Abstract
Objectives
To elucidate the pathogenesis of hyperintense arteries on Gd-enhanced 3D T1W BB FSE and their clinical significance in acute middle cerebral artery (MCA) stroke.
Methods
We retrospectively reviewed 20 patients with MCA infarction. We measured the contrast-to-noise ratio between hyperintense artery and adjacent grey matter on T2-FLAIR and Gd-enhanced 3D T1W BB FSE and compared them by using Student’s t test. The agreement of positive hyperintense artery between T2 FLAIR and Gd-enhanced 3D T1WI BB FSE was estimated with intraclass correlation coefficient. Our cohort was dichotomised into two groups depending on hyperintense artery scores, and clinical data were compared between two groups by using Student’s t test and chi-square test.
Results
The contrast between hyperintense artery and grey matter on Gd-enhanced 3D T1W BB FSE was significantly higher than that on T2-FLAIR (2.27 ± 1.65 versus 0.94 ± 0.86, p = 0.01). Overall, agreement of hyperintense arteries on T2-FLAIR and Gd-enhanced 3D T1W BB FSE was excellent (ρ = 0.76, p < 0.01). Patients with higher hyperintense artery scores had higher perfusion deficits that those with lower hyperintense artery scores (196.7 ± 41.4 vs 100.1 ± 130.1, p = 0.03).
Conclusion
Hyperintense arteries on Gd-enhanced 3D T1W BB FSE in acute MCA stroke may be associated with slow collateral flows. Their territories corresponded to those of FLAIR, but had a better contrast. The patients with hyperintense arteries in a wider territory showed larger perfusion deficit than those with hyperintense arteries in a narrower territory.
Key Points
• Hyperintense arteries on Gd-enhanced 3D T1W BB FSE are slow collateral flows.
• Hyperintense arteries on Gd-enhanced 3D T1W BB FSE are well matched with FLAIR hyperintense vessels.
• Hyperintense arteries are associated with perfusion deficit in stroke patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional catheter angiography and noninvasive luminal imaging techniques (MR angiography or CT angiography) can only identify abnormalities affecting vessel lumen [1, 2]. However, high-resolution black-blood images allow direct characterisation of vessel wall status in the setting of acute stroke. Intracranial artery atherosclerosis, dissection, moyamoya disease, vasculitis and reversible cerebral vasoconstriction syndrome can be diagnosed and differentiated by using high-resolution black-blood images [1, 3,4,5,6,7,8,9].
A variety of black-blood imaging techniques have been developed. 3D variable refocusing flip angle sequences (VISTA, Philips Healthcare, Best, the Netherlands; SPACE, Siemens Healthcare, Erlangen, Germany; CUBE, GE Healthcare, Milwaukee, USA) have been the most extensively studied 3D techniques to date, as these sequences provide blood suppression as well as excellent image quality and increased coverage in a shorter scan time [10,11,12,13]. Recently, additional blood suppression techniques have been employed with 3D variable refocusing flip angle. One such technique is motion-sensitised driven equilibrium, which uses flow-sensitive dephasing gradients to suppress flow [14,15,16]. Another technique is the delay alternating with nutation for tailored excitation pulse train, which uses a series of low flip angle non-selective pulses interleaved with gradient pulses with short repetition times that can result in both optimised blood and cerebrospinal fluid (CSF) suppression, without any effects on tissue contrast [17].
As 3D high-resolution black-blood images have been widely used, hyperintense arteries are frequently encountered on Gd-enhanced three-dimensional T1-weighted black-blood fast spin echo (3D T1W BB FSE) images in patients with stroke. We presume that this sign is associated with collateral sluggish flow in acute stroke, but none of the previous studies have revealed this phenomenon and evaluated its clinical impact. Various imaging techniques are used in acute stroke. CT angiography detects intracranial stenosis and occlusion site with high sensitivity (97–100%) and specificity (98–100%) [18, 19]. Digital subtraction angiography (DSA) is considered as the reference standard for detection of stenosis and occlusion. Fluid attenuated inversion recovery (FLAIR) hyperintense vessels reflect sluggish collateral flows [20,21,22]. They are strongly related to proximal large-vessel occlusion or severe stenosis [23, 24]. Recently, clinical application of FLAIR hyperintense vessels has been actively studied but not yet concluded [25, 26]. Perfusion images are not used to select patients for intravenous thrombolysis [27]. However, they may identify the salvageable brain parenchyma for newer therapy or treatment outside accepted time windows [28,29,30]. Moreover, perfusion images may predict a patient’s clinical outcome, detect the additional vascular territories at risk that may be otherwise normal on conventional imaging and differentiate stroke from its mimics [31,32,33]. Thus, in this study, by comparing these various imaging techniques, we might be able to elucidate the pathogenesis of hyperintense artery on 3D T1W BB FSE and its clinical significance in acute middle cerebral artery (MCA) stroke.
Materials and methods
Patients
We retrospectively screened consecutive patients who presented to our tertiary referral medical centre between January 2017 and December 2017. We included patients with acute MCA territory ischaemic stroke within 1 week of symptom onset. They all underwent stroke MR imaging. All of the patients included in the study showed restricted diffusion in the MCA territory on diffusion-weighted imaging. We excluded patients with transient ischaemic attack, multiple infarctions other than in MCA territories, or lacunar infarction. Our institutional review board approved this retrospective study. The following cerebrovascular risk factors were evaluated on admission: smoking, arterial hypertension, diabetes mellitus and hyperlipidaemia. Stroke severity was assessed by using the National Institutes of Health Stroke Scale (NIHSS). Functional outcome was assessed 3 months post-stroke by using the modified Rankin scale (mRS) score: favourable outcome was defined as mRS score of 2 or less. Arterial occlusions were categorised into proximal Internal carotid artery (ICA), distal ICA, MCA horizontal segment, MCA insular segment and MCA cortical segment.
Imaging protocols
All patients underwent both brain CT angiography (SOMATOM Definition AS+, Siemens Healthcare, Forchheim, Germany) and brain 3-T MRI (Discovery MR750; GE Healthcare, Milwaukee, USA). The brain CT angiography protocol included 1-mm non-contrast CT and CT angiography with the same thickness. The maximum intensity projection using source data was obtained. Our stroke MR imaging protocol for acute stroke included DWI and T2-FLAIR. In addition, dynamic susceptibility contrast (DSC) perfusion-weighted imaging and 3D T1W BB FSE were acquired sequentially after administration of a compact bolus (0.1 mmol/kg) of gadobutrol at an injection rate of 5 mL/s. The parameters for DWI were repetition time (TR)/echo time (TE) = 8000/65.6 ms; slice thickness = 3mm; gap = 0.1 mm; matrix size = 160 × 160; field of view (FOV) = 240 × 240 mm2; three directions; b value = 0 and 1000 s/mm2. T2-FLAIR images were acquired with the following parameters: TR/TE = 12,000/140 ms; inversion time (TI) = 2500 ms; slice thickness = 4 mm; gap = 1 mm; flip angle = 110°; FOV = 210 × 210 mm2; matrix = 352 × 353; and 30 contiguous sections for a total acquisition of 3 min 20 s. DSC perfusion MRI employed gradient echo-planar imaging and imaging parameters were TR/TE =1500/23 ms; slice thickness = 4 mm; gap = 1 mm; flip angle = 60°; FOV = 220 × 220 mm2; matrix = 100 × 100. The acquisition time was 1 min 40 s. To obtain 3D T1W BB FSE, motion-sensitised driven equilibrium prepared pulse was added in the front of 3D T1W FSE with variable flip angle. The scan parameters of 3D T1W FSE with variable flip angle were TR/TE = 500/24.5 ms; slice thickness = 1 mm; echo train length = 24; flip angle = variable; FOV = 220 × 220 mm2; matrix = 256 × 224; 170 axial slices. The acquisition time was 4 min 50 s.
Image analysis
Multimodality images of patients (CT angiography, cerebral angiography, advanced MRI) were reviewed to elucidate the pathogenesis of hyperintense arteries on Gd-enhanced 3D T1W BB FSE.
Two readers independently assessed either T2-FLAIR or Gd-enhanced 3D T1W BB FSE. The first review of images was randomly selected by the study coordinator. The remaining sequences were reviewed 1 week later. The reviewers were blinded to clinical history. Hyperintense arteries were defined as focal, tubular or serpentine hyperintensities in subarachnoid spaces with a typical arterial course of at least two consecutive axial sections of each of the seven Alberta Stroke Program Early CT Score (ASPECTS) territories (insula, M1–M6) [34]. An ASPECTS cortical area was considered positive when it coincided with hyperintense arteries. Hyperintense artery scores were defined as the total number of positive ASPECTS cortical areas on Gd-enhanced 3D T1W BB FSE.
To compare artery-to-grey matter contrast on both sequences, we calculated the contrast-to-noise ratio between hyperintense arteries and adjacent grey matter. The contrast-to-noise ratio was defined as (SIartery – SIGM)/(SD2artery + SD2GM)1/2, where SIartery and SIGM are signal intensities of hyperintense artery and adjacent grey matter respectively. SDartery and SDGM denote standard deviation of hyperintense artery and adjacent grey matter respectively [35].
The ischaemic lesion volume and perfusion abnormality volume were also measured as follows: DWI images were coregistered to Tmax of the perfusion map using FMRIB Software Library tools (FSL, Version 5.0; https://fsl.fmrib.ox.ac.uk/fsl). The free open-source toolkit ITK-SNAP (www.itksnap.org) was used for drawing regions of interest (ROIs). Two different reviewers drew ROIs separately along the borders of the high-signal area on registered DWI and yellow territories on coloured Tmax maps (Tmax > 6 s). The Tmax map was generated by using perfusion-processing software (Func-tool; GE Healthcare). Infarct growth was defined as DWI lesion volume after thrombolysis minus DWI baseline lesion volume.
Statistical analysis
Artery-to-grey matter contrasts on T2-FLAIR and Gd-enhanced 3D T1W BB FSE were compared by using Student’s t test. The agreement of hyperintense arteries on both sequences according to each MCA ASPECTS territory was estimated with the intraclass correlation coefficient using the two-way random effects model. Using an accepted standard, we defined intraclass correlation coefficient values below 0.40 as poor, between 0.40 and 0.59 as fair, between 0.60 and 0.74 as good, and over 0.74 as excellent [36]. Interobserver agreement of volume measurement was also performed with same method as above. Our cohort was dichotomised into two groups using median hyperintense artery scores (hyperintense artery score < 5 vs ≥ 5). We further compared clinical and imaging data (age, sex, hypertension, diabetes mellitus, hyperlipidaemia, smoking status, NIHSS score, baseline infarct volume, infarct growth, perfusion deficit and favourable outcome) between the two groups by using Student’s t test and chi-square test. All statistical analysis was performed by using Statistical Package for Social Sciences (SPSS) software (version 21, IBM Corp., Armonk, NY, USA). P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
Twenty patients fulfilled the inclusion criteria (Table 1). The mean age was 69.5 years, and 25% of patients were women. Eleven patients (50%) had hypertension, eight patients (40%) had diabetes mellitus, six patients (30%) had hyperlipidaemia, and six patients (30%) were current smokers. The median initial NIHSS score was 11 (interquartile range, 5.5–16.5). The median time from symptom onset to MR imaging was 360 min (interquartile range, 235–580 min). Occlusion sites included the following areas: the MCA horizontal segment (5/20, 25%), MCA insular segment (5/20, 25%), MCA cortical segment (4/20, 20%) and the proximal ICA (6/20, 30%).
Illustrative case of arterial hyperintensity with multimodality images
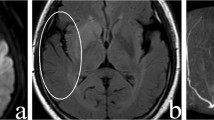
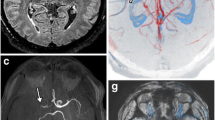
A 62-year-old man presented with left-sided weakness with an initial NIHSS score of 10. CT angiography and digital subtraction angiography showed occlusion in the right MCA, insular segment. Hyperintense artery was visualised distal to the occlusion site on post-contrast 3D T1W BB FSE, and not on the pre-contrast study. The velocity of hyperintense artery appeared to be slower than that of the vein, since the signal intensity of the posterior superior sagittal sinus was suppressed. Hyperintense artery was well matched with the retrograde collateral flow on digital subtraction angiography (Fig. 1).
a Maximum intensity projection image of CT angiography shows occlusion in the right MCA, insular segment (arrow). b Maximum intensity projection image of Gd-enhanced 3D T1W BB FSE shows hyperintense arteries (arrowheads) distal to the occlusion site. c Angiography in early phase shows occlusion in the right MCA, insular segment. d Retrograde collateral flow is filled in the delayed phase at the region where the hyperintense artery is seen. Hyperintense artery is not seen on the pre-contrast (e) but on the post-contrast 3D T1W BB FSE (f)
Comparison with hyperintense artery on T2-FLAIR
The contrast-to-noise ratio between the hyperintense artery and adjacent grey matter on Gd-enhanced 3D T1W BB FSE was significantly higher than that on T2-FLAIR (2.27 ± 1.65 versus 0.94 ± 0.86, p = 0.01, Fig. 2).
Overall, agreement of hyperintense arteries on T2-FLAIR and Gd-enhanced 3D T1W BB FSE was excellent (ρ = 0.76, p < 0.01, Fig. 3). Regarding each ASPECT territory, agreements were excellent in I, M1, M2, M3 and M4 (ρ = 0.87, 0.8, 0.78, 0.81 and 0.79 respectively, p < 0.01), good in M5 (ρ = 0.67, p = 0.01) and fair in M6 (ρ = 0.56, p = 0.04) (Table 2).
Clinical significance of hyperintense artery on Gd-enhanced 3D T1W BB FSE
Interobserver agreements for volume measurement of DWI and perfusion images were excellent (ρ = 0.99, p < 0.01). Patients with higher hyperintense artery scores had higher perfusion deficits that those with lower hyperintense artery scores (196.7 ± 41.4 vs 100.1 ± 130.1, p = 0.03) (Table 3). Age, sex, hypertension, diabetes mellitus, hyperlipidaemia, current smoking, NIHSS score, baseline infarct volume, infarct growth and favourable outcome were not significantly different between the two groups.
Discussion
This study showed that in patients with acute MCA stroke, the hyperintense artery sign on Gd-enhanced 3D T1W BB FSE may have resulted from a retrograde slow collateral flow and was well matched with the FLAIR hyperintense vessel sign, but showed a better contrast. The patients with hyperintense artery in a wider territory showed higher perfusion deficit than those with hyperintense artery in a narrower territory.
Gd-enhanced high-resolution black-blood T1-weighted imaging has been commonly used in stroke, and Jang et al showed that it improves detection of intraluminal thrombi [37]. However, hyperintense artery signs are rarely mentioned [13, 38,39,40]. Hui et al first mentioned the hyperintense artery sign as a “white snake sign” and they presumed that this sign may be due to a sluggish slow flow and just distal to a thrombus [41]. Our results agree with those of the previous study. Hyperintense artery corresponds to a sluggish collateral flow. This retrograde slow flow may result in loss of the flow void phenomenon, and vessels may appear hyperintense against the dark CSF background [42, 43]. However, according to our results, a collateral flow did not appear hyperintense on non-enhanced 3D T1W BB FSE. This implies that the appearance of hyperintense artery cannot be explained only by loss of the flow void phenomenon. We presume that the T1-shortening effect of Gd contributes to the underlying loss of the flow void phenomenon, making sluggish flows hyperintense.
Hyperintense artery is more prominent on Gd-enhanced 3D T1W BB FSE than on T2-FLAIR. The signal intensity of GM is lower than that of WM on T1WI, whereas the signal intensity of GM is higher than that of WM on T2-FLAIR [44, 45]. Thus, the contrast between hyperintense artery and adjacent grey matter was not distinct on T2-FLAIR. Overall, the regions of hyperintense artery distribution were well matched with those on T2-FLAIR. However, the degree of agreement between two sequences was decreased in M5 and M6, compared to other ASPECTS territories (I, M1, M2, M3, M4). Previous studies have reported that hyperintense artery on T2-FLAIR is prominent within the Sylvian fissure [23, 46, 47]. This might be because larger arteries have a slower flow speed than smaller distal arteries under the same perfusion pressure. Likewise, in our results, hyperintense artery on T2-FLAIR tended not to be observed in distal cortical regions (M4, M5 and M6), which may explain the slight discrepancy between the two sequences.
The clinical significance of hyperintense artery on Gd-enhanced 3D T1W BB FSE has not been reported. According to our results, in most territories, the greater the hyperintensity of the artery was, the larger the perfusion deficit was. However, the extent of hyperintense artery was not related to baseline infarct volume, infarct growth and patient outcome. Considering the similarities of hyperintense artery on Gd-enhanced 3D T1W BB FSE with that on T2-FLAIR regarding pathogenesis and extent, we suggest its clinical significance. Previous papers have frequently stated the association of FLAIR hyperintense vessels with large perfusion lesions and bigger mismatch volumes [23, 46, 48,49,50]. However, in determining the prognostic information provided by FLAIR hyperintense vessels, the data are seemingly contradictory. Some authors have noted that the presence of FLAIR hyperintense vessels is correlated with smaller infarct volume and better clinical outcomes [25, 51]. In contrast to these studies, Kufner et al observed that the presence of FLAIR hyperintense vessels was associated with larger perfusion deficits, larger growth and more severe hypoperfusion [26]. One reason for these differences may be that FLAIR hyperintense vessels imply a mixture of “poor” and “good” collaterals, hampering the differentiation between true penumbra and benign oligaemia [50]. Moreover, the occlusion sites in our cohort were heterogeneous. Specifically, patients’ outcomes may be different depending on occlusion site [52]. The clinical significance of hyperintense artery on Gd-enhanced 3D T1W BB FSE should be further investigated in future studies with larger cohorts and consistent occlusion sites.
In addition, application of new techniques may affect the extent of hyperintense artery. The Periodically Rotated Overlapping ParallEL Lines with Enhanced Reconstruction (PROPELLER) technique increases the extent of FLAIR hyperintense artery and its territory is more related to perfusion abnormality than that of conventional FLAIR [20]. Thus, there is a chance that other black-blood techniques such as Delay Alternating with Nutation for Tailored Excitation (DANTE) may change the extent of hyperintense artery.
Recently, some studies demonstrated a strong correlation between susceptibility-weighted imaging (SWI) and perfusion imaging, and they proposed that asymmetrical dilated vessel-like signal loss in the cortex on SWI could be a marker of penumbra [53, 54]. This sign on SWI may reflect the haemodynamic changes resulting from the dilatation of vessels in response to ischaemia and an elevated oxygen extraction fraction (OEF) following an increase of the ratio of deoxyhaemoglobin to oxyhaemoglobin [55]. Thus, it might be interesting to evaluate the relationship between hyperintense artery on black-blood images and dark vessels on SWI.
There was a limitation in our study. The number of cases was small and might not be sufficient to make a solid conclusion regarding its clinical significance. However, various imagine modalities clearly show that hyperintense artery on Gd-enhanced 3D T1 BB FSE is a sluggish collateral flow. We also believe that our preliminary results supporting its clinical significance may serve as a cornerstone for future studies with a larger population to validate and extend these results.
Conclusion
Hyperintense arteries on Gd-enhanced 3D T1W BB FSE in acute MCA stroke may be associated with a retrograde slow collateral flow. Their territories corresponded to those of FLAIR, but had a better contrast. The patients with hyperintense arteries in a wider territory showed larger perfusion deficit than those with hyperintense arteries in a narrower territory. However, their clinical significance should be further studied with larger and homogenous cohorts.
Abbreviations
- 3D T1W BB FSE:
-
Three-dimensional T1-weighted black-blood fast spin echo
- ASPECTS:
-
Alberta stroke program early CT score
- CSF:
-
Cerebrospinal fluid
- FLAIR:
-
Fluid attenuated inversion recovery
- Gd:
-
Gadolinium
- ICA:
-
Internal carotid artery
- MCA:
-
Middle cerebral artery
- mRS:
-
Modified Rankin scale
- NIHSS:
-
National Institutes of Health Stroke Scale
References
Kim YJ, Lee DH, Kwon JY et al (2013) High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol 20:1311–1318
Natori T, Sasaki M, Miyoshi M et al (2014) Evaluating middle cerebral artery atherosclerotic lesions in acute ischemic stroke using magnetic resonance T1-weighted 3-dimensional vessel wall imaging. J Stroke Cerebrovasc Dis 23:706–711
Choi YJ, Jung SC, Lee DH (2015) Vessel wall imaging of the intracranial and cervical carotid arteries. J Stroke 17:238–255
Saam T, Habs M, Pollatos O et al (2010) High-resolution black-blood contrast-enhanced T1 weighted images for the diagnosis and follow-up of intracranial arteritis. Br J Radiol 83:e182–e184
Saam T, Hatsukami TS, Takaya N et al (2007) The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology 244:64–77
Bley TA, Wieben O, Uhl M, Thiel J, Schmidt D, Langer M (2005) High-resolution MRI in giant cell arteritis: imaging of the wall of the superficial temporal artery. AJR Am J Roentgenol 184:283–287
Takano K, Hida K, Kuwabara Y, Yoshimitsu K (2017) Intracranial arterial wall enhancement using gadolinium-enhanced 3D black-blood T1-weighted imaging. Eur J Radiol 86:13–19
Ben Hassen W, Machet A, Edjlali-Goujon M et al (2014) Imaging of cervical artery dissection. Diagn Interv Imaging 95:1151–1161
Obusez EC, Hui F, Hajj-Ali RA et al (2014) High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 35:1527–1532
Busse RF, Brau AC, Vu A et al (2008) Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med 60:640–649
Kato Y, Higano S, Tamura H et al (2009) Usefulness of contrast-enhanced T1-weighted sampling perfection with application-optimized contrasts by using different flip angle evolutions in detection of small brain metastasis at 3T MR imaging: comparison with magnetization-prepared rapid acquisition of gradient echo imaging. AJNR Am J Neuroradiol 30:923–929
Fan Z, Zhang Z, Chung YC et al (2010) Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging 31:645–654
Qiao Y, Zeiler SR, Mirbagheri S et al (2014) Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 271:534–542
Korosec FR, Grist TM, Polzin JA, Weber DM, Mistretta CA (1993) MR angiography using velocity-selective preparation pulses and segmented gradient-echo acquisition. Magn Reson Med 30:704–714
Norris DG, Schwarzbauer C (1999) Velocity selective radiofrequency pulse trains. J Magn Reson 137:231–236
Zhu C, Graves MJ, Yuan J, Sadat U, Gillard JH, Patterson AJ (2014) Optimization of improved motion-sensitized driven-equilibrium (iMSDE) blood suppression for carotid artery wall imaging. J Cardiovasc Magn Reson 16:61
Wang J, Helle M, Zhou Z, Bornert P, Hatsukami TS, Yuan C (2016) Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med 75:831–838
Shrier DA, Tanaka H, Numaguchi Y, Konno S, Patel U, Shibata D (1997) CT angiography in the evaluation of acute stroke. AJNR Am J Neuroradiol 18:1011–1020
Nguyen-Huynh MN, Wintermark M, English J et al (2008) How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke 39:1184–1188
Ahn SJ, Suh SH, Lee KY, Kim JH, Seo KD, Lee S (2015) Hyperintense vessels on T2-PROPELLER-FLAIR in patients with acute MCA stroke: prediction of arterial stenosis and perfusion abnormality. AJNR Am J Neuroradiol 36:2042–2047
Lee SH, Seo KD, Kim JH, Suh SH, Ahn SJ, Lee KY (2016) Correlation between hyperintense vessels on FLAIR imaging and arterial circulation time on cerebral angiography. Magn Reson Med Sci 15:105–110
Ahn SJ, Lee KY, Ahn SS, Suh H, Kim BS, Lee SK (2016) Can FLAIR hyperintensevessel (FHV) signs be influenced by varying MR parameters and flow velocities? A flow phantom analysis. Acta Radiol 57:580–586
Kamran S, Bates V, Bakshi R, Wright P, Kinkel W, Miletich R (2000) Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology 55:265–269
Cosnard G, Duprez T, Grandin C, Smith AM, Munier T, Peeters A (1999) Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology 41:342–346
Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S (2009) Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology 72:1134–1139
Kufner A, Galinovic I, Ambrosi V et al (2015) Hyperintense vessels on FLAIR: hemodynamic correlates and response to thrombolysis. AJNR Am J Neuroradiol 36:1426–1430
Goyal M, Menon BK, Derdeyn CP (2013) Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology 266:16–21
Donnan GA, Baron JC, Ma H, Davis SM (2009) Penumbral selection of patients for trials of acute stroke therapy. Lancet Neurol 8:261–269
Kim JT, Yoon W, Park MS et al (2009) Early outcome of combined thrombolysis based on the mismatch on perfusion CT. Cerebrovasc Dis 28:259–265
Jovin TG, Liebeskind DS, Gupta R et al (2011) Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke 42:2206–2211
Inoue M, Mlynash M, Straka M et al (2012) Patients with the malignant profile within 3 hours of symptom onset have very poor outcomes after intravenous tissue-type plasminogen activator therapy. Stroke 43:2494–2496
Lin K, Do KG, Ong P et al (2009) Perfusion CT improves diagnostic accuracy for hyperacute ischemic stroke in the 3-hour window: study of 100 patients with diffusion MRI confirmation. Cerebrovasc Dis 28:72–79
Schellinger PD, Bryan RN, Caplan LR et al (2010) Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 75:177–185
Pexman JH, Barber PA, Hill MD et al (2001) Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 22:1534–1542
Kakeda S, Korogi Y, Hiai Y, Ohnari N, Sato T, Hirai T (2012) Pitfalls of 3D FLAIR brain imaging: a prospective comparison with 2D FLAIR. Acad Radiol 19:1225–1232
Streiner DL, Norman GR (1995) Health measurement scales: a practical guide to their development and use, 2nd edn. Oxford University Press, Oxford
Jang W, Kwak HS, Chung GH, Hwang SB (2018) Three-dimensional black-blood contrast-enhanced MRI improves detection of intraluminal thrombi in patients with acute ischaemic stroke. Eur Radiol. https://doi.org/10.1007/s00330-018-5323-4
Power S, Matouk C, Casaubon LK et al (2014) Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke 45:2330–2334
Mandell DM, Mossa-Basha M, Qiao Y et al (2017) Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 38:218–229
Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ (2013) Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol 34:299–304
Hui FK, Zhu X, Jones SE et al (2015) Early experience in high-resolution MRI for large vessel occlusions. J Neurointerv Surg 7:509–516
Storey P, Atanasova IP, Lim RP et al (2010) Tailoring the flow sensitivity of fast spin-echo sequences for noncontrast peripheral MR angiography. Magn Reson Med 64:1098–1108
Park J, Kim EY (2010) Contrast-enhanced, three-dimensional, whole-brain, black-blood imaging: application to small brain metastases. Magn Reson Med 63:553–561
Knight MJ, McCann B, Tsivos D, Couthard E, Kauppinen RA (2016) Quantitative T1 and T2 MRI signal characteristics in the human brain: different patterns of MR contrasts in normal ageing. MAGMA 29:833–842
Lu H, Nagae-Poetscher LM, Golay X, Lin D, Pomper M, van Zijl PC (2005) Routine clinical brain MRI sequences for use at 3.0 Tesla. J Magn Reson Imaging 22:13–22
Hohenhaus M, Schmidt WU, Brunecker P et al (2012) FLAIR vascular hyperintensities in acute ICA and MCA infarction: a marker for mismatch and stroke severity? Cerebrovasc Dis 34:63–69
Haussen DC, Koch S, Saraf-Lavi E, Shang T, Dharmadhikari S, Yavagal DR (2013) FLAIR distal hyperintense vessels as a marker of perfusion-diffusion mismatch in acute stroke. J Neuroimaging 23:397–400
Cheng B, Ebinger M, Kufner A et al (2012) Hyperintense vessels on acute stroke fluid-attenuated inversion recovery imaging: associations with clinical and other MRI findings. Stroke 43:2957–2961
Ebinger M, Kufner A, Galinovic I et al (2012) Fluid-attenuated inversion recovery images and stroke outcome after thrombolysis. Stroke 43:539–542
Gawlitza M, Gragert J, Quaschling U, Hoffmann KT (2014) FLAIR-hyperintense vessel sign, diffusion-perfusion mismatch and infarct growth in acute ischemic stroke without vascular recanalisation therapy. J Neuroradiol 41:227–233
Huang X, Liu W, Zhu W et al (2012) Distal hyperintense vessels on FLAIR: a prognostic indicator of acute ischemic stroke. Eur Neurol 68:214–220
Klijn CJ, Kappelle LJ, Algra A, van Gijn J (2001) Outcome in patients with symptomatic occlusion of the internal carotid artery or intracranial arterial lesions: a meta-analysis of the role of baseline characteristics and type of antithrombotic treatment. Cerebrovasc Dis 12:228–234
Luo S, Yang L, Wang L (2015) Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol 42:255–260
Dejobert M, Cazals X, Annan M, Debiais S, Lauvin MA, Cottier JP (2016) Susceptibility-diffusion mismatch in hyperacute stroke: correlation with perfusion-diffusion mismatch and clinical outcome. J Stroke Cerebrovasc Dis 25:1760–1766
Hermier M, Nighoghossian N (2004) Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke 35:1989–1994
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Kyung-Yul Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Lee, KY., Suh, S.H. & Ahn, S.J. Significance of hyperintense arteries on Gd-enhanced 3D T1W black-blood imaging in acute stroke. Eur Radiol 29, 1329–1337 (2019). https://doi.org/10.1007/s00330-018-5669-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5669-7