.
• The ability of diffusion-weighted imaging to evaluate bowel fibrosis decreased with increasing bowel inflammation.
• Diffusion-weighted imaging enabled accurate detection of the degree of fibrosis only in mildly inflamed bowel walls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease that can affect the entire digestive tract. Early in the disease course, bowel inflammation is dominant, whereas fibrosis in the affected bowel region tends to increase with time [1]. In the long term, approximately 40% of patients with CD develop a stricture [2]. A stricture is a complication of severe CD that significantly decreases the patient’s quality of life [3] and may require surgery. Differentiation between fibrotic and inflammatory strictures has important implications because inflammatory lesions can be relieved by anti-inflammatory treatment whereas fibrotic lesions are not and usually require endoscopic or surgical treatment [4]. Hence, detection and accurate quantification of bowel fibrosis is crucial.
MRI is used increasingly in the management of CD [5]. Several studies have agreed on the role of diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) in the diagnosis of active bowel inflammation [6,7,8,9,10,11,12,13,14,15]. A meta-analysis [16] found that DWI had high accuracy in detecting active bowel inflammation in CD with a summary sensitivity of 92.9% and a summary specificity of 91%. However, the relationship between bowel fibrosis and DWI remains undetermined, although a few small studies have reported a possible correlation between bowel fibrosis and ADC [17,18,19]. Another knowledge gap is whether bowel inflammation itself could have a confounding impact on the ADC when detecting fibrosis, given that bowel inflammation and fibrosis always coexist. The purpose of this study was to assess the role of DWI in the characterization of bowel fibrosis in patients with CD using surgical histopathology as the reference standard.
Materials and methods
This prospective study was approved by our institutional ethics review board and written informed consent was obtained from all participating patients. From July 2014 through December 2017, consecutive patients with a diagnosis of CD who were scheduled for elective surgery at our institution were considered for enrolment. The inclusion criteria were as follows: age 18 years or older; a diagnosis of CD based on standard clinical, endoscopic, imaging, and histologic criteria; preoperative MRI within a fortnight of elective surgery for a symptomatic bowel stricture that was refractory to medical treatment; and pathologic bowel segments identified on MRI at the same location as the abnormal histologic findings. The exclusion criteria were inadequate DWI quality or collapsed bowel segments on MRI (Fig. 1).
MRI protocol
Bowel preparation was performed as described previously [15]. One hour before the procedure, 1600–2000 mL of 2.5% mannitol solution was administered to achieve adequate distension of the bowel segments. Ten milligrams of raceanisodamine hydrochloride (Minsheng Pharmaceutical Group Co., Ltd.) was injected intramuscularly into the buttocks 10 min before magnetic resonance enterography.
MRI was performed using a 3.0-T MRI machine (Magnetom Trio, Siemens Healthineers) with multichannel phased array body coils. The scan protocol was the same as that described in a previous study [15]. Conventional MRI, including axial and coronal breath-held half-Fourier acquisition single-shot, turbo spin-echo, T2-weighted imaging (T2WI), was performed using a repetition time/echo time (TR/TE) of 1200 ms/87 ms, a 320 × 194 matrix, a slice thickness of 4 mm, and a flip angle of 160°. Fat-suppressed fast low angle shot T1-weighted imaging was performed using a TR/TE of 210 ms/2.18 ms, a 320 × 200 matrix, a slice thickness of 4 mm, and a flip angle of 70°. Axial and coronal free-breathing DWI was performed using a water-excited, single-shot, spin-echo, echo-planar sequence with a TR/TE of 5000–5900 ms/73–83 ms, a 192 × 115 or 192 × 154 matrix, and a slice thickness of 4–5 mm with three b values of 50, 400, and 800 s/mm2. The ADC map was generated automatically on the scanner console using a mono-exponential model. After intravenous injection of gadopentetate dimeglumine (Beilu Pharmaceuticals) 0.2 mL/kg at a rate of 2 mL/s, multiple coronal dynamic contrast-enhanced, fat-suppressed, three-dimensional volumetric interpolated breath-hold examinations were performed using a TR/TE of 4.37 ms/1.37 ms, a 320 × 217 matrix, a slice thickness of 2 mm, and a flip angle of 1° at 15, 40, 65, and 90 s from the beginning of injection.
MRI analysis
Diffusion-weighted imaging
The target segments on DWI were premarked using conventional MRI by a radiologist (CS) with 15 years’ experience in bowel MRI who was not blinded to the clinical, imaging, or surgical information. Another radiologist (XL) with 8 years’ experience in bowel MRI and blinded to the clinical and pathologic information then assessed the DWI and ADC maps of the designated segments on two occasions in a 3-month interval. The mean ADC was calculated by placing three regions of interest to include the full thickness with a maximum length of one third the circumference of the bowel wall in cross section on the ADC map [20]. The first measurements were used for the final analysis. The mean area of the regions of interest in the affected bowel walls was 107.49 ± 72.36 mm2.
Conventional MRI
The radiologist (XL) assessed the conventional MRI findings (T2WI signal intensity and enhancement pattern) of the affected bowel that could correlate with bowel inflammation. Hypointensity on T2WI was defined as being similar to that of the adjacent normal bowel wall and hyperintensity on T2WI as being greater than that of the adjacent normal bowel wall [21, 22]. The enhancement pattern included stratified enhancement (i.e., mucosal hyperenhancement with relatively low submucosal enhancement) and transmural enhancement (i.e., homogeneous enhancement in the entire bowel wall) [21, 22].
Region-by-region matching of DWI evaluation and histologic assessment
A matched evaluation of DWI and the histologic assessment was performed as described in a previous study [20] by a nonblinded radiologist (CS). The anatomic location of the resected segments was documented with respect to defined anatomic landmarks (e.g., the ileocecal valve or appendix) or gross lesions (e.g., the most stenosed area or bowel adhesion). Two to four bowel specimens were obtained from the strictured segments in each patient according to the extent of the disease. Bowel walls with normal surgical findings at the ends of the resection margin were used as controls.
Histopathologic evaluation
After fixation of the tissue in formalin, a full-thickness sample of the resected bowel segment was embedded in paraffin and sliced into several sections, each 4 μm in thickness. One section was stained with hematoxylin–eosin for the histologic inflammation score and another section with Masson’s trichrome for the histologic fibrosis score. A pathologist (QC) with 10 years’ experience in digestive tract pathology, who was blinded to all clinical, surgical, and MRI information, graded the histologic sections from areas with the most severe inflammation or fibrosis (Table 1) using a semiquantitative scoring system [20, 23].
Statistical analysis
The statistical analysis was performed by two-sided comparisons with significance defined as a p value < 0.05 using SPSS version 20.0 software (SPSS Inc.). The quantitative data are expressed as the mean ± standard deviation and the qualitative data as the absolute values or percentage. A bivariate correlation analysis was performed using Spearman’s rank correlation. Multiple linear regression was performed using the stepwise method to show the linear association between the dependent variable (ADC) and the independent variables (inflammation and fibrosis score). The standardized partial regression coefficient (b′) was used to describe the strength of the influence of the independent variable on the dependent variable. Differences in the ADC between the different histologic grading methods (inflammation and fibrosis) were tested using repeated-measures analysis of variance because of the inclusion of multiple segments per patient. The Bonferroni test was used for further pairwise comparisons. The differences in the conventional MRI findings associated with different inflammation scores were tested using the chi-square test. A receiver-operating characteristic curve analysis was performed, and the area under the curve was calculated to determine the diagnostic accuracy of the ADC for differentiating different grades of bowel fibrosis. The intraobserver agreement for the ADC was tested using the intraclass correlation coefficient.
Results
Demographic and clinical data
Of the 103 patients treated at our institution, 30 (17 men, 13 women; mean age 32.53 ± 8.58 years; 90 bowel segments) met the eligibility criteria for inclusion in the study. The average thickness of the resected bowel segments on T2WI was 7.92 ± 4.09 mm. The patient demographic and clinical characteristics are summarized in Table 2.
Histologic assessment
The fibrosis was graded as none (n = 12), mild (n = 8), moderate (n = 40), or severe (n = 30), and inflammation was graded as none (n = 0), mild (n = 22), moderate (n = 49), or severe (n = 19). There was a fair correlation between histologic fibrosis and inflammation scores (r = 0.458, p < 0.001), which highlighted the need for multiple linear regression analysis.
ADC for assessment of bowel inflammation
There was a fair correlation between the ADCs and the histologic inflammation scores (r = − 0.499, p < 0.001). Significant differences in the ADCs were found between mild ([1.38 ± 0.29] × 10−3 mm2/s), moderate ([1.19 ± 0.25] × 10−3 mm2/s), and severe ([0.99 ± 0.18] × 10−3 mm2/s) disease activity (F = 7.984, p = 0.002; Supplementary Table 1). The ADCs decreased with increasing CD activity.
ADC for assessment of bowel fibrosis
There was a fair correlation between the ADCs and the histologic fibrosis scores (r = − 0.464, p < 0.001; Fig. 2a). Given the existence of correlations between bowel inflammation and both the ADC and bowel fibrosis, multiple linear regression was performed to test further whether there was a definite correlation between ADC and bowel fibrosis, and if so, the extent of the influence of bowel fibrosis on the ADC when compared with that of bowel inflammation. The regression model (ADC = [1.633 − 0.339 × fibrosis score − 0.308 × inflammation score] × 10−3 mm2/s; F = 20.033, p < 0.001) indicated that the ADC indeed correlated negatively with bowel fibrosis. Bowel fibrosis was shown to have an effect on the ADC (b′ = − 0.339) that was similar to the effect of bowel inflammation (b′ = − 0.308).
A scatter plot showing statistically significant negative correlations between the ADC values and histologic fibrosis scores in (a) all 90 bowel wall segments (r = − 0.464, p < 0.001) and (b) mildly inflamed bowel wall segments (r = − 0.641, p = 0.001) but not in (c) moderately inflamed bowel wall segments (r = − 0.274, p = 0.255) or severely inflamed bowel wall segments (d; r = − 0.225, p = 0.120). ADC, apparent diffusion coefficient
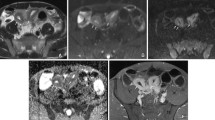
Among the 90 specimens, the ADCs for nonfibrotic bowel walls ([1.53 ± 0.27] × 10−3 mm2/s) were significantly higher than those for mildly ([1.19 ± 0.30] × 10−3 mm2/s; Fig. 3a–f), moderately ([1.20 ± 0.24] × 10−3 mm2/s), and severely ([1.06 ± 0.22] × 10−3 mm2/s; Fig. 3g–l) fibrotic bowel walls (F = 16.070, p < 0.001; Supplementary Table 2). There was a significant difference in the ADCs between nonfibrotic and mildly fibrotic bowel walls ([1.39 ± 0.32] × 10−3 mm2/s) and moderately to severely fibrotic bowel walls ([1.14 ± 0.24] × 10−3 mm2/s; F = 8.158, p = 0.008; Fig. 4a; Supplementary Table 3).
Images from a 41-year-old man with mildly fibrotic and severely inflammatory Crohn’s disease in the proximal ileum (a–f) and a 33-year-old man with severely fibrotic and severely inflammatory Crohn’s disease in the terminal ileum (g–l) show similarly low ADCs in the affected bowel wall segments. In the first patient, (a) coronal T2-weighted imaging and (b) postcontrast-enhanced T1-weighted imaging reveal marked bowel wall thickening with luminal narrowing in the proximal ileum (arrows). Hyperintensity on coronal diffusion-weighted imaging with b = 800 s/mm2 (c) and hypointensity on the corresponding ADC map (d) (ADC = 0.94 × 10−3 mm2/s) are shown in the same segment (arrows). Hematoxylin–eosin (e) and Masson’s trichrome (f) staining depict marked transmural inflammation (score = 3) and mild fibrosis (blue area; score = 1), respectively. In the second patient, (g) coronal T2-weighted imaging and (h) postcontrast-enhanced T1-weighted imaging also show bowel wall thickening and luminal narrowing in the terminal ileum (arrows). Hyperintensity on the coronal diffusion-weighted images with b = 800 s/mm2 (i) and hypointensity on the corresponding ADC map (j) (ADC = 1.08 × 10−3 mm2/s) are shown in the same segment (arrows). Hematoxylin–eosin (k) and Masson’s trichrome (l) staining depict marked transmural inflammation (score = 3) and marked transmural fibrosis (blue area; score = 3), respectively. (× 2 magnification). ADC, apparent diffusion coefficient
Apparent diffusion coefficients in nonfibrosis and mild fibrosis are significantly higher than those in moderate–severe fibrosis in (a) all 90 bowel wall segments (p = 0.008) and in (b) mildly inflamed bowel wall segments (p < 0.001) but not in (c) moderately–severely inflamed bowel wall segments (p = 0.613)
Ability of the ADC to assess bowel fibrosis according to the degree of bowel inflammation
Pairwise correlations between the ADCs and histologic fibrosis scores within the groups with varying degrees of bowel inflammation were analyzed to determine whether the severity of bowel inflammation affected the correlation between the ADC and bowel fibrosis. A significant correlation between the ADCs and histologic fibrosis scores (r = − 0.641, p = 0.001; Fig. 2b) existed only in the bowel walls with mild inflammation but not in those with moderate (r = − 0.274, p = 0.255; Fig. 2c) or severe (r = − 0.225, p = 0.120; Fig. 2d) inflammation. The strength of the correlation between the ADCs and histologic fibrosis scores decreased with increasing degree of bowel inflammation in the same bowel walls.
In bowel walls with mild inflammation, the ADCs in nonfibrotic and mildly fibrotic bowel walls (n = 12; [1.53 ± 0.26] × 10−3 mm2/s) were significantly higher than those in bowel walls with moderate–severe fibrosis (n = 10; [1.19 ± 0.20] × 10−3 mm2/s; F = 10.443, p < 0.001; Fig. 4b; Supplementary Table 4); moreover, the ADCs had good accuracy (area under the curve 0.867; 95% confidence interval, 0.714–1.000; p = 0.004) for distinguishing nonfibrotic and mildly fibrotic bowel walls from bowel walls with moderate–severe fibrosis (Fig. 5). However, in bowel walls with moderate–severe inflammation, no significant difference in the ADCs was found between nonfibrotic and mildly fibrotic bowel walls (n = 8; [1.18 ± 0.29] × 10−3 mm2/s) and moderate–severe bowel fibrosis (n = 60; [1.13 ± 0.25] × 10−3 mm2/s; F = 0.549, p = 0.613; Fig. 4c; Supplementary Table 5). The efficacy of the ADC for differentiating different grades of fibrosis in the bowel wall weakened with increasing degree of bowel inflammation.
Validating the ability of ADC for assessing bowel fibrosis in different degrees of inflammation defined by conventional MRI
We randomly selected a resected bowel wall from each patient to evaluate the relationship of histologic inflammation with T2WI signal intensity and enhancement pattern. Only the T2WI signal intensity can differentiate mildly from moderately to severely inflamed segments (Z = − 3.820, p < 0.001). The mural T2WI hypointensity always indicated mild inflammation (8/11, 72.73%), while T2WI hyperintensity was linked to the presence of moderate–severe inflammation (18/19, 94.74%) (Table 3). In mildly inflamed segments defined by T2WI hypointensity, ADCs strongly correlated with histologic fibrosis scores (r = − 0.690, p = 0.019), while no significant correlation between ADCs and fibrosis scores was found in moderately to severely inflamed segments defined by T2WI hyperintensity (r = − 0.449, p = 0.054).
Intraobserver agreement
There was good intraobserver agreement for the ADC measurement (intraclass correlation coefficient 0.831; 95% confidence interval, 0.754–0.885; p < 0.001).
Discussion
Our study showed that the ADC correlated negatively with bowel fibrosis in patients with CD. ADC enabled differentiation of nonfibrosis and mild fibrosis from moderate–severe fibrosis in pathologic bowel walls. However, the strength of the correlation between the ADC and bowel fibrosis and the ability of the ADC to distinguish nonfibrosis and mild fibrosis from moderate–severe fibrosis decreased with increasing degree of bowel inflammation within the same bowel segment. The ADC had good accuracy for detecting bowel fibrosis in bowel walls with mild inflammation.
DWI can be performed rapidly and has been widely used in the routine management of patients with CD [9, 13, 15, 24]. As gastroenterologists are focusing more and more on discrimination of the different types of strictured CD, imaging is expected to provide information on the pathological processes, including inflammation and, more importantly, fibrosis. Consistent with previous studies [13, 15, 24], our study confirmed that ADC is able to assess the degree of inflammatory activity in CD.
To date, only a few studies with small samples [17,18,19] have investigated the potential role of ADC in the characterization of fibrotic lesions in patients with CD. Kovanlikaya et al [17] and Rosenbaum et al [19] reported that the ADC was significantly lower in the fibrostenotic phenotype than in the inflammatory or normal phenotype, in pediatric patients with CD. Tielbeek et al [18] found a significant difference in the ADC for nonfibrotic and fibrotic bowel wall in adult patients with CD. However, the reference standard used in the study by Rosenbaum et al [19] was MRI rather than histopathology, and a comparison of ADC according to the degree of histologic fibrosis was lacking in the other two studies [17, 18]. Conversely, our study had the benefit of pathology as the gold standard and showed a negative correlation between histologic grade of fibrosis and ADC. Increasing deposition of collagen in the fibrotic bowel wall leads to a reduction in the extracellular space and then restricts the diffusion of water molecules, reducing ADC [17, 18]. In our study, ADC could also discriminate nonfibrosis and mild fibrosis from moderate–severe fibrosis in the affected bowel wall. This is an important added value of ADC for the assessment of the pathologic processes occurring in the bowel wall in patients with CD. The ability of ADC to detect and grade fibrosis may help in the differentiation of fibrotic from inflammatory strictures, which would be conducive to selection of optimal treatment strategies.
As bowel inflammation and fibrosis coexist to varying degrees in patients with CD, the question is whether the evaluation of fibrosis with ADC remains valid whatever the grade of inflammation. Interestingly, we found that the strength of the correlation of ADC with fibrosis and the ability of ADC to distinguish different grades of fibrosis decreased with increasing degree of inflammation. ADC had acceptable efficacy in terms of quantitatively detecting deposition of collagen fibers in mildly inflamed bowel walls but had no diagnostic accuracy in case of moderate to severe inflammation. The value of the ADC for diagnosis of bowel fibrosis needs to be interpreted according to the degree of inflammation. Combination with conventional MRI sequences can be useful: if the bowel wall contains mild inflammation according to T2WI hypointensity, ADC can be used to quantitatively assess the degree of fibrosis; if the bowel wall has severe inflammation according to T2WI hyperintensity, the use of ADC to evaluate bowel fibrosis is irrelevant.
Our study has some limitations. First, the surgical specimens used as the reference standard for more severe disease resulted in a smaller subset of bowel segments with absent or mild fibrosis. We selected some specimens with normal surgical findings. However, no histopathologically normal bowel wall segments were found in our study because inflammation and fibrosis always coexisted in specimens resected from surgical patients. Second, an accurate point-by-point comparison of the intestine on DWI with the resected specimen was difficult but was partially addressed by hypotonic bowel preparation, decreased peristalsis of the affected bowel, and a short interval between DWI and surgery. We used region-by-region correlation of DWI results with surgical specimens by identifying the gross lesion or anatomic structure in the same slice.
Conclusion
In this study, we found that ADC could be used to assess bowel inflammation and fibrosis in patients with CD. However, the ability of ADC to evaluate bowel fibrosis decreased with increasing degrees of bowel inflammation. In patients with CD, ADC enables accurate detection of the degree of fibrosis only in the bowel wall with mild inflammation and not in case of moderate to severe inflammation.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CD:
-
Crohn’s disease
- DWI:
-
Diffusion-weighted imaging
- MRI:
-
Magnetic resonance imaging
- TE:
-
Echo time
- TR:
-
Repetition time
- T2WI:
-
T2-weighted imaging
References
Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ (2013) Crohn’s disease complicated by strictures: a systematic review. Gut 62:1072–1084
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140:1785–1794
Shukla R, Thakur E, Bradford A, Hou JK (2018) Caregiver burden in adults with inflammatory bowel disease. Clin Gastroenterol Hepatol 16:7–15
Latella G, Di Gregorio J, Flati V, Rieder F, Lawrance IC (2015) Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol 50:53–65
Miles A, Bhatnagar G, Halligan S et al (2018) Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: patient acceptability and perceived burden. Eur Radiol. https://doi.org/10.1007/s00330-018-5661-2
Hectors SJ, Gordic S, Semaan S et al (2018) Diffusion and perfusion MRI quantification in ileal Crohn’s disease. Eur Radiol. https://doi.org/10.1007/s00330-018-5627-4
Klang E, Kopylov U, Ben-Horin S et al (2018) Assessment of patency capsule retention using MR diffusion-weighted imaging. Eur Radiol 27:4979–4985
Park SH, Huh J, Park SH, Lee SS, Kim AY, Yang SK (2017) Diffusion-weighted MR enterography for evaluating Crohn’s disease: effect of anti-peristaltic agent on the diagnosis of bowel inflammation. Eur Radiol 27:2554–2562
Dohan A, Taylor S, Hoeffel C et al (2016) Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging 44:1381–1396
Buisson A, Hordonneau C, Goutte M, Boyer L, Pereira B, Bommelaer G (2015) Diffusion-weighted magnetic resonance imaging is effective to detect ileocolonic ulcerations in Crohn’s disease. Aliment Pharmacol Ther 42:452–460
Klang E, Kopylov U, Eliakim R et al (2017) Diffusion-weighted imaging in quiescent Crohn’s disease: correlation with inflammatory biomarkers and video capsule endoscopy. Clin Radiol 72:797–798
Kim KJ, Lee Y, Park SH et al (2015) Diffusion-weighted MR enterography for evaluating Crohn’s disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis 21:101–109
Seo N, Park SH, Kim KJ et al (2016) MR enterography for the evaluation of small-bowel inflammation in crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 278:762–772
Buisson A, Joubert A, Montoriol PF et al (2013) Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther 37:537–545
Li XH, Sun CH, Mao R et al (2017) Diffusion-weighted MRI enables to accurately grade inflammatory activity in patients of ileocolonic Crohn’s disease: results from an observational study. Inflamm Bowel Dis 23:244–253
Choi SH, Kim KW, Lee JY, Kim KJ, Park SH (2016) Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 22:669–679
Kovanlikaya A, Beneck D, Rose M et al (2015) Quantitative apparent diffusion coefficient (ADC) values as an imaging biomarker for fibrosis in pediatric Crohn’s disease: preliminary experience. Abdom Imaging 40:1068–1074
Tielbeek JA, Ziech ML, Li Z et al (2014) Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 24:619–629
Rosenbaum DG, Rose ML, Solomon AB, Giambrone AE, Kovanlikaya A (2015) Longitudinal diffusion-weighted imaging changes in children with small bowel Crohn’s disease: preliminary experience. Abdom Imaging 40:1075–1080
Li XH, Mao R, Huang SY et al (2018) Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology 287:494–503
Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA (2010) MR enterographic manifestations of small bowel Crohn disease. Radiographics 30:367–384
Zappa M, Stefanescu C, Cazals-Hatem D et al (2011) Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 17:984–993
Adler J, Punglia DR, Dillman JR et al (2012) Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis 18:849–856
Hordonneau C, Buisson A, Scanzi J et al (2014) Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 109:89–98
Acknowledgements
The authors thank Zhongwei Zhang, a diagnostic medical physics doctor from the Radiology Department at the University of Florida, for reading and commenting on the paper. They are also grateful to Jian Zhang, a statistician from the Clinical Research Center at ZhongShan Ophthalmic Center, Sun Yat-Sen University, for valuable suggestion about statistical analysis.
Funding
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (81600508, 81770654, 81500501, 81870451, 81771908, 81571750).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shi-Ting Feng, Can-hui Sun.
Conflict of interest
The authors declare that they have no conflicts of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Li, Xh., Mao, R., Huang, Sy. et al. Ability of DWI to characterize bowel fibrosis depends on the degree of bowel inflammation. Eur Radiol 29, 2465–2473 (2019). https://doi.org/10.1007/s00330-018-5860-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5860-x