Abstract
Objectives
CTP images analyzed with the Alberta stroke program early CT scale (ASPECTS) have been shown to be optimal predictors of clinical outcome. In this study we compared two biomarkers, the cerebral blood volume (CBV)-ASPECTS and the CTA-ASPECTS as predictors of clinical outcome after thrombectomy.
Methods
Stroke patients with thrombosis of the M1 segment of the middle cerebral artery were included in our study. All patients underwent initial multimodal CT with CTP and CTA on a modern CT scanner. Treatment consisted of full dose intravenous tissue plasminogen activator, when applicable, and mechanical thrombectomy. Three neuroradiologists separately scored CTP and CTA images with the ASPECTS score.
Results
Sixty-five patients were included. Median baseline CBV-ASPECTS and CTA-ASPECTS for patients with favourable clinical outcome at follow-up were 8 [interquartile range (IQR) 8-9 and 7-9 respectively]. Patients with poor clinical outcome showed a median baseline CBV-ASPECTS of 6 (IQR 5-8, P < 0.0001) and a median baseline CTA-ASPECTS of 7 (IQR 7-8, P = 0.18). Using CBV-ASPECTS and CTA-ASPECTS raters predicted futile reperfusions in 96 % and 56 % of the cases, respectively.
Conclusions
CBV-ASPECTS is a significant predictor of clinical outcome in patients with acute ischemic stroke treated with mechanical thrombectomy.
Key Points
• CBV-ASPECTS is a significant predictor of clinical outcome.
• Single phase CTA-ASPECTS has low predictive value.
• Using CBV-ASPECTS, raters identified futile reperfusions in 96 % of the cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patient selection is essential for the improvement of clinical outcome in treating stroke patients with mechanical thrombectomy [1, 2]. Until publication of the latest randomized trials, studies had shown the feasibility of mechanical thrombectomy in restoring perfusion distal to the initial occlusion site, but had failed to report a substantial increase in favourable clinical outcome [3, 4]. Even in the latest randomized trial, which proved the superiority of combined mechanical thrombectomy and intravenous thrombolysis versus intravenous thrombolysis alone, functional independence was observed in only 32.6 % of treated patients after 90 days [5]. In a recent study, it was suggested that cerebral blood volume (CBV) maps, evaluated with the Alberta stroke program early CT score (ASPECTS), are strong predictors of outcome [6]. Hence, selection of patients based on CTP can reduce futile reperfusions. In this study we compared the CBV-ASPECTS with the CTA-ASPECTS as final outcome predictors in a highly select group of stroke patients treated with mechanical thrombectomy. We additionally introduced a novel scoring system combining neurological and CTP-findings as well as age to further improve the selection process.
Materials and methods
Patients were retrospectively identified from our university hospital’s institutional review board-approved stroke database according to the following inclusion criteria:
-
1.
Acquisition of a complete multi-modal CT examination between January of 2011 and December of 2014 including noncontrast computed tomography (NCCT) of the head, near whole-brain CTP, and single phase CTA of the head and neck.
-
2.
Presence of M1 occlusion on initial angiogram, as defined by modified thrombolysis in cerebral infarction score (mTICI) of 0 or 1, a scale used to grade reperfusion of distal tissue bed [7]. Carotid bifurcation occlusions were excluded from our study.
-
3.
Successful reperfusion of the middle cerebral artery as defined by mTICI 2b or 3 on the final angiogram after mechanical thrombectomy.
Exclusion criteria were incomplete CTP datasets in our department’s picture archiving system, datasets with severe motion artefacts, patient age <21 years and reperfusion of the middle cerebral artery on initial angiograms.
Stroke imaging was acquired with a 128-slice multidetector CT scanner (Siemens Definition AS+; Siemens Healthcare Sector, Forchheim, Germany) and included a NCCT, followed by near whole-brain CTP and single phase CTA of extra- and intracranial arteries. CTP consisted of 30 consecutive spiral scans of the brain (96 mm z-axis coverage, 2 s delay after start of contrast agent injection, 45 s total acquisition time, 80 kV, 200mAs and effective dose of ~5 mSv). Thirty-six mL of contrast agent were injected with a rate of 6 mL/s through a cubital vein, followed by 30 mL of saline chaser. CTP data were reconstructed with a slice thickness of 5 mm every 3 mm (H20f Kernel, 512 matrix) and were further processed by a neuroradiologist using a commercial analysis package (Volume Perfusion CT Neuro; Siemens) with a delay-invariant deconvolution method, automatic motion correction and a dedicated noise reduction technique for dynamic data. For CTA (120 kV, 120 reference mAs, 0.3 s rotation time, 0.6 pitch, 2 × 64 × 0.6 mm collimation, ~3 mSv) 45 mL of contrast agent were injected, followed by 30 mL of saline chaser. CTA was acquired with a single phase protocol using bolus triggering in the aortic arch (100 HU threshold; 5 s delay for bolus watch, 3 s delay after reaching threshold). CTA data were then reconstructed to multiplanar images with a section width of 5 mm and increment of 3 mm.
According to protocol, patients presenting within 4.5 hours of symptom onset with no significant infarction or haemorrhage detected on NCCT were eligible for intravenous recombinant tissue plasminogen activator (IV rtPA). IV rtPA (0.9 mg/kg over 60 min) was then administered directly after noncontrast CT. Following stroke imaging, patients were transferred to the angiography suite (Artis Zee, Siemens Healthcare, Forchheim, Germany) where they underwent mechanical thrombectomy either with a Penumbra MAX™ reperfusion catheter (Penumbra, Alameda, California) or with a combination of aspiration with reperfusion catheters and retrievable stents (Aperio, Acandis, Pforzheim, Germany; Trevo, Stryker, Mountain View, California; 3D Separator, Penumbra). In cases of rapid transport to angiography and fast mechanical thrombectomy the remaining rtPA was administred intraarterially either before or after recanalization at the discretion of the interventional neuroradiologist. Appropriate anaesthesia (intravenous conscious sedation or general anaesthesia) was obtained as per standard practice.
All data used in this study (demographic data, vascular risk factors, mTICI scores, various times, and neurological scores) were extracted from the aforementioned database. Neurological scores (National Institutes of Health Stroke Scale (NIHSS), modified Rankin scale (mRS), etc.) were assessed by a certified stroke neurologist at hospital admission, hospital discharge, and discharge from the rehabilitation unit. Interventional and stroke imaging scores were assigned in consensus by three neuroradiologists (two with more than 5 years of experience). The neuroradiologists separately rated NCCT, CTP, and CTA scans with the ASPECTS, a 10-point scoring system of the middle cerebral artery (MCA) territory. For every MCA region with acute ischemic signs, 1 point is subtracted from 10, resulting in an ASPECTS of 10 for a scan without ischemic lesions and an ASPECTS of 0 for complete MCA-infarctions (www.aspectsinstroke.com, Fig. 1).
We also calculated a new score, the Goettinger stroke scale (GSS) according to the following formula: CBV-ASPECTS – (NIHSS/4 + age/20).
The introduction of this novel score was based on the observation that each of the parameters admission NIHSS, age, and CBV-ASPECTS already predicted outcome fairly well on its own. A combination of all three should have the potential to further improve predictability.
Scans of every patient were divided into two groups, which were rated with a 90-day gap in between: At first NCCT scans were rated simultaneously with CTA images; the second group consisted of coloured CTP parameter maps including cerebral blood flow (CBF), CBV, mean transit time (MTT), and time to drain (TTD) maps. The latter is a very sensitive parameter depicting the total extent of perfusion lesions [8]. Additionally, raters were retrospectively asked to predict futile reperfusions based either on NCCT, CTA or on CTP images. NCCT/CTA-ASPECTS, or CBV-ASPECTS lower than 7 was the primary criterion for futile reperfusions in the groups. Cases of disagreement were settled by consensus. Raters were blinded to all clinical outcomes.
Study parameters were compared between patients with follow-up mRS >2 (poor outcome) and with follow-up mRS ≤2 (favorable outcome). In cases with missing follow-up mRS values, discharge mRS was used instead [9]. Continuous parameters were compared either with the Welch t test, in cases of normal distribution, or with the Mann-Whitney U test, in case of non-normal or ordinal distribution. Categorical variables were compared between the two groups by the Fischer exact test. Additionally, we calculated the probability of good outcome at follow-up by stepwise logistic regression using all variables that were significantly different between groups in the univariate analysis. Selected variables were further examined by receiver operating characteristic analysis. Interobserver agreement was calculated with the interclass correlation coefficient (ICC). Analyses were performed with the MedCalc statistical package (MedCalc, Mariakerke, Belgium). The significance level for all tests was set to α = 0.05.
Results
Of the 72 patients identified, seven were excluded because of incomplete imaging data sets. Sixty-five patients were included for analysis (26 women; median age 72 years; interquartile range (IQR) 68-76). Median NIHSS at admission was 17 (IQR, 12-21) and median mRS at admission was 5 (IQR, 4-5). All patients had successful reperfusion on final angiograms (mTICI2b or 3). In the majority of cases (56 %) mechanical thrombectomy was combined with IV and IA rtPA therapy; IV rtPA and mechanical thrombectomy was combined in 26 %, with sole mechanical thrombectomy accounting for 18 % of cases. At discharge, NIHSS was reduced to a median of 7 (IQR, 4-12) and mRS to a median of 4 (IQR, 2-5). The median follow-up mRS was 3 (IQR, 2-4). Overall, 40 % of our patients had a favourable outcome at follow-up.
Of the analyzed medical comorbidities, only diabetes mellitus (P = 0.013) and obesity (P = 0.04) were significantly different between patients with favourable and poor outcome (Table 1). Regarding imaging variables, the CBV-ASPECTS and the various mismatch scores achieved high significance levels with P = 0.0014 and P < 0.01, respectively (Table 1). NCCT-ASPECTS and GSS values were also significantly different between the two groups with P = 0.03 and P < 0.0001, respectively. Patients with favourable outcome had a baseline CTA-ASPECTS of 8 (IQR, 7-9), while patients with poor follow-up outcome had a baseline CTA-ASPECTS of 7 (IQR, 7-8; P = 0.18). There was no significant difference in time from symptom onset or groin puncture to successful reperfusion between the two groups (P = 0.16 and P = 0.91).
When asked to predict futile reperfusions based on NCCT and CTA images, raters were able to recognize 56 % of the patients with poor outcome. The percentage was significantly higher when raters based their decisions on CTP images, as they were able to recognize poor outcomes in 96 % of the cases (Table 2). CBV-ASPECTS based decisions were also better for the prediction of favourable outcome, as raters recognized 61 % of patients with favourable outcome as opposed to 39 % of the cases with CTA-ASPECTS based decisions. Receiver operating characteristic analysis showed similar results. Sensitivity, specificity, positive predictive and negative predictive values (PPV, NPV) for NCCT-ASPECTS <7 were 8 %, 100 %, 100 %, and 42 %. For CTA-ASPECTS <7 sensitivity, specificity, PPV and NPV were 23 %, 77 %, 60 %, and 40 %. CBV-ASPECTS showed an optimal criterion value of <7 with a sensitivity of 49 %, specificity of 96 %, PPV of 95 %, and NPV of 56 % (Fig. 2).
In the stepwise logistic regression analysis, only admission NIHSS (P = 0.04; odds ratio 1.14; 95 % confidence interval [CI] 1.00 to 1.31) and CBV-ASPECTS (P = 0.006; odds ratio 0.45; 95 % CI 0.26 to 0.79) were significant contributors to the prediction of outcome. Agreement between the three raters was very good with ICC values of 0.92 for CBV-ASPECTS (95 % CI, 0.89-0.95), 0.9 for CBF-ASPECTS (95 % CI, 0.85-0.93), 0.7 for NCCT-ASPECTS (95 % CI, 0.59-0.79), 0.88 for CTA-ASPECTS (95 % CI, 0.82-0.92), 0.71 for MTT-ASPECTS (95 % CI, 0.6-0.8) and 0.85 for TTD-ASPECTS (9 5 % CI, 0.78-0.9).
Discussion
Our results support previously published studies supporting the use of CBV-ASPECTS as a biomarker in acute ischemic stroke treatment [10, 11]. CBV-ASPECTS was significantly different in the univariate analysis and a significant predictor of outcome in the logistic regression model with P = 0.006 and an odds ratio of 0.45. When using CBV-ASPECTS, raters were able to identify futile reperfusions in 96 % of the cases. The other significant predictor of outcome was the admission NIHSS. Our results disagree with previous publications showing that CTA-ASPECTS, acquired with a modern fast CT scanner, is a significant predictor of outcome (Fig. 3) [12]. It is worth mentioning that CTA images in the publication by Lum et al. were extracted out of a CTP dataset, using a predefined point on the contrast-time curve. In our study we used a single phase acquisition protocol for CTA with a bolus-adapted delay, which is a widely used protocol for the detection of large vessel occlusion. Studies with older, slower scanners have shown no significant difference in volume lesion between CTA and CBV images [13]. With the introduction of fast multisection scanners there have been reports that CTA images are CBF- rather than CBV-weighted, thus overestimating infarct core [14]. Our results support these findings, as CTA-ASPECTS was not significantly different between patients with favourable and poor outcomes and was not a significant predictor of outcome in the logistic regression model. When asked to retrospectively predict futile reperfusions based on CTA images, raters were successful in 56 % of the cases.
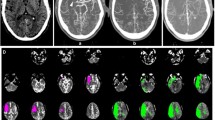
Images of a 75-year-old woman presenting with an admission NIHSS of 16 and admission mRS of 5. NCCT scan (a, b) shows initial signs of cerebral ischemia in the right insular cortex and was scored with an NCCT-ASPECTS of 9. CTA images (C, D) depict hypodense regions of the right caudate and lentiform nuclei, insular ribbon and frontal operculum resulting in a CTA-ASPECTS of 6. CBV images (E, F) show reduction of the cerebral blood volume on the right insular ribbon and were scored with a CBV-ASPECTS of 9. After swift and successful mechanical thrombectomy of the M1 occlusion (groin to TICI2b time of 45 minutes) patient showed significant improvement of her clinical symptoms and was discharged with and mRS of 1
Five randomized trials published in the last months have shown the superiority of endovascular treatment compared to IV lysis alone in the treatment of stroke patients with large vessel occlusions [5, 15–18] .The rate of favourable 90-day outcome after endovascular treatment varied from 33 % (MR-CLEAN) to 71 % (EXTEND-IA). The trial with the lowest percentage of favourable outcomes had the widest inclusion criteria and did not require demonstration of a small core on imaging, while the trial with the highest percentage used CTP to select patients. The great difference in favourable outcome percentages emphasizes the importance of proper imaging for patient selection and reduction of futile reperfusions. Using CBV-ASPECTS as a selection criterion has led to a significant increase in favourable outcomes in our department the last two years, with 34 % of favourable outcomes between 2007 and 2010 compared to 60 % of good outcomes from 2012 to 2014 [3]. We increased the median CBV-ASCPECTS by 2 points during this time frame with a median of 6 in 2007-2010 and a median of 8 in 2012-2014 [6]. After publication of the aforementioned trials in 2015 we stopped using CBV-ASPECTS as a selection criterion for thrombectomy treatment within the first 6 hours after symptom onset. However, we still use a CBV-ASPECTS >6 to select patients for mechanical thrombectomy in the 6 to 12 hour window after stroke symptom onset or in case of wake-up stroke.
The main argument against a tight selection strategy in treating acute stroke patients with large artery occlusions is the reduction of the number of patients treated with the most effective therapy. Untreated patients may still have profited from treatment, but at a lower percentage than those with a positive imaging profile. Around 30 % of patients with large vessel occlusions that would have probably been randomized in the NCCT-based trials were not included in EXTEND-IA based on imaging characteristics. This fact reduces the value of high percentage of favourable outcomes in a tight selection environment, as the absolute number of patients with favourable outcomes is probably the same as in the MR-CLEAN paradigm. The relevant difference, as shown by the shift analysis in MR-CLEAN, is the consistent shift towards better outcomes in favour of the intervention for all categories of the mRS.
In our study, the only patient with CBV-ASPECTS <7 and favourable outcome after successful reperfusion was a young patient (27y) with a moderate admission NIHSS score (10). This finding led us to the description of the GSS, a score combining admission NIHSS and CBV-ASPECTS, as strong predictors of outcome, together with age. The GSS was statistically different (P < 0.0001) between patients with favourable and poor clinical outcome with a median of 1.6 (IQR 0.25-2.4) and -1.5 (IQR -3.6- -0.01), respectively. Although initial results are promising, the predictive or complementary value of this score has to be validated in further studies. Other authors have proposed the combination of multiple diagnostic parameters in a new score for the prediction of futile recanalizations [19]. However, as CTA collaterals and CT perfusion imaging potentially depict the same biological feature about ischemic tissue sustenance [20], we are not sure about the feasibility of the score proposed by Espinosa de Rueda et al. Physicians should also be aware of the potential risk of CT collateral scoring on single-phase CTA, as single-phase acquisitions are dependent on bolus timing and can underestimate collateral state. This risk can be eliminated with use of multiphase CTA [21].
The primary limitation of our study is the retrospective character of our analysis and the highly select group of patients. Only patients with successful reperfusion were included in our study. Data were extracted from a prospectively collected database and consecutive patients were included in our analysis, but CTP images were used in treatment decisions between September 2012 and December 2014. Noncontrast CT was the primary imaging selection criterion before September 2012. This imaging selection praxis probably explains the fact that time (either onset to recanalization or groin to recanalization) was not a significant predictor of outcome in our study. Our results may only apply to CTP data acquired and reconstructed with the specific protocols and software stated in methods, as other authors have found significant differences between various vendors [22]. Furthermore, although studies have presented better predictive value of combined CTP and CTA we did not analyze a combined ASPECTS score of these variables [23].
Our study suggests that CBV-ASPECTS is a significant predictor of clinical outcome in patients with acute ischemic stroke treated with mechanical thrombectomy. We discourage the use of single phase CTA-ASPECTS, acquired with a modern fast multisection CT scanner, as an imaging biomarker because of its low predictive value. Obviously, our results do not apply to CTA images extracted out of a CTP scan or to a multiphase CTA protocol.
Abbreviations
- ASPECTS:
-
Alberta stroke program early CT score
- CBF:
-
Cerebral blood flow
- CBV:
-
Cerebral blood volume
- GSS:
-
Goettinger stroke scale
- ICC:
-
Interclass correlation coefficient
- IQR:
-
Interquartile range
- IV rtPA:
-
Intravenous recombinant tissue plasminogen activator
- MCA:
-
Middle cerebral artery
- mTICI:
-
Modified thrombolysis in cerebral infarction
- MTT:
-
Mean transit time
- mRS:
-
Modified Rankin scale
- NIHSS:
-
National Institutes of Health stroke scale
- NCCT:
-
noncontrast computed tomography
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- TTD:
-
Time to drain
References
Cloft HJ (2011) Death and destruction in the intra-arterial battle with acute ischemic stroke. AJNR Am J Neuroradiol 32:1769–1770
Lansberg MG, Straka M, Kemp S et al (2012) MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 11:860–867
Psychogios MN, Kreusch A, Wasser K, Mohr A, Groschel K, Knauth M (2012) Recanalization of large intracranial vessels using the penumbra system: a single-center experience. AJNR Am J Neuroradiol 33:1488–1493
Nogueira RG, Lutsep HL, Gupta R et al (2012) Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 380:1231–1240
Berkhemer OA, Fransen PS, Beumer D et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372:11–20
Psychogios MN, Schramm P, Frolich AM et al (2013) Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke 44:2188–2193
Zaidat OO, Yoo AJ, Khatri P et al (2013) Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 44:2650–2663
Abels B, Klotz E, Tomandl BF, Kloska SP, Lell MM (2010) Perfusion CT in acute ischemic stroke: a qualitative and quantitative comparison of deconvolution and maximum slope approach. AJNR Am J Neuroradiol 31:1690–1698
Ovbiagele B, Saver JL (2010) Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis 29:50–56
Aviv RI, Mandelcorn J, Chakraborty S et al (2007) Alberta stroke program early CT scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol 28:1975–1980
Kim JT, Park MS, Choi KH et al (2010) The CBV-ASPECT score as a predictor of fatal stroke in a hyperacute state. Eur Neurol 63:357–363
Lum C, Ahmed ME, Patro S et al (2014) Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke 45:2683–2688
Schramm P, Schellinger PD, Klotz E et al (2004) Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 35:1652–1658
Sharma M, Fox AJ, Symons S, Jairath A, Aviv RI (2011) CT angiographic source images: flow- or volume-weighted? AJNR Am J Neuroradiol 32:359–364
Goyal M, Demchuk AM, Menon BK et al (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030
Campbell BC, Mitchell PJ, Kleinig TJ et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372:1009–1018
Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med
Jovin TG, Chamorro A, Cobo E et al (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med
Espinosa de Rueda M, Parrilla G, Manzano-Fernandez S et al (2015) Combined multimodal computed tomography score correlates with futile recanalization after thrombectomy in patients with acute stroke. Stroke 46:2517–2522
Vagal A, Menon BK, Foster LD et al (2015) Association between CT angiogram collaterals and CT perfusion in the interventional management of stroke III trial. Stroke
Menon BK, d'Esterre CD, Qazi EM et al (2015) Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 275:510–520
Fahmi F, Marquering HA, Streekstra GJ et al (2012) Differences in CT perfusion summary maps for patients with acute ischemic stroke generated by 2 software packages. AJNR Am J Neuroradiol 33:2074–2080
Lee JH, Kim YJ, Choi JW et al (2013) Multimodal CT: favorable outcome factors in acute middle cerebral artery stroke with large artery occlusion. Eur Neurol 69:366–374
Acknowledgments
The scientific guarantor of this publication is Dr. Marios Nikos Psychogios. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. We would like to inform you that part of the current patient collective (about one-third of the population) has been subject to previous analyses: Frölich AM, Wolff SL, Psychogios MN et al. (2014) Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol 24(2):390-6. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsogkas, I., Knauth, M., Schregel, K. et al. Added value of CT perfusion compared to CT angiography in predicting clinical outcomes of stroke patients treated with mechanical thrombectomy. Eur Radiol 26, 4213–4219 (2016). https://doi.org/10.1007/s00330-016-4257-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4257-y