Abstract
Background
Magnetic resonance imaging (MRI) is the diagnostic cornerstone for precisely identifying acute ischaemic strokes and locating vascular occlusions, especially since mechanical thrombectomy has become a reference treatment. We observed that a post-contrast three-dimensional turbo-spin-echo T1-weighted sequence showed striking post-contrast vascular hyperintensities (PCVH) in ischaemic territories. We aimed to evaluate the prevalence and the meaning of this finding.
Methods
This retrospective single centre study included 130 consecutive patients admitted for acute ischaemic stroke with a 3-T MRI performed in the first 12 h of symptom onset from September 2014 through September 2016. Two neuroradiologists blinded to clinical data analysed the first MRI assessments. The association between PCVH and clinical, radiological and follow-up findings was assessed, as well as inter- and intra-observer agreements.
Results
Of 130 patients, 105 (81%) had PCVH in the ischaemic territory. PCVH were associated with the presence of thrombus on susceptibility weighted imaging (p < 0.0001) and vascular occlusions on MR angiography (p < 0.0001). All patients with a visible thrombus had PCVH closely surrounding the clot. PCVH were associated with higher initial (p < 0.01) and follow-up (p < 0.01) National Institutes of Health Stroke Scale score, and higher mRS score (p < 0.05). Thrombectomy was the reference treatment for all patients with arterial occlusions. Inter- and intra-observer agreements for the detection of PCVH were excellent (κ = 0.95 and κ = 0.91, respectively).
Conclusions
PCVH during acute strokes are a striking sensitive and reproducible tool for diagnosing and locating vascular occlusions. It may help triage patients who can benefit from thrombectomy.
Key points
• Post-contrast vascular hyperintensities (PCVH) are a sensitive MR finding in acute stroke
• PCVH are strongly associated with the presence and location of arterial occlusions
• Inter- and intra-observer agreements for the detection of PCVH are excellent
• PCVH are visible even in the case of significant motion artefacts
• PCVH may help triage patients who can benefit from mechanical thrombectomy
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the most devastating of all neurological conditions, affecting 15 million people worldwide every year. It accounts for approximately 5.5 million deaths annually, with 44 million disability-adjusted life-years lost. The stroke prevalence is expected to rise as the global population older than 65 years of age continues to increase by approximately 9 million people per year [1]. Improved prognoses rely on early recanalisation of the occluded artery, which allows reperfusion and revascularisation of the suffering brain parenchyma. In recent years, mechanical thrombectomy (MT) in combination with intravenous thrombolysis (IVT), when used jointly with non-invasive arterial imaging, has proved to have a beneficial effect on morbidity, functional impact (autonomy and independence) and quality of life at 90 days in patients with acute ischaemic strokes and proximal vascular occlusions [2,3,4].
Cerebral and vascular imaging is a prerequisite for making the indication for MT [4]. Performing accurate and early diagnosis of brain parenchymal ischaemia and a precise vascular occlusion description is the cornerstone of the management of acute ischaemic strokes. The American Heart Association/American Stroke Association (AHA/ASA) and the European Stroke Organisation (ESO) recommend performing diagnostics using a computed tomography (CT) scan or magnetic resonance imaging (MRI) [5, 6]. MRI is now preferred by some professional societies because of its higher sensitivity and specificity [6,7,8,9].
A brain parenchymal hyperintensity on diffusion-weighted imaging (DWI) associated with a low signal on the apparent diffusion coefficient (ADC) map is currently the imaging “gold standard” for the diagnosis. Other associated prognostic findings have been described, such as a large volume of parenchymal ischaemia [10], the presence of fluid attenuated inversion recovery (FLAIR) vascular hyperintensities (FVH) [10, 11] or a perfusion/diffusion mismatch [7]. The depiction of a proximal vascular occlusion is requested to perform an MT and usually relies on time-of-flight (TOF) and/or contrast-enhanced MR angiography (CE-MRA) sequences. The presence of a visible clot on T2* or susceptibility-weighted imaging (SWI) sequences may also be helpful.
We noticed that a post-contrast three-dimensional (3D) turbo spin echo (TSE) T1-weighted MR sequence showed striking post-contrast vascular hyperintensities (PCVH) in the ischaemic territory in many patients, especially those with arterial occlusions. We hypothesised that this imaging evidence might offer valuable information for both accurate diagnosis and prognosis, knowing that acute occlusion of a large intracranial artery is associated with an increased risk of poor outcome [12].
The main objective of this study was to assess the prevalence of the PCVH in acute ischaemic stroke patients. Secondary objectives were to assess the correlation between PCVH and the presence of an arterial occlusion in addition to patient functional outcome.
Material and methods
This retrospective non-interventional study was approved by our site’s institutional review board, which waived the need for individualised informed consent. It follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [13].
Inclusion and exclusion criteria
This analysis was based on a prospectively collected single centre registry of consecutive adult patients admitted to our hospital for acute ischaemic stroke from September 2014 through September 2016. The inclusion criteria were:
-
Patients aged 18 and older at onset of an acute ischaemic stroke
-
Availability of at least one pre-therapeutic MRI performed in our centre within 12 h of stroke onset, including a post-contrast 3D TSE T1-weighted sequence.
The exclusion criteria were:
-
Patients with posterior fossa strokes
-
Patients with known intracranial vasculitis or MoyaMoya disease
-
Presence of severe MR imaging artefacts preventing correct interpretation
Among the 1,454 patients admitted to our institution for stroke during the study time, 1,158 did not meet inclusion criteria (752 with an MRI performed at a different centre, 324 with an MRI performed more than 12 h after stroke onset and 82 with an MRI without a post-contrast 3D TSE T1-weighted sequence). Secondary exclusion occurred in 137 patients with posterior fossa strokes, 27 patients with intracranial vasculitis, and 2 patients with MoyaMoya disease. Finally, we included 130 patients.
Age, sex, arterial blood pressure, smoking status, diabetes mellitus, hyperlipidaemia and stroke mechanism (cardio-embolism versus extra-cranial macroangiopathy) were recorded. National Institutes of Health Stroke Scale (NIHSS) score before treatment and 24 h after treatment onset were collected. Outcome at 24 h and 3 months after initial care was assessed using the modified Rankin Scale (mRS).
All patients with suspected acute strokes were sent to our centre, where they had a first fast neurological clinical examination by a neurologist with stroke expertise, who then asked for brain MRI.
MRI protocol
Initial MRI was performed using a single 3-T MR scanner (Ingenia; Philips Healthcare, Best, The Netherlands) with a 16-channel neurovascular head coil. Our centre’s MRI stroke protocol included axial DWI (b0-b1,000-b2,000), sagittal 3D FLAIR, intracranial 3D TOF, CE-MRA of cervical and intracranial arteries, fast axial SWI, and a post-contrast 3D TSE T1-weighted sequence (3D BrainView). Total acquisition time for this protocol was 8 min and 54 s. The CE-MRA required a bolus single dose (0.1 mmol/kg) of Gadobutrol (Gadovist; Bayer HealthCare, Berlin, Germany) injected into an antecubital vein with a flow rate of 2 mL/s, by using an automatic injector (Spectris Solaris® EP MR Injection System; Medrad, Warrendale, PA, USA). The arrival of the contrast agent in the arterial circulation was visually detected by trained technicians on fast serial 2D images before starting acquisition of the 3D CE-MRA in the coronal plane with elliptical k-space encoding. The post-contrast 3D TSE T1-weighted sequence required 1 min 19 s and was performed 2 min and 25 s after intravenous contrast injection. This sequence used a train of non-spatially selective refocusing RF pulses with variable flip angles, allowing an extended echo train and minimising the blurring effect. No vascular signal suppression was added to the inherent vascular signal suppression of turbo spin echo sequences. Detailed acquisition parameters are listed in Table 1.
Treatment protocol
All patients with a confirmed acute ischaemic stroke and without clinical or imaging contraindication received an IVT immediately after the MR exam. Based on clinical and imaging data, interventional neuroradiologists and neurologists discussed the opportunity to perform a MT in emergency. All patients considered for MT were treated in a neuroangiography suite under conscious sedation (n = 33) or general anaesthesia (n = 1) after quick evaluation by the anaesthesiology team. Selective cerebral angiogram was performed on an Allura flat-detector based biplane or single plane angiographic system (FD20/10 or FD20, respectively; Philips Healthcare) with acquisitions in at least two orthogonal incidences (anteroposterior and lateral). Methods used for recanalisation were stent retriever in 19 patients, using Solitaire (Medtronic Neurovascular, Irvine, CA, USA) and Trevo (Stryker Neurovascular, Fremont, CA, USA) devices, and direct aspiration in 15 patients using a Penumbra 5MAX aspiration catheter (Penumbra, Alameda, CA, USA). The median MRI-to-needle time was 34 min [interquartile range (IQR), 16 min].
Image analysis
All MR images were stored in a PACS (Picture Archiving and Communication System) and reviewed by one junior radiologist (L.D., 4 years’ experience) and one senior neuroradiologist (A.L., 8 years’ experience), both blinded to clinical, therapeutic and follow-up data. Images were interpreted using a DICOM viewer on a dedicated workstation (Carestream Vue Solutions®, version 12.1.0.2041; Carestream Health, Rochester, NY, USA).
A standardised form was completed by each reader, who had open access to all MR sequences of the exam. The primary endpoint was the presence or absence of PCVH on the post-contrast 3D TSE T1-weighted sequence. Positive results were marked when there were tubular or serpentine hyperintensities in the ischaemic territory corresponding to the typical course of arteries and not visible on the contralateral hemisphere (Fig. 1). Other reported items included:
-
Location, volume, and side of the arterial ischaemic territory on DWI.
-
Presence and location of a vascular hypointense thrombus on SWI.
-
Presence of hypointense dilated cortical veins in the ischaemic territory on SWI.
-
Presence and location of a vascular occlusion on TOF and CE-MRA sequences.
-
Presence of vascular collaterals on CE-MRA, marked positive if the distal vessels in the ischaemic territory were enhanced.
-
Presence of FVH, marked positive in the case of tubular or serpentine hyperintensities located in the ischaemic territory, corresponding to the typical course of arteries and not visible on the contralateral hemisphere.
-
FLAIR-DWI mismatch defined as positive DWI and concomitant negative FLAIR.
-
FVH-DWI mismatch defined as FVH without adjacent parenchymal hyperintensity on DWI.
-
Diagnostic confidence in visualising PCVH, classified as follows: 0 (poor), 1 (moderate), 2 (excellent).
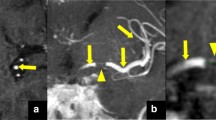
PCVH and correlation to the arterial occlusion on SWI and CE-MRA (part 1). MR performed 1.5 h after stroke onset in a 63-year-old man suffering from acute left hemiparesis. a Axial DWI b2,000 showing a right middle cerebral artery territory infarct (black asterisks). b Axial 3D FLAIR sequence showing mild corresponding hyperintensities. c Axial 3D TOF reconstruction and d axial maximal intensity projection (MIP) of CE-MRA showing right M1 occlusion (white arrows). e Axial MIP of post-contrast 3D TSE T1-WI showing proximal PCVH (white arrowheads) in the right middle cerebral artery territory surrounding the arterial occlusion. f Fusion of axial CE-MRA (red) and 3D TSE T1 (blue) sequences showing the location of PCVH (white arrowheads) relative to the arterial occlusion (white arrow). g Fusion of axial SWI and 3D TSE T1 (blue) sequences showing the location of PCVH relative to the hypointense clot on SWI (black arrowhead); (H) Axial MIP of post-contrast 3D TSE T1-WI showing distal PCVH in collateral vessels (black arrows)
All MR images were reviewed a second time by the junior reader eight weeks after the first reading to assess the intra-observer reproducibility.
Statistical analysis
Quantitative variables were presented as mean [standard deviation (SD)] and median (IQR). Categorical variables were presented as number (percentages). The binary variable PCVH was compared to quantitative variables using a non-parametric Wilcoxon test and to categorical variables using a Fisher exact test. The Cohen’s Kappa coefficient (κ) was used to assess inter-observer and intra-observer agreements for PCVH. According to the Landis and Koch guidelines, values of κ < 0 were considered to show no agreement, 0–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial and 0.81–1 excellent agreement. A p value less than 0.05 was considered significant. Analyses were performed using R 3.3.2 [14].
Results
Population
One hundred and thirty patients were included from September 2014 to September 2016. Strokes involved the territories of a middle cerebral artery in 109/130 (84%), a posterior cerebral artery in 19/130 cases (14%), and an anterior cerebral artery in 2/130 cases (2%). The median time from stroke onset to initial MR imaging was 2 h 44 mins (range, 44 min to 11 h 33 min). Patients’ characteristics are summarised in the Table 2.
Prevalence of PCVH and clinical correlations
Among the 130 patients analysed, 105 had PCVH (81%). PCVH were depicted in the middle (Fig. 1), posterior, and anterior cerebral artery territories (Fig. 2). The initial NIHSS score was significantly higher in patients with PCVH (median 9, IQR 13) than without (median 3, IQR 5), p < 0.001. Among patients who were subsequently treated by MT, all had positive PCVH (p < 0.001). The presence of PCVH was significantly associated with a subsequent MT (p < 0.01). Both NIHSS and mRS at 24 h were significantly higher in patients with PCVH (median 3 versus 1, p < 0.01 and 2 versus 1, p < 0.05 respectively). Detailed data are available in Table 3.
PCVH in the posterior and anterior cerebral artery territories in a 59-year-old man with occlusion of ACA and PCA confirmed on CE-MRA and TOF. a Axial DWI b2,000 showing a right occipital acute ischaemic stroke (black asterisk) and a right frontal stroke (not shown on the image). b Axial 3D FLAIR sequence showing mild corresponding hyperintensities. c Axial MIP of CE-MRA showing a distal right posterior cerebral artery occlusion (white arrowhead). d Axial SWI showing a hypointense arterial clot in the P3 and P4 portions of the right posterior cerebral artery (white arrow). e Axial MIP of post-contrast 3D TSE T1-WI showing PCVH in the right posterior cerebral artery upstream (black arrow) and downstream (black arrowhead) from the clot. f Axial and g sagittal MIP of 3D TSE T1-WI showing PCVH in the right anterior cerebral artery (white arrowheads). h The corresponding acute stroke on DWI
Correlation between PCVH and arterial occlusion
PCVH were strongly associated with the presence of a thrombus on SWI (p < 0.0001) and of an arterial occlusion on TOF and/or CE-MRA sequences (p < 0.0001). Among the 105 patients with PCVH, 26 (25%) had neither a thrombus on SWI nor an arterial occlusion on CE-MRA or TOF sequences. Twenty-five (99%) of them had PCVH exclusively located on the third portion of a middle cerebral artery. The latter had significantly smaller ischaemic cores on DWI [median (IQR), 5 (13) versus 15 (30) ml, p = 0,03], better initial NIHSS [median (IQR), 3 (6) versus 12 (13), p < 0.001], better NIHSS at 24 h [median (IQR), 2 (4) versus 3 (10), p = 0.03], better mRS at 24 h [median (IQR), 2 (1) versus 3 (3), p = 0.02] and all were treated with IVT alone.
All the patients with a visible thrombus on SWI presented with PCVH in close proximity to the clot, depicted immediately upstream and downstream from the thrombus, as shown in Figs. 1 and 2. PCVH were significantly more often depicted when the stroke mechanism was cardio-embolism compared to extra-cranial macroangiopathy [39/41 (95%) versus 66/89 (74%), p < 0.01]. After MT, no residual PCVH was depicted. One patient had visible PCVH with no hyperintensity on DWI. A digitalised arteriography confirmed a vascular occlusion in the PCVH territory and an MT was performed, with a follow-up MRI 24 h later showing a positivity of the DWI (Fig. 3).
Early positive PCVH with still negative DWI in a 50-year-old man with an imaging delay from stroke onset of 1 h. a Axial DWI and b axial ADC map showing no evidence of acute ischaemic stroke. c Axial MIP of post-contrast 3D TSE T1-WI showing distal PCVH in the left middle cerebral artery territory (white arrows). d Coronal view of digital subtraction angiography (DSA) showing a left M2 occlusion (black arrow). e Coronal view of post-thrombectomy DSA showing complete recanalisation of the left middle cerebral artery (black arrowhead). f Axial DWI and g axial 3D FLAIR sequence 24 h after treatment showing a recent ischaemic infarct in the left middle cerebral artery (black asterisks)
The detailed correlation between the location of the occluded artery on CE-MRA and/or TOF and the location of PCVH is available in Table 4.
Correlation between PCVH and collateral vessels
PCVH were strongly associated with the presence of collateral vessels on CE-MRA (p < 0.01) and FVH (p < 0.0001). PCVH were significantly more frequent than FVH [105 (81%) versus 70 (54%) respectively, p < 0.0001] and were depicted in all cases where FVH was positive, but for one.
Correlation between PCVH and other imaging findings
PCVH were strongly associated with higher ischaemic volumes on DWI (p < 0.0001). Among the 25 patients with no PCVH, all patients had a small ischaemic volume on DWI (median, 1 ml; IQR, 2 ml). None of them had a visible thrombus or an arterial occlusion on CE-MRA. PCVH were also associated with dilated cortical veins on SWI (p < 0.0001) and with FVH-DWI mismatch (p < 0.01) but not significantly with FLAIR-DWI mismatch (p = 0.8). Correlations between PCVH and other imaging findings are summarised in Table 3.
Inter-observer and intra-observer agreements
The inter- and intra-observer agreements for identifying PCVH were excellent (κ = 0.95 and κ = 0.91, respectively).
Diagnostic confidence about PCVH
Reader-reported confidence for determining the presence of PCVH was excellent in 104/130 cases (80%), moderate in 21/130 (16%) and poor in 5/130 cases (4%). PCVH were also seen despite significant motion artefacts, as shown in Fig. 4.
Visibility of PCVH despite significant motion artefacts. a Axial DWI b2,000 showing a left middle cerebral artery infarct (black asterisks). b Axial fast single shot FLAIR sequence showing no evidence of abnormality. Axial MIP of post-contrast 3D TSE T1-WI showing proximal (c) and distal (d) PCVH on the left middle cerebral artery (white arrows) suggestive of proximal occlusion, despite significant motion artefacts. e Axial SWI and f axial CE-MRA are more sensitive to motion artefacts and do not allow to diagnose an occlusion. g Coronal view of digital subtraction angiography (DSA) showing a left M1 occlusion (black arrow). h Coronal view of post-thrombectomy DSA showing complete recanalisation of the left middle cerebral artery (black arrowhead)
Discussion
In this retrospective study, we found a high prevalence of PCVH on 3-T MRI during acute ischaemic strokes and a strong association of PCVH with the presence and location of arterial occlusions and the severity of the stroke. PCVH might be useful for planning accurate therapeutic management, especially those requiring a MT given its strong association with vascular occlusion and its robustness even in cases of CE-MRA or TOF artefacts.
All the patients who had evidence of a proximal vascular occlusion on CE-MRA or 3D TOF had sharp PCVH closely surrounding the clot. Interestingly, 34 patients who had no evidence of vascular occlusion on CE-MRA or 3D TOF presented with PCVH exclusively located on the third portion of a middle cerebral artery: this might be related to a current small distal clot and suggests that the post-contrast 3D TSE T1-weighted sequence is more sensitive than CE-MRA or TOF sequences to show distal occlusions (8/34 had evidence of a distal thrombus on SWI); alternatively, it might be related to a recent clot that has undergone spontaneous thrombolysis (26/34 had no evidence of thrombus on SWI).
In recent years, MT has become the reference treatment for acute ischaemic stroke with proximal vascular occlusion. In combination with IVT in some patients with acute ischaemic strokes occurring in proximal anterior circulation, MT has proven to have a beneficial effect on morbidity, functional impact and quality of life at 90 days [2,3,4]. The indication for MT essentially relies on brain MRI findings [4, 9]. Initial MR assessment is intended to confirm the diagnosis of ischaemic stroke and to measure the core infarct, as there is no expected benefit from MT if the ischaemic core is larger than 70 ml [15]. It also excludes differential diagnoses like stroke mimics. Above all, MRI asserts the presence of proximal vascular occlusion (basilar trunk, internal carotid artery or proximal middle cerebral artery), which is mandatory to perform an MT. Currently, the diagnosis of proximal vascular occlusion relies on the TOF or CE-MRA sequences. According to the current guidelines, a prompt assessment of intra- and extra-cranial arteries using MR angiography is recommended to provide a vascular roadmap for an emergency endovascular treatment but also to detect a threatening arterial narrowing or an arterial occlusion which could necessitate an emergency endovascular or surgical treatment to avoid the risk of recurrence and to improve the patient’s outcome [5, 9]. Some neurovascular units provide CE-MRA findings as early as the initial MR assessment. It allows for a better analysis of intracranial vessels than the TOF sequence since it is less sensitive to vascular slow flow artefacts. It also allows a complete mapping of intra- and extra-cranial vessels, which provides data used by interventional neuroradiologists to plan and guide the MT.
In consultation with interventional neuroradiologists and vascular neurologists, we chose to systematically include a post-contrast 3D TSE T1-weighted sequence after the CE-MRA sequence because it strengthens diagnostic confidence in the presence of a vascular occlusion. It also allows to properly appreciate other known prognostic factors, like the precise location and the length of the thrombus [16, 17], which are essential to choose the appropriate technique and equipment for MT. It seems particularly interesting in case of discrepancy between TOF and CE-MRA, since no false-positive or false-negative PCVH have been depicted when a proximal occlusion is present. It is also very useful in case of blurred or non-interpretable TOF or CE-MRA sequence because it remains interpretable despite a high level of motion artefacts. During acute ischaemic strokes, “time is brain” and the whole MR should not exceed 15 min [9, 18]. Nevertheless, although our stroke protocol routinely includes a CE-MRA and a post-contrast 3D TSE T1-weighted sequence, its overall duration remains short, around 9 min.
Patients with PCVH had a higher NIHSS at diagnosis and after 24 h, as well as a higher mRS. This suggests that PCVH might be of potential prognostic significance. The presence of PCVH was also associated with larger core infarcts on DWI, evidence of thrombus on SWI and vascular occlusions on CE-MRA, findings that are known as independent factors of poorer outcomes [12, 15]. However, as an exploratory endpoint and given the retrospective nature of the study, we are not allowed to conclude that the presence of PCVH is an independent prognostic factor of patient’s outcome. Dedicated prospective studies might be interesting to assess the PCVH prognostic value.PCVH detection was strong and robust, with a high rate of diagnostic confidence in 80% of all cases as well as excellent inter- and intra-observer agreements, even between junior and senior readers. In a few cases, an incomplete suppression of the vascular signal of normal vessels was responsible for lower diagnostic confidence, but most of the time this phenomenon was clearly identified and did not prevent a correct delineation of PCVH. This problem may be solved by using a post-contrast 3D TSE T1-weighted sequence prepared by a motion sensitised driven equilibrium (MSDE) technique, which increases the suppression of the signal arising from normal structures and increases the contrast of abnormal vessels [19].
PCVH seemed to appear early in strokes, as observed in one of our patients where an MRI performed 1 h after the onset of a right motor deficit and aphasia showed PCVH in the left middle cerebral artery territory, despite normal DWI findings. A subsequent arteriography confirmed a proximal occlusion, which was treated by MT, and a follow-up MRI showed a positivity of the DWI 24 h later in the same territory where PCVH were present.
The pathophysiological meaning of PCVH remains unclear. Considering their vascular distribution, it could represent opened slow or retrograde flow. In our study, PCVH were visible on the feeding artery immediately upstream and downstream from the thrombus, but also in the distal vascular branches and collateral vessels, which support a haemodynamic mechanism. We previously included a non-enhanced 3D T1 TSE-weighted sequence in a few patients with proximal vascular occlusion and found that none of them had hyperintense vascular signal in the ischaemic territory (unpublished data), confirming that PCVH are not spontaneously hyperintense vessels. PCVH were significantly more frequent than FVH, although FVH are supposed to be consistent with slow or retrograde flow in collaterals. We may have expected to find a better correlation between these two findings. It is not a technical issue because although 3D sequences were previously reported to prevent the visualisation of FVH, we observed FVH in 54% of our patients, which is consistent with the literature focusing on 2D-FLAIR sequences [11]. Our results suggest that PCVH could be more sensitive than FVH, since only 66% of the patients with PCVH had FVH and only one patient had FVH without PCVH. PCVH were also significantly more depicted when there was a DWI-FVH mismatch, which has been previously associated with perfusion-diffusion mismatch.
Our study has several limitations. First, it is a retrospective study, with potential bias, despite the fact that we analysed a single-centre cohort and consecutive patients. All patients had the same routine protocol and were imaged on the same MRI machine, which decreased confusion of potential technical issues over multiple cases. Second, we had a few patients who were lost to follow-up and missing data about mRS at the 3-month follow-up visit, which precluded an accurate analysis of the correlation between the presence of PCVH and the middle-term outcome. It is not clear whether this sign can really serve as an outcome predictor. The most important outcome predictors in acute ischaemic stroke remain vessel occlusion, time to recanalisation, infarct size and individual collateral status. Further studies accounting for these outcome variables are required to determine the clinical consequences of PCVH. Third, we only performed 3D TSE T1-weighted imaging on a 3-T machine, thus limiting the generalisation of our results.
Conclusions
We found a high prevalence of PCVH and a strong association between PCVH and the presence and location of arterial occlusions in acute ischaemic stroke. We think it is important for both diagnostic and interventional neuroradiologists to be aware of this new sign, which may provide valuable information about vascular occlusion, particularly if there is a discrepancy between the TOF sequence and CE-MRA, or if there is a high degree of motion artefacts. We also found that PCVH might be linked to stroke severity and patient outcome. However, further studies are needed to confirm our results and prospectively assess its interest. On the one hand, it will be interesting to carry out prospective multicentre studies on large cohorts with different MR scanners to assess the reproducibility of this sign. On the other hand, it will be useful to conduct clinically oriented studies, preferably in a homogeneous and controlled population, to study the impact of PCVH on patient outcome.
Abbreviations
- FVH:
-
FLAIR Vascular Hyperintensities
- MSDE:
-
Motion Sensitised Driven Equilibrium
- MT:
-
Mechanical Thrombectomy
- NIHSS:
-
National Institutes of Health Stroke Scale
- PCVH:
-
Post-contrast Vascular Hyperintensities
References
Mukherjee D, Patil CG (2011) Epidemiology and the global burden of stroke. World Neurosurg 76:S85–S90
Nogueira RG, Jadhav AP, Haussen DC et al (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Jovin TG, Chamorro A, Cobo E et al (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372:2296–2306
Haute Autorité de Santé (2016) Thrombectomie des artères intracrâniennes par voie endovasculaire, France. https://www.has-sante.fr/portail/upload/docs/application/pdf/2016-11/rapport_thrombectomie.pdf. Accessed 12 Nov 2017
Powers WJ, Derdeyn CP, Biller J et al (2015) 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment. Stroke 46:3020–3035
European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee (2008) Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 25:457–507
Schellinger PD, Bryan RN, Caplan LR et al (2010) Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 75:177–185
Chalela JA, Kidwell CS, Nentwich LM et al (2007) Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369:293–298
Haute Autorité de Santé (2009) Accident vasculaire cérébral (AVC): prise en charge précoce, France. https://www.has-sante.fr/portail/upload/docs/application/pdf/2009-07/avc_prise_en_charge_precoce_-_recommandations.pdf. Accessed 12 Nov 2017
Schaefer PW, Pulli B, Copen WA et al (2015) Combining MRI with NIHSS thresholds to predict outcome in acute ischemic stroke: value for patient selection. AJNR Am J Neuroradiol 36:259–264
Hodel J, Leclerc X, Rodallec M et al (2013) Fluid-attenuated inversion recovery vascular hyperintensities are not visible using 3D CUBE FLAIR sequence. Eur Radiol 23:1963–1969
Smith WS, Tsao JW, Billings ME et al (2006) Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit Care 4:14–17
von Elm E, Altman DG, Egger M et al (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
R Core Team (2015) R: a language and environment for statistical computing. http://www.r-project.org/. Accessed 4 Nov 2017
Yoo AJ, Verduzco LA, Schaefer PW et al (2009) MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke 40:2046–2054
Friedrich B, Gawlitza M, Schob S et al (2015) Distance to thrombus in acute middle cerebral artery occlusion. Stroke 46:692–696
Campbell BCV (2016) Clot length assessment in stroke therapy decisions. Stroke 47:643–644
Oppenheim C, Naggara O, Arquizan C et al (2005) MRI of acute ischemic stroke. J Radiol 86:1069–1078
Yoneyama M, Nakamura M, Takahara T et al (2014) Improvement of T1 contrast in whole-brain black-blood imaging using motion-sensitized driven-equilibrium prepared 3D turbo spin echo (3D MSDE-TSE). Magn Reson Med Sci 13:61–65
Acknowledgments
Laura McMaster provided professional English-language medical editing of this article. Malek Ben Maacha provided valuable data about mechanical thrombectomy devices.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Julien Savatovsky.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Marie Astrid Metten kindly provided statistical advice for this manuscript.
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Duron, L., Savatovsky, J., Obadia, M. et al. Magnetic resonance post-contrast vascular hyperintensities at 3 T: a new highly sensitive sign of vascular occlusion in acute ischaemic stroke. Eur Radiol 28, 2903–2913 (2018). https://doi.org/10.1007/s00330-018-5312-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5312-7