Abstract
Background
We evaluated the diagnostic performance of 18F-NaF PET/MRI in patients with suspected cardiac amyloidosis (CA).
Methods
Twenty-seven consecutive patients underwent myocardial PET 1 hour after injection of 4 MBq/kg 18F-NaF with simultaneous MRI including cine-MRI, T1 and T2 mapping, first-pass and late gadolinium enhancement (LGE). 18F-NaF uptake was measured visually and semi-quantitatively by calculating myocardium-to-blood pool (M/B) ratios. CA was confirmed histologically.
Results
Transthyretin (TTR)-CA was diagnosed in 16 patients, light-chain (AL)-CA in 7, and no-CA in 4. Visual interpretation of 18F-NaF images revealed a relative increase in myocardial uptake in only 3 patients, all with TTR CA, and a relative decrease in 13, including 7 AL CA, 3 no-CA, and 3 TTR CA. M/B ratios were significantly higher in TTR CA (1.00 ± 0.12) than in AL CA (0.81 ± 0.06, P = 0.001) or in no-CA (0.73 ± 0.16, P = 0.006). The optimal M/B cut-off to distinguish TTR CA from AL CA was ≥ 0.90 (Fischer, P = 0.0005). By comparison, classification of patients using 99mTc-HMDP heart-to-mediastinum ratios with the previously published cut-off ≥ 1.21 reached higher significance (P < 0.0001). Among MRI parameters, myocardial T1, LGE score, and extracellular volume were higher in CA than in no-CA patients, 1409 ± 76 vs 1278 ± 35 ms (P = 0.004), 10.35 ± 5.30 vs 3.50 ± 3.42 (P = 0.03), and 46 ± 10 vs 33 ± 8 % (P = 0.01), respectively.
Conclusion
18F-NaF PET/MRI shows good diagnostic performance when semi-quantification is used. However, contrast is low and visual interpretation may be challenging in routine. PET/MRI could constitute a one-stop-shop evaluation of amyloid load and cardiac function in patients needing rapid work-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyloidosis is a set of diseases due to the deposition of insoluble fibril within the extracellular matrix of tissues and organs. To date, more than thirty-six responsible proteins are identified, but the most common types of amyloidosis are due to deposition of transthyretin (TTR) or light chains of immunoglobulins (AL).1 The diagnosis is histological, based on the identification of amyloid deposits by microscopy, with common features: a Congo Red stain, as well as a birefringence in polarized light.2 The prognosis is correlated with myocardial and renal involvements.
Transthyretin-related cardiac amyloidosis (TTR CA) is a systemic disease in which mutant (m-TTR) and/or wild-type (wt-TTR) proteins assemble into the myocardial interstitium. More than 100 different amyloidogenic mutations in the TTR gene have been described, and each is associated with clear multisystem involvement, including life-threatening heart failure. Endomyocardial biopsy (EMB) remains the gold standard for definitive diagnosis of TTR CA. Once amyloid deposits are found, additional testing with immunohistochemistry and/or sequence analysis by mass spectroscopy can identify the precursor protein. EMB may be difficult to perform in elderly patients as complications such as arrhythmia, perforation with pericardial effusion, accidental arterial puncture, and pneumothorax may occur in approximately 6% of patients.3
Imaging techniques may progressively surrogate EMB.4 MRI is useful to evaluate ventricular function, wall thickening, and myocardial perfusion.5 Myocardial scintigraphy using 99mTc labeled diphosphonates have demonstrated excellent diagnostic performance to identify TTR accumulation in the myocardium.6 Recently, Van Der Gucht et al. showed the first case report of potential diagnostic and technical values of 18F-sodium fluoride (18F-NaF) PET/CT in CA.7 Indeed, 18F-NaF PET/CT was able to differentiate TTR CA from AL CA, and was suggested to be more efficient as it demonstrates faster kinetics and then faster imaging time compared to conventional 99mTc-diphosphonates imaging. These preliminary data were later confirmed in a series of 5 TTR CA and 2 AL CA patients.8
Recent developments of hybrid PET/MRI systems make possible to evaluate simultaneously myocardial metabolism and ventricular function within a single exam. In a pilot study including 4 TTR CA and 3 AL CA patients, Trivieri et al. demonstrated this proof of concept.9 Therefore, we sought to evaluate the diagnostic performance of 18F-NaF PET/MRI in a larger cohort of patients with suspected CA, using visual and semi-quantitative image analysis.
Materials and Methods
Study Population
Between June 2017 and November 2018, 27 consecutive patients referred to our reference center (GRC Amyloid Research Institute) with strong clinical suspicion of CA underwent 18F-NaF PET/MRI imaging. Mean age was 70 ± 10 years and 19 were male. Patients with contra-indications for MRI were not included. All had a glomerular filtration rate ≥ 30 mL/min/1.73 m2 compatible with contrast agent injection. Data collection was approved by the Direction Interrégionale de la Recherche Clinique Ile-de-France (DC 2009-930) and by our local institutional review board, and all patients gave informed written consent.
PET/MRI Imaging
Simultaneous acquisition of chest 18F-NaF PET and cardiac MRI was performed on a Biograph mMR 3T scanner (Siemens Healthcare, Erlangen, Germany), starting 1 hour after injection of 4 MBq/kg of 18F-NaF. Emission images were recorded during 60 minutes on a single 26-cm height thoracic step, without gating. MRI sequences included (1) a T1 Dixon sequence during mid-exhalation breath-hold for attenuation correction purposes; (2) an unenhanced cine steady-state free-precession (SSFP) sequence, acquired in the long-axis 2/3/4-chamber view, followed by a short-axis view encompassing the left ventricle (LV), with retrospective 25-phase ECG gating for LV kinetics measurements; (3) a T1 mapping before and 15 minutes after 0.2 mmol/kg gadolinium injection (Dotarem, Guerbet, Aulnay-sous-Bois, France) in the short and long axes, within a single breath-hold, using the modified look-locker inversion recovery (MOLLI) sequence for tissue characterisation (fibrosis and amyloid load); (4) a T2 mapping using a prototype balanced SSFP sequence with an adiabatic T2 preparation, and acquired at the same conditions than T1 maps, for tissue characterization (edema);10 (5) a dedicated inversion recovery time scouting sequence to adjust the optimal time of inversion; and (6) late gadolinium enhancement (LGE) images covering the LV in the short and long axes were obtained 10 minutes after gadolinium injection using a segmented 3D inversion recovery gradient-echo T1-weighted sequence and followed by phase-sensitive inversion recovery (PSIR) images, because of suboptimal nulling of the myocardial signal may be encountered in CA.11 Detailed MRI acquisition parameters are summarized in Table 1.
PET Image Analysis
Emission images were reconstructed using an iterative 3D ordered subset expectation maximization algorithm with attenuation correction and point-spread function correction. Myocardial 18F-NaF uptake was measured by consensus of two nuclear physicians (MA & EI), qualitatively (presence or absence of myocardial 18F-NaF uptake), and semi-quantitatively by drawing a 2-dimensional anatomical region-of-interest (ROI) encompassing the left myocardium on the 4-cavity MRI image. Another ROI was drawn inside the left ventricle to allow calculation of the myocardium-to-blood pool (M/B) ratio (Figures 1 and 2). Additionally, a basal-to-apical ratio was calculated by drawing a circular ROI over a basal segment and the apex.
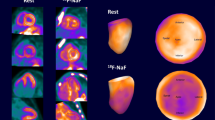
Example of a 81-year-old man with wt-TTR CA. Maximum intensity projection (A) shows faint 18F-NaF uptake over the myocardium (arrow). Axial PET (B), Dixon water MRI (C) and fusion (D) show a relatively homogeneous distribution of the radiotracer over the left myocardium (arrows). The M/B ratio is 1.13, while early-phase 99mTc-HMDP projection (E) shows much higher contrast with a H/M ratio calculated at 1.82. The average myocardial T1 calculated on the parametric map (F) is 1460 ms and PSIR image (G) shows diffuse late Gadolinium enhancement over the right and left myocardium with a QALE score of 14 (arrowheads)
Example of a 68-year-old man with AL CA. Maximum intensity projection (A) shows absence of 18F-NaF uptake over the myocardium (arrow). Axial PET (B), Dixon water MRI (C) and fusion (D) show a net decrease of myocardial uptake compared to the neighboring blood pool (arrows). The M/B ratio is 0.72 and the H/M ratio calculated on early-phase 99mTc-HMDP projection (E) is 0.86. The average myocardial T1 calculated on the parametric map (F) is 1450 ms and PSIR image (G) shows late Gadolinium enhancement predominating over the septum with a QALE score of 10 (arrowheads)
HMDP Image Analysis
Early-phase 99mTc-HMDP scintigraphy was also performed in 24 patients. On the planar thoracic projection, a squared ROI was drawn over the heart and another over the mediastinum, and heart-to-mediastinum (H/M) ratios were calculated, as previously described.12
MRI Image Analysis
MRI images were interpreted by consensus of two experienced radiologists (JFD & IS), blinded to clinical data, on a dedicated syngo.via platform (Siemens Healthcare, Erlangen, Germany). Left ventricular ejection fraction (LVEF), indexed end-diastolic volume (EDV), indexed end-systolic volume (ESV), and indexed myocardial mass were calculated on the cine short-axis images using a dedicated software (cvi42, Circle Cardiovascular Imaging Inc, Calgary, Canada). Maximal septal thickening was measured on the end-diastolic short-axis cine image acquired at the middle part of the left ventricle, T1 and T2 maps were obtained after applying a fast variational non-rigid registration algorithm to correct for residual cardiac and respiratory motion between images; then all T1- and T2-prepared frames were aligned to the center frame, and T1 and T2 maps were generated from these motion-corrected images by fitting a mono-exponential decay curve at each pixel. The mid-ventricular short-axis and the 4-chamber images were manually contoured to outline the endocardium and epicardium, and the average myocardial T1 and T2 values were calculated for all patients. LGE measurement was evaluated on the short-axis PSIR sequence at the base, mid-ventricle, and apical portions; the Query Amyloid Late Enhancement (QALE) score was calculated at each level on a 5-point scale (0, absent; 1, non-specific or non-circumferential subendocardial; 2, circumferential subendocardial; 3, non-circumferential transmural; and 4, circumferential transmural). then summed for a maximum of 12 points, to which 6 points were added if right ventricle LGE was also present, as previously reported.10,13 Finally, the extracellular volume fraction was calculated with the following formula14:
Diagnosis of Cardiac Amyloidosis
All patients underwent a physical examination, electrocardiogram, transthoracic echocardiography, and standard biological tests (including serum assays of ultrasensitive cardiac troponin T, N-terminal pro-brain natriuretic peptide [NTproBNP], and creatinine). Diagnosis of CA was confirmed by endomyocardial biopsy or by the association of clinical, biological and imaging abnormalities, together with peripheral tissue biopsy. Pathologic specimens were interpreted after proper staining by Congo red, anti-TTR, anti-κ, and anti-λ antibodies, as previously described.6
Statistical Analysis
Statistical analyses were performed with MedCalc 12.2.1.0 (MedCalc Software bvba, Ostend, Belgium). Continuous variables were compared between patient groups using the Mann-Whitney test and categorical variables using the Fischer exact test. A P value < 0.05 indicated statistical significance.
Results
Population Characteristics
Population characteristics are summarized in Table 2. TTR CA was diagnosed in 16 patients (7 wt-TTR, 9 m-TTR), AL CA in 7 patients and no-CA in 4 patients. There was a trend for older age in TTR than in AL CA patients. A carpal tunnel syndrome was significantly more frequent in TTR than in AL CA patients, and NTproBNP levels were significantly higher in TTR than in AL CA patients. The other clinical, electrocardiographic, echocardiographic, and biological parameters were not significantly different between patient groups.
PET Results
Visual interpretation of PET images revealed a relative increase in myocardial 18F-NaF uptake compared to neighboring blood pool in only 3 patients, all with TTR CA (3/16), and a relative decrease in myocardial uptake in 13 patients, including 7 with AL CA (7/7), 3 without CA (3/4), and 3 with TTR CA (3/16). Representative examples of a positive and a negative PET patient are shown in Figures 1 and 2. The remaining patients had myocardial uptake similar to the blood pool. Using semi-quantification, M/B ratios (Table 3, Figure 3) were significantly higher in TTR CA patients (1.00 ± 0.12) than in AL CA patients (0.81 ± 0.06. P = 0.001) or in patients without CA (0.73 ± 0.16. P = 0.006). M/B ratios were also significantly higher in CA patients put together than in no-CA patients (P = 0.02. Table 3). Among TTR CA patients, M/B ratio was slightly higher in wt-TTR (1.01 ± 0.13) than in m-TTR patients (0.98 ± 0.12), although not significantly. The optimal 18F-NaF M/B cut-off by ROC analysis to distinguish TTR CA from AL CA was ≥ 0.90 (area under the ROC curve, AUC = 0.937; sensitivity 81.2%, specificity 100.0%). Classification of patients between TTR and AL using this cut-off reached significance with P = 0.0005. By comparison, classification of patients using 99mTc-HMDP H/M ratio with the previously published cut-off ≥ 1.21 reached higher significance with P < 0.0001 (Figure 3). The mean basal-to-apical 18F-NaF ratio among patients above the 0.90 cut-off averaged 1.45±0.43, indicating relative apical sparing of bone tracer uptake.
MRI Results
Among MRI parameters, the T1 was significantly longer in CA patients, compared with no-CA patients, 1409 ± 76 vs 1278±35 ms, respectively (P = 0.004, Table 3). The optimal T1 cut-off by ROC analysis to distinguish CA from no-CA was ≥1315 ms (AUC = 0.962; sensitivity 91.3%, specificity 100.0%). Classification of patients between CA and no-CA using this cut-off reached significance with P = 0.0009. The LGE QALE score was also significantly higher in CA than in no-CA patients, 10.35 ± 5.30 vs 3.50 ± 3.42, respectively (P = 0.03). The optimal QALE cut-off by ROC analysis to distinguish CA from no-CA was ≥ 5 (AUC = 0.848; sensitivity 82.6%. specificity 75.0%) and classification of patients using this cut-off reached significance with P = 0.03. Finally, the extracellular volume fraction was significantly higher in CA than in no-CA patients, 46 ± 10 vs 33 ± 8 % (P = 0.01). The optimal cut-off by ROC analysis to distinguish CA from no-CA was ≥ 32 % (AUC = 0.897; sensitivity 95.5%. specificity 75.0%) and classification of patients using this cut-off reached significance with P = 0.005. The other MRI parameters did not differ significantly between CA and no-CA patients, including LVEF, myocardial mass, and septal thickening. There was no significant difference in MRI parameters between TTR CA and AL CA patients, except for the left ventricle ESV, which appeared significantly lower in AL CA than in TTR CA, 24 ± 9 vs 41±21 mL/m2, respectively (P = 0.03, Table 3).
Discussion
Our study shows that 18F-NaF PET is able to distinguish TTR from AL/no CA patients, with equivalent performance compared with 99mTc-HMDP, but only when M/B semi-quantification is used, as visual interpretation shows lower contrast. In addition, MRI can distinguish patients with CA from no-CA patient with a strong suspicion of CA, using the T1 mapping and LGE. Simultaneous PET/MRI imaging allows full cardiac assessment in a one-stop-shop setting.
The diagnostic value of 18F-NaF PET, based on the assumption that the type of amyloid protein affects local homeostasis of calcium, has been a matter of debate in the recent literature. Indeed, the first two case reports showed discrepant results.7,15 In the first one, the authors found moderate myocardial 18F-NaF uptake on PET/CT in a patient with mutant (Val122Ile) TTR CA, while PET/CT was negative in another patient with AL CA. To note, myocardial-to-background ratios showed a slow decrease between early (20 minutes post-injection) and delayed (1 hour post-injection) PET/CT images. In the second one, PET/CT did not show significant myocardial 18F-NaF uptake above background in two patients with TTR CA, one with an m-TTR (Ile68Leu) and one with a wt-TTR, on a 3-phase imaging (0, 15, and 60 minutes post-injection). However, no semi-quantification was provided; by reviewing the article figures, there is actually some 18F-NaF uptake, undistinguishable from the blood pool uptake, and therefore, we can assume that the M/B ratio would be around 1.00 in both patients, which is concordant with the mean M/B ratio found in the present study. In two recent series,8,9 including 7 CA patients each (5 TTR + 2 AL and 4 TTR + 3 AL, respectively), the authors confirmed the higher myocardial 18F-NaF uptake in TTR CA than in AL CA patients. In the first series, conducted with PET/CT 1 h post-injection, the mean SUV over the entire myocardium was 58% higher in TTR CA than in AL CA patients, and slightly higher in wt-TTR than in m-TTR patients. To note, the degree of uptake varied according to myocardial segment. In the second series, conducted with PET/MRI using a dynamic acquisition over 90 minutes starting 5 minutes post-injection, myocardial target-to-background ratios after 1 h were 48% higher in TTR CA than in AL CA patients, with a patchy pattern of uptake. In our series, M/B ratio was only 24% higher despite we measured it within the same delay post-injection.
Cardiac MRI has been a reference tool for the diagnosis and functional evaluation of cardiac amyloidosis over the last decade, as reported in many publications.16,17,–18 In our study, all patients underwent extensive cardiac MRI, including parametric imaging and semi-quantitative evaluation of late gadolinium enhancement. In line with previous reports, we found a significant increase of native myocardial T1 and extracellular volume fraction in patients with CA compared to those without CA, secondary to amyloid protein deposits in myocardial tissue. Several authors have also reported different MRI characteristics between TTR and AL CA patients, with a trend for higher QALE score in the former than in the latter,11 whereas native myocardial T1 was longer in AL than in TTR CA patients, probably because of myocardial edema.10 Unfortunately, we did not find such differences in our study.
Although semi-quantification of 18F-NaF uptake can reliably distinguish TTR CA from AL CA in our series, visual interpretation was much more challenging. Indeed, a higher myocardial uptake compared to background was seen in only 3/16 patients with TTR CA (the remaining patients having a myocardial uptake equivalent or slightly lower to the blood pool), while a relative decrease in myocardial uptake was observed in all (7/7) patients with AL CA. Contrast is then very different from what is observed with 99mTc-labeled diphosphonate imaging. Our study allows direct comparison between 18F-NaF and 99mTc-HMDP uptake in 24/27 patients: all TTR CA patients were strongly positive while all AL CA patients were negative on visual interpretation, without help of semi-quantification. Therefore, in view of performing PET/MRI one-stop-shop imaging, other 18F-labeled tracers should probably be preferred, such as markers of the amyloid plaque. Park et al. studied the specific binding of 18F-florbetapir to human myocardial AL and TTR amyloid deposits in 20 CA patients (10 TTR + 10 AL) and 10 non-amyloid controls on autopsy myocardial specimen by autoradiography.19 They demonstrated higher uptake of the radiotracer in AL CA patients than in TTR CA and controls. These findings were later confirmed in vivo by Law at al. using 18F-florbetaben PET/CT and measurement of the retention index in 10 CA patients (5 TTR + 5 AL) and 4 hypertensive controls.20 In addition, Wagner et al. showed the very high specificity of 18F-florbetapir for cardiac and extracardiac amyloid involvements in 17 CA patients (2 TTR + 15 AL) and suggested that this tracer may become the main tracer for the diagnosis of systemic amyloidosis in the future.21 Finally, the recent pilot study from Dietemann et al. in 9 patients (1 AL + 8 TTR) has shown high tumor-to-background ratios in CA patients compared to no-CA patients (n = 3) using 18F-flutemetamol, 1.46 vs 1.06, respectively (P = 0.03).22
Our study has some limitations. Despite a relatively large cohort of patients with CA (n = 23), the number of patients with non-amyloid cardiopathies is limited (n = 4), which makes it difficult to compare metabolic, morphological and functional parameters between CA and non-CA patients. Segmental assessment of 18F-NaF distribution within the myocardium was not possible because the contrast was too low to build polar maps; however, by simply drawing circular ROIs above a basal segment and the apex, we found a mean basal-to-apical ratio of 1.45 ± 0.43, indicating relative apical sparing of bone tracer uptake, as previously described using 99mTc-HMDP.23 There is also a recruitment bias as many candidates for 18F-NaF imaging (approximately 30% of patients in our experience) could not undergo MRI at 3 T due to the presence of a pacemaker or other metallic devices, which are not contra-indicated when using PET/CT. However, this technique could be useful for a one-stop-shop evaluation of bone tracer uptake, tissue characterization, and cardiac function in patients needing rapid diagnostic work-up.
Conclusion
18F-NaF PET/MRI shows comparable diagnostic performance as 99mTc-HMDP when M/B semi-quantification is used. However, contrast is much lower, and visual interpretation may not be able to distinguish TTR from AL/no CA in routine with enough reliability. This technique could be useful for a one-stop-shop evaluation of bone tracer uptake, tissue characterization, and cardiac function in patients needing rapid diagnostic work-up.
New Knowledge Gained
18F-NaF PET is able to distinguish patients with TTR cardiac amyloidosis from those with AL or without cardiac amyloidosis
Visual analysis of 18F-NaF uptake is challenging because of low contrast between myocardium and blood pool but semi-quantification demonstrates good performances
MRI with T1 mapping, late gadolinium enhancement, and calculation of the extracellular volume is able to distinguish patients with cardiac amyloidosis from those without cardiac amyloidosis
Simultaneous assessment of PET and MRI may be useful for a one-stop-shop evaluation of bone tracer uptake, tissue characterization, and cardiac function in patients needing rapid diagnosis
Abbreviations
- 18F-NaF:
-
18F-sodium fluoride
- PET/MRI:
-
Positron emission tomography/magnetic resonance imaging
- TTR:
-
Transthyretin
- AL:
-
Light-chain
- CA:
-
Cardiac amyloidosis
- 99mTc-HMDP:
-
99mTc-hydroxymethylene diphosphonate
- SUV:
-
Standardized uptake value
- LGE:
-
Late gadolinium enhancement
- NTproBNP:
-
N-terminal pro-brain natriuretic peptide
- ROC:
-
Receiver operating characteristics
References
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med 2003;349:583-96.
Mohty D, Damy T, Cosnay P, Echahidi N, Casset-Senon D, Virot P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis 2013;106:528-40.
Holzmann M, Nicko A, Kuhl U, Noutsias M, Poller W, Hoffmann W, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: A retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008;118:1722-8.
Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404-12.
Deux JF, Damy T, Rahmouni A, Mayer J, Plante-Bordeneuve V. Noninvasive detection of cardiac involvement in patients with hereditary transthyretin associated amyloidosis using cardiac magnetic resonance imaging: A prospective study. Amyloid 2014;21:246-55.
Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, et al. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid 2015;22:210-20.
Van Der Gucht A, Galat A, Rosso J, Guellich A, Garot J, Bodez D, et al. [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol 2016;23:846-9.
Morgenstern R, Yeh R, Castano A, Maurer MS, Bokhari S. (18)Fluorine sodium fluoride positron emission tomography, a potential biomarker of transthyretin cardiac amyloidosis. J Nucl Cardiol 2017;25:1559-67.
Trivieri MG, Dweck MR, Abgral R, Robson PM, Karakatsanis NA, Lala A, et al. (18)F-sodium fluoride PET/MR for the assessment of cardiac amyloidosis. J Am Coll Cardiol 2016;68:2712-4.
Ridouani F, Damy T, Tacher V, Derbel H, Legou F, Sifaoui I, et al. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J Cardiovasc Magn Reson 2018;20:58.
Dungu JN, Valencia O, Pinney JH, Gibbs SD, Rowczenio D, Gilbertson JA, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging 2014;7:133-42.
Galat A, Van der Gucht A, Guellich A, Bodez D, Cottereau AS, Guendouz S, et al. Early phase 99Tc-HMDP scintigraphy for the diagnosis and typing of cardiac amyloidosis. JACC Cardiovasc Imaging 2017;10:601-3.
Wan K, Sun J, Han Y, Liu H, Yang D, Li W, et al. Increased prognostic value of query amyloid late enhancement score in light-chain cardiac amyloidosis. Circ J 2018;82:739-46.
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75.
Gagliardi C, Tabacchi E, Bonfiglioli R, Diodato S, Nanni C, Guidalotti P, et al. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol 2016;24:746-9.
Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson 2008;10:54.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 2013;6:488-97.
Fontana M, Chung R, Hawkins PN, Moon JC. Cardiovascular magnetic resonance for amyloidosis. Heart Fail Rev 2015;20:133-44.
Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V, et al. 18F-florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: Autoradiography study. Circ Cardiovasc Imaging 2015;8:e002954.
Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-florbetaben PET: A pilot study. J Nucl Med 2016;57:1733-9.
Wagner T, Page J, Burniston M, Skillen A, Ross JC, Manwani R, et al. Extracardiac (18)F-florbetapir imaging in patients with systemic amyloidosis: More than hearts and minds. Eur J Nucl Med Mol Imaging 2018;45:1129-38.
Dietemann S, Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: A pilot study using (18)F-flutemetamol positron emission tomography. Ann Nucl Med 2019;33(8):624-8.
Van Der Gucht A, Cottereau AS, Abulizi M, Guellich A, Blanc-Durand P, Israel JM, et al. Apical sparing pattern of left ventricular myocardial (99m)Tc-HMDP uptake in patients with transthyretin cardiac amyloidosis. J Nucl Cardiol 2018;25:2072-9.
Disclosures
All authors have approved the manuscript, and agree with its submission to the Journal of Nuclear Cardiology. There is no financial or other relation that could lead to a conflict of interest.
Ethical approval
The procedure followed was in accordance with the ethical standards guidelines of the responsible committee on human experimentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abulizi, M., Sifaoui, I., Wuliya-Gariepy, M. et al. 18F-sodium fluoride PET/MRI myocardial imaging in patients with suspected cardiac amyloidosis. J. Nucl. Cardiol. 28, 1586–1595 (2021). https://doi.org/10.1007/s12350-019-01885-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01885-8