Abstract
Objective
To measure myocardium iodine concentration (MIC) in patients with cardiac amyloidosis (CA) using dual-energy computed tomography (DECT).
Methods
Twenty-two patients with CA, 13 with non-amyloid hypertrophic cardiomyopathies (CH) and 10 control patients were explored with pre-contrast, arterial and 5-minute DECT acquisition (Iomeprol; 1.5 mL/kg). Inter-ventricular septum (IVS) thickness, blood pool iodine concentration (BPIC), MIC (mg/mL), iodine ratio and extra-cellular volume (ECV) were calculated.
Results
IVS thickness was significantly (p < 0.001) higher in CA (17 ± 4 mm) and CH (15 ± 3 mm) patients than in control patients (10 ± 1 mm). CA patients exhibited significantly (p < 0.001) higher 5-minute MIC [2.6 (2.3–3.1) mg/mL], 5-minute iodine ratio (0.88 ± 0.12) and ECV (0.56 ± 0.07) than CH [1.7 (1.4–2.2) mg/mL, 0.57 ± 0.07 and 0.36 ± 0.05, respectively] and control patients [1.9 (1.7–2.4) mg/mL, 0.58 ± 0.07 and 0.35 ± 0.04, respectively). CH and control patients exhibited similar values (p = 0.9). The area under the curve of 5-minute iodine ratio for the differential diagnosis of CA from CH patients was 0.99 (0.73–1.0; p = 0.001). With a threshold of 0.65, the sensitivity and specificity of 5-minute iodine ratio were 100% and 92%, respectively.
Conclusion
Five-minute MIC and iodine ratio were increased in CA patients and exhibited best diagnosis performance to diagnose CA in comparison to other parameters.
Key point
• Dual-energy computed tomography can be used to detect cardiac amyloidosis
• Five-minute myocardial iodine concentration and iodine ratio increase in cardiac amyloidosis
• Among iodine parameters, 5-minute iodine ratio has the best diagnosis performance
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic amyloidosis is a multisystem disease caused by the deposition of abnormal proteins within tissues and organs [1, 2]. Cardiac amyloidosis (CA) is relatively rare, but associated with a high rate of morbidity and mortality [3]. The most common imaging techniques used to non-invasively diagnose CA are transthoracic echocardiography, bone scintigraphy and magnetic resonance imaging (MRI) [1, 4,5,6,7]. Diagnostic accuracy of transthoracic echocardiography is relatively limited because of thickening of the cardiac wall that is less specific [8]. Bone scintigraphy exhibits the highest diagnostic performance for diagnosis of transthyretin amyloidosis (ATTR) with a sensitivity and specificity close to 100%, but its role is limited for diagnosis of light-chain amyloidosis (AL). Cardiac MRI can identify CA with a good specificity and sensitivity, frequently reported higher than 80% [2,3,4,5, 7]. MRI of CA typically reveals thickening of the cardiac wall and sub-endocardial or diffuse myocardial late gadolinium enhancement (LGE) due to an increase in extra-cellular volume (ECV) induced by amyloid protein deposit [3, 9, 10]. However, quantification of amyloid burden is challenging because of difficulty to detect a normal myocardial area [11, 12]. To overcome this problem, new quantitative MRI parameters such as measurement of myocardial T1 on a mapping sequence [10, 13, 14] and calculation of ECV [9, 10] have been proposed.
Cardiac multi-detector computed tomography (MDCT) has experienced rapid technological developments in recent years [15, 16]. Among them, dual-energy computed tomography (DECT) offers the possibility to non-invasively measure myocardium iodine concentration (MIC) after administration of iodine contrast [9, 17,18,19,20]. DECT offers some theoretical benefits to diagnose CA in comparison to MRI: MIC is a quantitative parameter that may reflect myocardial involvement, it provides high-resolution images and it is not limited by specific MR contra-indications or limitations such as pacemakers or implanted cardioverter defibrillators. A few studies have reported higher ECV [19, 21] and an increase in myocardial attenuation after iodine administration in CA [22]. We hypothesised that CA patients would exhibit greater myocardial uptake of iodine contrast medium because of the increased ECV. Therefore, we aimed to: 1) quantify MIC using DECT in patients with CA compared to patients with non-amyloid cardiac hypertrophy and control patients, and 2) evaluate the diagnostic accuracy of MIC measurement for CA diagnosis.
Materials and methods
The study was approved by our local research ethics committee. From January 2015 through March 2016, 25 consecutive patients with CA and 14 consecutive patients with non-amyloid hypertrophic cardiomyopathy (CH) addressed in our institution for cardiac CT were considered for this study. Exclusion criteria were difficulties to obtain cardiac synchronisation (n = 2) and severe renal insufficiency (creatinine > 150 μmol/L; n = 2). Thirty-five patients (22 patients with CA and 13 patients with CH) were finally included and explored with DECT. Each included patient provided written informed consent. Ten control patients referred in our centre for cardiac CT were also included and explored with DECT.
Population characteristics (Table 1)
Among the 22 CA patients included, 15 had transthyretin amyloidosis [12 with senile amyloidosis and 3 with familial transthyretin amyloidosis (ATTR)] and 7 had AL. All the ATTR and AL patients had histological confirmation of systemic amyloidosis with tissue sample. For the AL patients (n = 7), diagnosis of CA was based on a positive endomyocardial biopsy (EMB; n = 4; 57%) or typical pattern of CA on MRI defined as a left ventricular (LV) myocardium thickening (end-diastolic thickness of LV wall ≥12 mm) associated with a diffuse or circumferential enhancement of the LV myocardium on LGE sequences (n = 7; 100%). For the patients with senile amyloidosis (n = 12), diagnosis of CA was based on significant heart retention of 99mTc-HMDP bone tracer on scintigraphy (n = 12; 100%) [3,4,5, 7]. All patients with ATTR amyloidosis (n = 3) had a positive EMB that confirmed CA. Among the 22 patients with CA, 6 (27%) had an intra-cardiac device (ICD) and were not explored by cardiac MRI.
Among the 13 CH patients, 5 had sarcomeric hypertrophic cardiomyopathy (HCM), 3 had hypertrophy due to aortic stenosis, 3 had hypertensive hypertrophy and 2 had mixed aetiology (aortic stenosis plus hypertensive hypertrophy).
The 10 control patients had been referred for cardiac CT because of suspicion of thrombus within a left appendage (n = 8), suspicion of an intra-cardiac mass (n = 1) and suspicion of coronary stenosis (n = 1). All but two cardiac CTs were considered normal: these two patients exhibited a thrombus within the left appendage.
All CA and CH patients underwent echocardiography for measurement of global longitudinal strain (- %), diastolic inter-ventricular septum thickness (IVS; mm) and left ventricular ejection fraction (LVEF). NT-pro BNP level (ng/L), creatinine level (μmol/L), haematocrit and troponin (pg/mL) levels were also recorded. The same parameters were recorded for the control patients except for global longitudinal strain. All patient characteristics are reported in Table 1.
DECT protocol
All patients were scanned by 128-slice DECT (HD 750; General Electric, MI, USA). Gantry rotation time was 330 ms and a 64 × 1.25-mm-wide detector collimation was used. Prospective ECG triggering was used to reduce X-ray exposure. A gemstone spectral imaging (GSI) protocol was performed. This protocol uses a rapid (every 140 micro-seconds) kilo-voltage switching (80/140 kVp) and a specific gemstone detector to perform dual-energy acquisitions. Tube current was fixed to 400 mA, both for 80- and 140-kVp acquisitions.
The GSI protocol was performed three times for all patients and control subjects: before contrast medium injection, at the arterial phase and 5 minutes after iodine contrast administration [injection of 1.5 mL/kg of contrast medium (Iomeprol 400; Bracco Imaging, France) flushed by saline, injection rate was 4.0 mL/s]. To achieve adequate vascular arterial enhancement, bolus tracking was performed by placing a region of interest (ROI) in the aortic arch with a trigger threshold of 200 Hounsfield units (HU). A 50% adaptive statistical iterative reconstruction (ASIR) technology (GE; MI; USA) was used for image reconstruction. Dose–length product (DLP) was recorded for all patients. The effective radiation dose (mSv) was calculated by multiplying the DLP (mGy.cm) by the conversion factor for the thorax k (k = 0.014 mSv/mGy/cm).
Image analysis
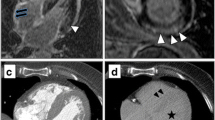
Images were analysed in consensus in a blinded manner on a dedicated platform (ADW 4.6; General Electric, MI, USA) by two readers (V.C. and J.F.D.) with 2 and 15 years of experience in cardiac imaging, respectively. One reader (V.C.) performed the measurements and was approved by the other. IVS thickness was measured on a multi-planar reconstruction performed in the mid-short axis plane of the left ventricle on arterial images. A volume of interest of at least 5 mL was manually defined in the middle part of the IVS from manual contouring of all contiguous axial slices of the arterial data set, and pasted on the unenhanced 5-minute images. Segmentation of unenhanced and 5-minute volumes was manually adjusted if the heart was not exactly at the same position. A volume of interest of at least 5 mL was also defined in the blood pool of the left ventricle on each data set. Mean attenuations (HU) of the IVS and blood pool were noted for each acquisition. The mean MIC (mg/mL) and the mean blood pool iodine concentration (BPIC; mg/mL) were calculated from each data set by using commercially dedicated software (GSI viewer; GE, MI, USA) that extracts iodine concentration (iodine-water as the basic substance; Fig. 1). The iodine concentration ratio was also calculated by dividing MIC by BPIC on arterial (arterial iodine ratio) and 5-minute (5-minute iodine ratio) acquisitions.
Examples of arterial (a, c) and 5-minute (b, d) DECT images obtained in one patient with CA (top row) and one patient with CH (bottom row). Myocardium iodine concentration (MIC) measured on arterial acquisition within the inter-ventricular septum (red line) was in the same range in the CA (2.76 mg/mL) and CH (3.28 mg/mL) patient. The CA patient exhibited a much higher MIC on delayed acquisition (3.44 mg/mL) than the CH patient (1.86 mg/mL). Note the lower contrast between blood pool and myocardium in the CA patient compared to the CH patient on delayed acquisition
Finally, ECV was calculated from haematocrit and attenuation values of the myocardium and blood pool using the following formula [9, 19, 21, 23]:
Statistical analysis
Continuous variables are expressed as median (inter-quartile), mean ± standard deviation or percentage ± standard deviation depending of normal or abnormal distribution. Because of the low number of patients, we used a nonparametric Kruskal–Wallis test to evaluate the significance of differences between the three groups. In case of significance, a Mann–Whitney U test with Bonferroni correction was used. Sub-group analysis between ATTR and AL amyloidosis was performed with a non-parametric Mann–Whitney U test. Associations between iodine parameters and clinical parameters were evaluated by calculating Pearson correlation coefficients. Receiver operating characteristic (ROC) analysis, with corresponding measures of statistical uncertainty (i.e., 95% confidence intervals), was applied to arterial MIC, 5-minute MIC, arterial iodine ratio, 5-minute iodine ratio and ECV to identify the cut-off values for CA diagnosis. Because the most relevant clinical question for the CA diagnosis was to exclude CA from other causes of left ventricular hypertrophy, the control group not considered for the ROC analysis. The cut-off values were determined using Youden’s test. Differences were considered significant if p < 0.05. Analyses were performed with SPSS 16.0 software (SPSS Inc., IBM, Chicago, IL, USA).
Results
Population characteristics
Patients with CA and CH were older and more likely to be male in comparison to control patients. They exhibited significantly higher levels of NT pro-BNP, troponin and creatinine and higher values of IVS thickness than the control subjects. LVEF and global longitudinal strain tended to be lower in CA than in CH patients, but there were no significant differences between these two groups. All data are reported in Table 1.
Radiation exposure
Mean DLP was in the same range (p = 0.9) for all patients: 567 ± 220, 568 ± 140 and 600 ± 170 mGy.cm for CA, CH and control patients, respectively. The effective radiation dose was 7.9 ± 3.1 mSv for CA, 8.0 ± 2.0 mSv for CH and 8.4 ± 2.4 mSv for control patients.
Comparison of DECT parameters between groups (Table 2)
Both CA and CH patients exhibited significantly (p < 0.001) higher values of IVS thickness (17 ± 4 and 15 ± 3 mm, respectively) than control subjects (10 ± 1 mm). Before injection, MIC and BPIC were in the same range in all three groups (Table 2). At the arterial phase, MIC did not exhibit significant differences between groups [3.3 (3.1–3.9), 3.3 (2.8–3.7) and 3.9 (3.3–5.0) mg/mL for CA, CH and control subjects, respectively], but BPIC was significantly (p = 0.01) higher in CA [19. 2 (16.2–22.4) mg/mL] than in CH patients [14.3 (11.0–16.9) mg/mL] and control subjects [15.6 (10.8–19.5) mg/mL]. The arterial iodine ratio was significantly (p < 0.001) lower in CA patients (0.18 ± 0.04) than in CH patients (0.24 ± 0.08) and control subjects (0.30 ± 0.06).
On the 5-minute acquisition, MIC was significantly higher (p < 0.001) in CA patients [2.6 (2.3–3.1) mg/mL] than CH [1.7 (1.4–2.2) mg/mL] and control subjects [1.9 (1.7–2.4) mg/mL]. Five-minute iodine ratio was also significantly higher (p < 0.001) in CA patients (0.88 ± 0.12) than CH (0.57 ± 0.07) and control subjects (0.58 ± 0.07). ECV exhibited similar differences: it was significantly higher (p < 0.001) in CA patients (0.56 ± 0.07) than CH (0.36 ± 0.05) and control subjects (0.35 ± 0.04). CH and control patients did not exhibit significant differences for 5-minute MIC, 5-minute iodine ratio and ECV. Five-minute BPIC was in the same range for the three groups. Examples of arterial and 5-minute DECT segmentations on images for a CA and CH patient are reported in Fig. 1.
ROC curve analysis (Fig. 2)
Receiver operating characteristic (ROC) curves to detect the optimal threshold of MIC on 5-minute acquisition. AUC values were 0.53, 0.72, 0.84 and 0.99, respectively, for arterial MIC (blue line), arterial iodine ratio (green line), 5-minute MIC (yellow line) and 5-minute iodine ratio (purple line)
Patients with CA formed the positive case group and CH patients were considered as negative cases. Control patients were excluded so as to reflect clinical practice. The areas under curve (AUCs) of arterial and 5-minute MIC for diagnosed CA patients as opposed to CH patients were 0.53 (95% CI: 0.32–0.75, p = 0.7), and 0.84 (95% CI: 0.72–0.99, p < 0.005), respectively. With a cut-off of 2.2 mg/mL, the sensitivity and specificity of 5-minute MIC to diagnose CA were 82% and 75%, respectively. Higher performances were noticed for iodine ratios: the AUCs of arterial and 5-minute iodine ratio were 0.72 (95% CI: 0.53–0.91, p < 0.05) and 0.99 (95% CI: 0.96–1.02, p < 0.001), respectively. With a threshold of 0.65, the sensitivity and specificity of 5-minute iodine ratio were 100% and 92%, respectively. For ECV, a value of 0.45 resulted in 100% discrimination between CA and CH patients.
Relationship between iodine parameters and clinical parameters
Weak, but significant, correlations were observed between 5-minute MIC, IVS thickness (r = 0.34; p = 0.02) and global longitudinal strain (r = -0.32; p = 0.03). The 5-minute iodine ratio was also correlated with IVS thickness (r = 0.42; p < 0.05), global longitudinal strain (r = -0.47; p < 0.05), troponin level (r = 0.34; p < 0.05) and NT-pro BNP level (r = 0.37; p = 0.02). In terms of blood pool measurements, arterial BPIC was negatively correlated with LVEF (r = -0.43, p = 0.004). Five-minute iodine ratio and ECV were highly correlated with each other (r = 0.92; p < 0.0001).
Sub-group analysis
ECV and 5-minute iodine ratio trended to be higher in patients with TTR (0.58 ± 0.07 and 0.90 ± 0.13, respectively for ECV and 5-minute iodine ratio) than in those with AL (0.52 ± 0.04 and 0.83 ± 0.08, respectively for ECV and 5-minute iodine ratio), but these differences did not reach statistical significance (p = 0.05 and 0.1, respectively for ECV and 5-minute iodine ratio).
Discussion
We report here that measurement of iodine concentration within the myocardium with DECT may be of interest to detect CA in patients with myocardial hypertrophy. The significant increase in 5-minute MIC, 5-minute iodine ratio and ECV that we observed in the CA patients is probably related to the abnormal deposit of amyloid proteins within the myocardium [23, 24]. Greater ECV facilitates passive diffusion of iodine contrast medium within the myocardium [25], as for gadolinium, and tends to increase local concentrations [9, 26]. Among the iodine parameters, the 5-minute iodine ratio emerged as being especially relevant for detecting CA with performances in the same range as ECV. It is relatively easy to calculate, can be obtained from a single delayed acquisition and does not require haematocrit value (unlike ECV). In addition, it was significantly correlated with biological and clinical parameters, suggesting that this parameter may be considered as a tracker of amyloid burden, a major marker of outcome in systemic amyloidosis [1, 12, 24, 26,27,28]. Further studies will be necessary to confirm this hypothesis that has not been evaluated in this study.
Differences in iodine parameters between CA, CH and control patients were more subtle at the arterial phase. CA patients exhibited a higher value of BPIC than CH and control patients probably because their relatively low LVEF slowed down the dilution of iodine within the left ventricular cavity. This hypothesis is reinforced by the negative relationship between BPIC and LVEF that we observed. CA (and CH) patients also tended to have lower values of MIC in comparison to control patients. This difference may be related to the slow diffusion of iodine contrast within the thickened myocardium, as recently suggested [16, 22]. Altogether, these contrasting variations in MIC and BPIC explain the lower value of the iodine ratio observed in CA patients compared to CH and control patients. Although significant, the lower arterial iodine ratio exhibited a lower diagnostic performance (AUC = 0.72) than the 5-minute iodine ratio (AUC = 0.99) to detect CA.
In the present work, we observed that ECV was of excellent diagnostic value for CA. Our results are in line with Treibel et al. [21] who reported a significant increase in ECV in 26 patients with CA using MDCT. More recently, Lee et al. [19] reported similar results using DECT in patients with different sorts of non-ischemic cardiomyopathies. Our sub-group analysis revealed that ECV trended to be higher in patients with ATTR than in those with AL. Interestingly, such a difference has previously been reported using cardiac MRI [29]. The authors hypothesise that this difference is related to the differential myocyte response to amyloid infiltration, with true hypertrophy and infiltration in TTR amyloidosis, whereas, in AL, the increase in ventricular wall thickness is caused by extracellular expansion alone. To the best of our knowledge, this difference between types of CA has never been reported using CT.
While DECT is not a first-line examination to diagnose CA, our results reinforce the fact that it may be considered as an alternative technique, especially in case of difficulties to perform cardiac MRI, for instance, for patients with claustrophobia or an ICD [5]. In the present study, a significant percentage of patients (27%) had an ICD. Other advantages can be mentioned: image acquisition is faster and DECT examination is cheaper than cardiac MRI. Quantitative parameters can also be extracted more easily from DECT images than from MRI in which signal intensity is not directly related to gadolinium concentrations. While it is true that DECT is less widely available than standard MDCT, it offers great opportunities in terms of material characterisation and detection of myocardial ischemia and will hopefully become more widespread.
Some limitations of our study deserve to be mentioned. First, the sample size is relatively small because of the scarcity of CA. Second, EMB was not systematically performed in our patients and diagnosis of CA was based on several diagnosis tests. Therefore, it was not possible to perform correlations with histological parameters and to evaluate precisely diagnosis performance of DECT in comparison to other tests. The lack of EMB reflects clinical practice in our centre: we do not systematically perform EMB because of potential sampling errors and risks pertaining to the procedure. In addition, some authors suggest that it should no longer be used as gold standard in CA [30]. Third, three DECT acquisitions were performed in our protocol, resulting in significant X-ray exposure. However, our results suggest that a single 5-minute acquisition may be sufficient to diagnose CA and that this would reduce X-ray exposure. Lastly, new MR parameters such as native T1 myocardial measurement with mapping sequences were not available, precluding correlations with iodine parameters.
Measurement of MIC is feasible using DECT in patients with CA and the 5-minute iodine ratio is of good diagnostic performance. DECT could represent an alternative diagnostic tool for patients with suspected CA, for instance in, case of contraindication to cardiac MRI.
Abbreviations
- BPIC:
-
Blood pool iodine concentration
- CA:
-
Cardiac amyloidosis
- CH:
-
Cardiac hypertrophy
- DECT:
-
Dual-energy computed tomography
- EMB:
-
Endo-myocardial biopsy
- ECV:
-
Extra-cellular volume
- GSI:
-
Gemstone spectral imaging
- HCM:
-
Hypertrophic cardiomyopathy
- IVS:
-
Inter-ventricular septum
- AL:
-
Light-chain amyloidosis
- MIC:
-
Myocardium iodine concentration
- ATTR:
-
Transthyretin amyloidosis
References
Gertz MA, Benson MD, Dyck PJ et al (2015) Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J Am Coll Cardiol 66:2451–2466
Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet Lond Engl 387:2641–2654
Quarta CC, Kruger JL, Falk RH (2012) Cardiac amyloidosis. Circulation 126:e178–e182
White JA, Fine NM (2016) Recent Advances in Cardiovascular Imaging Relevant to the Management of Patients with Suspected Cardiac Amyloidosis. Curr Cardiol Rep 18:77
Quintana-Quezada RA, Yusuf SW, Banchs J (2016) Use of Noninvasive Imaging in Cardiac Amyloidosis. Curr Treat Options Cardiovasc Med 18:46
Deux J-F, Damy T, Rahmouni A et al (2014) Noninvasive detection of cardiac involvement in patients with hereditary transthyretin associated amyloidosis using cardiac magnetic resonance imaging: a prospective study. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis 21:246–255
Gillmore JD, Maurer MS, Falk RH et al (2016) Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 133:2404–2412
Falk RH, Quarta CC (2015) Echocardiography in cardiac amyloidosis. Heart Fail Rev 20:125–131
Bandula S, White SK, Flett AS et al (2013) Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology 269:396–403
Kammerlander AA, Marzluf BA, Zotter-Tufaro C et al (2016) T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc Imaging 9:14–23
Banypersad SM, Sado DM, Flett AS et al (2013) Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging 6:34–39
Boynton SJ, Geske JB, Dispenzieri A et al (2016) LGE Provides Incremental Prognostic Information Over Serum Biomarkers in AL Cardiac Amyloidosis. JACC Cardiovasc Imaging 9:680–686
Neilan TG, Coelho-Filho OR, Shah RV et al (2013) Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging 6:672–683
Karamitsos TD, Piechnik SK, Banypersad SM et al (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6:488–497
den Harder AM, Willemink MJ, de Jong PA et al (2016) New horizons in cardiac CT. Clin Radiol 71:758–767
Langer C, Both M, Harders H et al (2015) Late enhanced computed tomography in Hypertrophic Cardiomyopathy enables accurate left-ventricular volumetry. Eur Radiol 25:575–584
Delgado Sánchez-Gracián C, Oca Pernas R, Trinidad López C et al (2016) Quantitative myocardial perfusion with stress dual-energy CT: iodine concentration differences between normal and ischemic or necrotic myocardium. Initial experience. Eur Radiol 26:3199–3207
Patino M, Prochowski A, Agrawal MD et al (2016) Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiogr Rev Publ Radiol Soc N Am Inc 36:1087–1105
Lee H-J, Im DJ, Youn J-C et al (2016) Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 280:49–57
Schwarz F, Ruzsics B, Schoepf UJ et al (2008) Dual-energy CT of the heart--principles and protocols. Eur J Radiol 68:423–433
Treibel TA, Bandula S, Fontana M et al (2015) Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr 9:585–592
Deux J-F, Mihalache C-I, Legou F et al (2015) Noninvasive detection of cardiac amyloidosis using delayed enhanced MDCT: a pilot study. Eur Radiol 25:2291–2297
Nacif MS, Kawel N, Lee JJ et al (2012) Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology 264:876–883
Kristen AV, Brokbals E, Aus dem Siepen F et al (2016) Cardiac Amyloid Load: A Prognostic and Predictive Biomarker in Patients With Light-Chain Amyloidosis. J Am Coll Cardiol 68:13–24
Nacif MS, Liu Y, Yao J et al (2013) 3D left ventricular extracellular volume fraction by low-radiation dose cardiac CT: assessment of interstitial myocardial fibrosis. J Cardiovasc Comput Tomogr 7:51–57
Wong TC, Piehler K, Meier CG et al (2012) Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 126:1206–1216
Fontana M (2016) Prognosis in Cardiac Amyloidosis by LGE: Ready for Prime Time? JACC Cardiovasc Imaging 9:687–689
Gallego-Delgado M, González-López E, Muñoz-Beamud F et al (2016) Extracellular volume detects amyloidotic cardiomyopathy and correlates with neurological impairment in transthyretin-familial amyloidosis. Rev Esp Cardiol (Engl Ed). doi:10.1016/j.rec.2016.02.027
Fontana M, Banypersad SM, Treibel TA et al (2015) Differential Myocyte Responses in Patients with Cardiac Transthyretin Amyloidosis and Light-Chain Amyloidosis: A Cardiac MR Imaging Study. Radiology 277:388–397
Fontana M, Pica S, Reant P et al (2016) Response to Letters Regarding Article, “Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis.”. Circulation 133:e450–e451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jean-François Deux.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise.
Ethical approval
Institutional review board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• prospective
• experimental
• performed at one institution
Rights and permissions
About this article
Cite this article
Chevance, V., Damy, T., Tacher, V. et al. Myocardial iodine concentration measurement using dual-energy computed tomography for the diagnosis of cardiac amyloidosis: a pilot study. Eur Radiol 28, 816–823 (2018). https://doi.org/10.1007/s00330-017-4984-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4984-8