Abstract
Objectives
The aim of this study was to prospectively evaluate effective dose (E) of operators performing transjugular intrahepatic portosystemic shunts (TIPS) in a single centre. Patients’ radiation exposure was also collected.
Methods
Between 8/2015 and 6/2016, 45 consecutive TIPS were performed in adult patients using a flat-panel detector-based system (FPDS) and real-time ultrasound guidance (USG) for portal vein targeting. Electronic personal dosimeters were used to measure radiation doses to the primary and assistant operators, anaesthesia nurse and radiographer. Patients’ radiation exposure was measured with dose area product (DAP); fluoroscopy time (FT) was also collected.
Results
Mean E for the primary operator was 1.40 μSv (SD 2.68, median 0.42, range 0.12 – 12.18), for the assistant operator was 1.29 μSv (SD 1.79, median 0.40, range 0.10 – 4.89), for the anaesthesia nurse was 0.21 μSv (SD 0.67, median 0.10, range 0.03 – 3.99), for the radiographer was 0.42 μSv (SD 0.71, median 0.25, range 0.03 – 2.67). Mean patient DAP was 59.31 GyCm2 (SD 56.91, median 31.58, range 7.66 – 281.40); mean FT was 10.20 min (SD 7.40, median 10.40, range 3.8 – 31.8).
Conclusion
The use of FPDS and USG for portal vein targeting allows a reasonably low E to operators performing TIPS.
Key points
• The operators’ E vary according to the complexity of the procedure.
• FPDS and USG allow a reasonably low E to TIPS operators.
• FPDS and USG have an important role in reducing the occupational exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transjugular intrahepatic portosystemic shunt (TIPS) is considered as being one of the most radiation-intensive procedures in abdominal interventional radiology to patients and medical staff [1, 2]. Operators can receive relatively high doses, both from the primary radiation source and from scattered radiation.
The most technically challenging step of the procedure, and frequently the longest, is the puncture of the portal vein following hepatic vein access. Several approaches have been proposed to minimize the number of needle passes required to catheterize the intrahepatic portal venous system. Longo et al. [3] have described real-time sonographic guidance (USG) for portal vein targeting during TIPS creation in 1992. To date, this technique has not gained global acceptance despite it being relatively simple. USG targeting of the portal venous system during TIPS creation resulted in a significantly lower patient radiation exposure when compared to fluoroscopic-guided portal vein targeting in a recent study encompassing a large patient cohort [4]. Overall, patient radiation exposure was significantly below the results reported in the RAD-IR study, the largest series available at the time the manuscript was published [1], and below the proposed reference level [5]. Any technique that reduces the patient dose area product (DAP) will also reduce scatter levels and operator exposure [6, 7].
The aim of this study was to prospectively evaluate the effective dose (E) of the operators during TIPS creation in a single centre using a FPDS and USG targeting of the portal venous system.
Methods
Radiation doses to operators performing TIPS in our centre from August 2015 to June 2016 were prospectively collected. For this type of study, our institutional review board waived formal consent; however, an informed consent specific to TIPS was obtained in all cases.
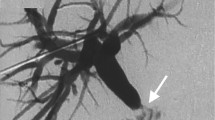
TIPS were performed in FPDS angiographic suite (Innova 4100, General Electric Medical Systems, USA). All procedures were carried out, as primary operator, by 2 faculty level radiologists with 15 and 12 years of experience in abdominal interventional radiology at the time of writing of this manuscript. All procedures were performed under general anaesthesia, using the ring transjugular intrahepatic access set (Cook Medical, Bloomington, IN, USA) with USG for portal vein targeting as previously described (4) using the 3.5-MHz curvilinear transducer of a GE Logiq E9 ultrasound machine (GE Healthcare, Milwaukee, WI; Fig. 1). An e-PTFE-covered stent (Viatorr®; W.L.GORE & Associates, Inc. Flagstaff, AZ, USA) was deployed and ballooned in all cases. Variceal embolization was performed in one case after TIPS creation, using coils.
Two sterile fields are prepared, one over the right jugular area, the other over the right anterolateral abdominal wall, with two corresponding windows. The abdominal sterile area is used during real-time sonographic visualisation of the Colapinto needle, and in guiding transit of the needle from the hepatic vein to the intra-hepatic portal vein branch. The ultrasound probe is covered with a sterile probe cover and manoeuvred by the primary operator who remains at the head of the patient. Of note are the nearly equal distances to the patient of primary and assistant operators
The medical and paramedical staff involved in the procedure always wore protective aprons with thyroid shields (0.5 mm lead equivalent) and protective glasses with side protection (0.75 mm lead equivalent). Low-frame rate pulsed fluoroscopy (at 7.5 frames/sec) was routinely employed. A high frame rate (up to 15–30 frames/sec) and/or magnification were used for a brief period in 10 cases. A low-dose fluoroscopy protocol was used in all cases and optimized with the automatic exposure control system, designed to automatically determine the optimal parameters such as kV (range 61 – 84), mA (range 3.6 – 10) and spectral filtration (range 0.1 – 0.3 mmCu).
Electronic personal dosimeters (EPD MK2.3, Thermo Fisher Scientific, Benham, UK), able to respond to photon energies down to 15 KeV, with a valid calibration and quality control certificate according to ISO/IEC guide 98 and to EA-4/02 standards requirements, were used to measure radiation dose to operators during TIPS creation. The dosimeters were placed outside the lead apron at the left upper chest position of the primary and assistant operators, anaesthesia nurse, and radiographer. The Hp(10), the personal dose equivalent at a depth of 10 mm of tissue, registered by the detectors at the end of every procedure was systemically recorded. Patients' radiation exposure was measured through the DAP, measured with a dual-channel DIAMENTOR M4-KDK DAP/dose meter transmission ion chamber (PTW, Freiburg, Germany) fixed to the collimator with a valid calibration and quality control certificate. DAP (or Kerma-area-product) was considered as a surrogate measurement of the entire amount of energy delivered to the patient by the radiation beam during the procedure, as recommended by the International Commission on Radiation Units [8]. Fluoroscopy time (FT) was also recorded and considered as surrogate marker of procedural complexity.
Operators’ E was determined using a modified Niklason algorithm (E = 0.03∙Hp(10)) [9, 10] and given in μSv.
The dosimetric data was inputted in an Excel 2007 spreadsheet (Microsoft, Richmond, WA, USA). Operators’ E and patients’ DAP- and FT-related data was analyzed for each group and expressed as the mean value ± standard deviation (SD), median and range. The differences in E within the two primary operators was analyzed using two-tailed t tests with statistical results considered significant if p values of less than 0.05 were reached.
Results
Forty-five consecutive TIPS creations were performed during the study period. All procedures were done in adult patients with complications of portal hypertension in the radiology unit of a single transplant centre (male 25, age 57 ± 11.1 y/o, and weight 70.1 ± 10.3 kg). Indications for TIPS creations were refractory ascites (n = 29), prophylaxis of variceal rebleeding (n = 15) and acute variceal bleeding (n = 1).
One radiologist performed 27 TIPS creations as the primary operator; the mean E was 1.65 μSv (SD 3.65, median 0.42, range 0.12 – 12.18). The second radiologist performed 18 TIPS creations as the primary operator; the mean E was 1.20 μSv (SD 0.50, median 0.75, range 0.33 – 2.90). Mean E was not statistically different between the two primary operators (p = 0.45). Overall, considering all 45 procedures, mean E for the primary operator was 1.40 μSv (SD 2.68, median 0.42, range 0.12 – 12.18).
Mean E for the assistant operator, a radiology resident, was 1.29 μSv (SD 1.79, median 0.40, range 0.10 – 4.89).
Mean E for the anaesthesia nurse was 0.31 μSv (SD 0.67, median 0.10, range 0.03 – 3.99), and mean E for the radiographer was 0.42 μSv (SD 0.71, median 0.12, range 0.03 – 2.67) (Table 1).
Mean patient DAP was 59.31 GyCm2 (SD 56.91, median 31.58, range 7.66 – 281.40) and mean FT was 10.20 min (SD 7.40, median 10.40, range 3.8 – 31.8).
Discussion
Although the magnitude of radiation exposure to the operator performing interventional radiology procedures is much smaller than that of the patients, the cumulative dose resulting in the span of a whole career might be substantial. Some clinical reports have suggested an increased risk of radiation-related cataracts in physicians who use fluoroscopy [11]. Recently, it has been shown that the original data used to infer the linear no-threshold model for genetic effects and carcinogenesis from low-dose ionizing radiation were either incorrect or based on extrapolations that proved inaccurate or inconclusive [12]; however, a systematic assessment of skin and brain (cancer and non-cancer) effects of chronic low-dose radiation exposure in operators performing fluoroscopy-guided procedures has been recently recommended [13–16].
Few reports are available on occupational doses in non-cardiac interventional procedures. The operator E in TIPS creation is known to be among the highest in non-cardiac fluoroscopic guided procedures, and ranges from 2.5 – 74 (median = 17) μSv per case [2]. The currently available data is based on few studies [7, 17–19]. These studies performed TIPS using different angiographic equipment, used different radiological techniques for portal vein targeting, and report a relatively long TIPS fluoroscopy time (range 32–78, median 59 minutes).
Our study showed a reasonably low radiation exposure to the medical and paramedical staff involved in the procedure. As expected, the operator radiation dose in our cohort of procedures varied according to the complexity of the procedure. Of note, the mean E values of the primary and assistant operators were very similar due to a nearly equal distance to the patients of both the operators. Although it is difficult to compare occupational dosimetric results among studies, overall results are inferior to what was previously reported [2] (median E per procedure for the primary operator 0.42 μSv vs. 17 μSv). In our opinion, this result is due to the reduced length of the procedure secondary to the use of ultrasound guidance for portal vein targeting (median FT 10.40 minutes vs. 59 minutes). Overall, reduced DAP to the patient (median 31.58 GyCm2 vs. 230 GyCm2) is probably lower secondary to both ultrasound guidance and to the use of low-dose fluoroscopy protocols available in the FPDS.
The number of procedures analyzed in our study is relatively low. This is a potential limitation; however, the majority of the TIPS-related dosimetric studies [7, 17–19] were performed with similar cohorts. We do not have a direct comparison of staff radiation exposure in a control group of TIPS performed by the same staff without US guidance for portal vein targeting. All procedures were performed by two faculty level radiologists with several years of experience in TIPS creation, with potential impact on fluoroscopy time. Another limitation is that calculation of effective dose would have been more accurate with the addition of a second dosimeter under the lead apron.
In conclusion, the use of an FPDS angiographic suite using dedicated low-dose protocols and USG to target the portal venous system allow a reasonably low radiation exposure to operators performing TIPS. This could have an important role in maintaining the occupational exposure to the eyes of the operators below the threshold of 20 mSv/y as recently recommended [20]. Further studies should investigate if reasonably low radiation exposure to the medical and paramedical staff can be achieved also with new techniques recently described for portal vein targeting such as C-arm CT-targeted puncture of the portal vein in patients with a patent portal vein [21], or percutaneous transhepatic balloon-assisted TIPS in patients with portal vein thrombosis [22].
Abbreviations
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- DAP:
-
Dose area product
- FT:
-
Fluoroscopy time
- E:
-
Effective dose
- FPDS:
-
Flat-panel detector based system
- USG:
-
Real-time ultrasound guidance
References
Miller DL, Balter S, Cole PE et al (2003) Radiation doses in interventional radiology procedures: the RAD-IR study: part I: overall measures of dose. J Vasc Interv Radiol 14(6):711–727
Kim KP, Miller DL, Berrington de Gonzalez A et al (2012) Occupational radiation doses to operators performing fluoroscopically-guided procedures. Health Phys 103(1):80–99
Longo JM, Bilbao JI, Rousseau HP et al (1992) Color Doppler-US guidance in transjugular placement of intrahepatic portosystemic shunts. Radiology 184:281–284
Miraglia R, Maruzzelli L, Cortis K et al (2015) Radiation Exposure in Transjugular Intrahepatic Portosystemic Shunt Creation. Cardiovasc Intervent Radiol
Miller DL, Kwon D, Bonavia GH (2009) Reference levels for patient radiation doses in interventional radiology: proposed initial values for U.S. practice. Radiology 253(3):753–764
Williams JR (1997) The interdependence of staff and patient doses in interventional radiology. Br J Radiol 70:498–503
Boone JM, Levin DC (1991) Radiation exposure to angiographers under different fluoroscopic imaging conditions. Radiology 180:861–865
Wambersie A (2005) International Commission on Radiological Units and Measurements. Patient dosimetry for X-rays used in medical imaging. ICRU Report 74. J ICRU 5(2):iv–vi
Padovani R, Rodella CA (2001) Staff dosimetry in interventional cardiology. Radiat Prot Dosimetry 94:99–103
Niklason LT, Marx MV, Chan HP (1994) The estimation of occupational effective dose in diagnostic radiology with two dosimeters. Health Phys 67:611–615
Vano E, Gonzalez L, Fernández JM, Haskal ZJ (2008) Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology 248:945–953
Siegel JA, Pennington CW, Sacks B, Welsh JS. (2015) The Birth of the Illegitimate Linear No-Threshold Model: An Invalid Paradigm for Estimating Risk Following Low-dose Radiation Exposure. Am J Clin Oncol
Picano E, Vano E, Domenici L, Bottai M, Thierry-Chef I (2012) Cancer and non-cancer brain and eye effects of chronic low-dose ionizing radiation exposure. BMC Cancer 12:157
Eagan JT Jr, Jones CT (2010) Cutaneous cancers in an interventional cardiologist: a cautionary tale. J Interv Cardiol 24:49–55
Finkelstein MM (1998) Is brain cancer an occupational disease of cardiologists? Canadian J Cardiol 14:1385–1388
Matanoski GM, Seltser R, Sartwell PE et al (1975) The current mortality rates of radiologists and other physician specialists: specific causes of death. Am J Epidemiol 101:199–210
Hidajat N, Wust P, Kreuschner M et al (2006) Radiation risks for the radiologist performing transjugular intrahepatic portosystemic shunt (TIPS). Br J Radiol 79(942):483–486
Martin CJ, Whitby M (2003) Application of ALARP to extremity doses for hospital workers. J Radiol Prot 23:405–421
Zweers D, Geleijns J, Aarts NJM et al (1998) Patient and staff radiation dose in fluoroscopy-guided TIPS procedures and dose reduction, using dedicated fluoroscopy exposure settings. Br J Radiol 71:672–676
International Commission on Radiological Protection (2012) ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs—threshold doses for tissue reactions in a radiation protection context. ICRP Publication 118. Ann. ICRP 41(1–2). Elsevier
Ketelsen D, Groezinger G, Maurer M et al. (2016) Three-dimensional C-arm CT-guided transjugular intrahepatic portosystemic shunt placement: Feasibility, technical success and procedural time. Eur Radiol
Chen Y, Ye P, Li Y et al (2015) Percutaneous transhepatic balloon-assisted transjugular intrahepatic portosystemic shunt for chronic, totally occluded, portal vein thrombosis with symptomatic portal hypertension: procedure technique, safety, andclinical applications. Eur Radiol 25(12):3431–7
Acknowledgements
The scientific guarantor of this publication is Dr. Angelo Luca. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: prospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miraglia, R., Gerasia, R., Maruzzelli, L. et al. Radiation doses to operators performing transjugular intrahepatic portosystemic shunt using a flat-panel detector-based system and ultrasound guidance for portal vein targeting. Eur Radiol 27, 1783–1786 (2017). https://doi.org/10.1007/s00330-016-4558-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4558-1