Abstract
Purpose
There is increasing use of automated computed tomography perfusion (CTP) to aid thrombectomy decision in emergent large vessel occlusion. It is important to understand the performance of these software packages in predicting ischemic core and tissue-at-risk in the real-world setting. The aim of this study was to evaluate whether ischemic core on non-contrast CT (NCCT) and automated CTP correspond and predict infarct extent after thrombectomy for ischemic stroke.
Methods
Consecutive patients with acute anterior circulation large vessel occlusion undergoing successful thrombectomy (TICI 2b/3) were studied. All patients had baseline CT, CTP with RAPID post-processing software (RAPID-CTP), and post-thrombectomy 24 h CT. Ischemic cores were assessed by two blinded raters independently using the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on each modality. The interrater agreement for ASPECTS, and correlation between baseline CT-ASPECTS, RAPID-CTP-ASPECTS, and 24h CT-ASPECTS were calculated.
Results
A total of 86 patients with a mean age of 70.3 years (SD 16.5) were studied. The median baseline CT-ASPECTS was 9.5 (interquartile range, IQR 8–10), median RAPID-CTP-ASPECTS was 9 (IQR 8–10), and mean RAPID-CTP-ischemic core volume was 14.4 ml (SD 27.9 ml). The mean mismatch volume (difference of Tmax > 6s and cerebral blood flow (CBF) < 30%) was 128.6 ml (SD 126.0 ml). There was substantial correlation between baseline and 24h CT-ASPECTS (rs: 0.62; p < 0.001), but poor correlation between RAPID-CTP-ASPECTS and RAPID-CTP ischemic core volume with 24h NCCT-ASPECTS (rs: 0.21; p = 0.06 and −0.16; p = 0.15 respectively). The positive predictive value of any established infarct for baseline CT-ASPECTS was 81%, while that of RAPID-CTP-ASPECTS was 64%.
Conclusion
In this series of successfully revascularized patients, ischemic core as estimated by RAPID-CTP-ASPECTS did not correlate with the baseline CT and tended to depict a larger infarct core than the infarct extent as assessed by 24h CT-ASPECTS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Determining the extent of the ischemic core is important for identifying ischemic stroke patients who may benefit from revascularization therapy. In recent landmark trials for thrombectomy including EXTEND-IA, DAWN, and DEFUSE-3, patients were excluded if there was a large ischemic core as determined by the fully automated CT perfusion (CTP) software platform RAPID (iSChemaView Inc, Golden Park, CA, USA; RAPID-CTP) [1,2,3,4]. This led to an increasing role of automated CTP software packages in providing information on ischemic core volume and mismatch volume to help select emergent large vessel occlusion (ELVO) patients with salvageable brain tissue and acceptable risk of hemorrhagic transformation for endovascular thrombectomy. The real-world diagnostic performance of RAPID-CTP was studied and compared with Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on non-contrast CT in assessing final ischemic core in a cohort of ELVO patients successfully revascularized with thrombectomy.
Material and Methods
Study Design
This was a retrospective study of consecutive patients with angiogram-confirmed anterior circulation large vessel occlusion treated with mechanical thrombectomy. Inclusion criteria were the following: 1) baseline pretreatment NCCT, CT angiogram and RAPID-CTP, 2) angiogram-confirmed anterior circulation large vessel occlusion defined as internal carotid artery (ICA), M1 or M2 segment of the middle cerebral artery (MCA), 3) successful reperfusion with mechanical thrombectomy (defined as TICI 2b/3), and 4) had follow-up CT 24 h after thrombectomy. Exclusion criteria were the following: 1) posterior circulation stroke, 2) poor quality CT or RAPID-CTP images unsatisfactory for analysis or 3) unsuccessful thrombectomy (defined as TICI 0–2a).
Imaging Protocol and Image Interpretation
As part of routine practice at this institution, all acute ischemic stroke patients underwent CT, CT angiogram and CTP imaging on 320-slice scanners (Aquillion One, Toshiba, Tochigi, Japan). The CTP image was then processed by a fully automated software platform (RAPID, version 4.7.1, iSChemaView Inc.) to generate the extent and ischemic core volume using the cerebral blood flow (CBF) < 30% and cerebral blood volume (CBV) < 34% thresholds. Patient selection for thrombectomy was based primarily on clinical symptoms and CT-ASPECTS. After mechanical thrombectomy, non-contrast CT at 24 h was used to assess the extent of final ischemic core.
For all patients, the ischemic core extent on 1) baseline CT, 2) RAPID-CTP with CBF < 30% and CBV < 34% of normal tissue and, 3) 24 h follow-up CT were assessed independently by two raters (one stroke neurologist and one neuro-interventionist) with more than 5 years of experience using the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) in a blinded fashion, and discrepancies were resolved by consensus reading.
Subgroup Analysis
Because of the notion that ischemic changes may not be readily apparent on CT among patients who present early, and CTP may add value in early identification of infarcted tissue, a subgroup analysis of patients who had CT and RAPID-CTP within 90 min from symptom onset was performed. A subgroup analysis was also performed on patients who had TICI 2b and TICI 3 revascularization.
Statistical Analysis
The inter-rater agreement on ASPECTS reading was assessed with weighted-kappa score (Kw). Spearman’s rank order correlation coefficient (rs) was used to analyze the correlation between the baseline CT, RAPID-CTP and 24h-CT ischemic cores. Statistical analyses were performed with SPSS version 21 (IBM, Armonk, NY, USA).
Results
Patient Characteristics
There were 101 consecutive anterior circulation LVO patients treated in the study period. After excluding 12 patients who did not achieve TICI 2b/3 reperfusion and 3 who had uninterpretable images, 86 patients (31 male, 55 female) with a mean age of 70.3 years (SD 16.5 years) were included for analysis. All patients achieved successful thrombectomy with TICI 2b/3 reperfusion. The median baseline and 24 h National Institutes of Health stroke scale (NIHSS) were 13 (interquartile range, IQR 8–18.25) and 4 (IQR 1–10), respectively. The median time from onset-to-CT and CT-to-reperfusion were 90 min (IQR 64–252 min) and 105 min (IQR 81–137 min), respectively. Other patient and imaging characteristics are summarized in Table 1.
Ischemic Core Assessment
The median baseline CT-ASPECTS was 9.5 (IQR 8–10). The mean RAPID-CTP-ischemic core volume was 14.4 ml (SD 27.9 ml) defined by CBF < 30%, and 12.6 ml (SD 33.2 ml) defined by CBV < 34%. The mean mismatch volume (Tmax > 6s and CBF < 30%) was 128.6 ml (SD 126.0 ml). The median RAPID-CTP-ASPECTS was 9 (IQR 8–10) with CBF < 30%, and 10 (IQR 9–10) with CBV < 34% thresholds. The median 24h CT-ASPECTS was 9 (IQR 7–10). The inter-rater agreement was substantial for CT-ASPECTS (Kw: 0.71) and excellent for RAPID-CTP-ASPECTS (Kw: 0.94).
There was substantial correlation between baseline and 24h CT-ASPECTS (rs: 0.62; p < 0.001), but no significant correlation between RAPID-CTP-ASPECTS or ischemic core volume defined by CBF < 30% and 24h CT-ASPECTS (rs: 0.21; p = 0.06 and, rs: −0.16; p = 0.15, respectively; Fig. 1). Data for CBV < 34% threshold were available for 52 patients, and there was no significant correlation between RAPID-CTP-ASPECTS or ischemic core volume defined by CBV < 34% and 24h CT-ASPECTS (rs: 0.21; p = 0.13 and rs: −0.22; p = 0.12, respectively).
Correlations between a baseline CT-ASPECTS score, and b RAPID-CTP-ASPECTS score with final ischemic core (24 h CT-ASPECTS score) and the 95% confidence interval (circles represent numbers of patients). CT-ASPECTS Computed Tomography-Alberta Stroke Program Early Computed Tomography Score, RAPID-CTP-ASPECTS RAPID-Computed Tomography-Alberta Stroke Program Early Computed Tomography Score
There was also no significant correlation between baseline CT-ASPECTS and RAPID-CTP-ASPECTS (rs: 0.18; p = 1.0). Both positive predictive value and specificity for any established infarct at 24 h were higher in baseline CT-ASPECTS than RAPID-CTP-ASPECTS (81.4% vs. 64.4% and 77.1% vs. 54.3%, respectively). The 24h CT-ASPECTS was higher than RAPID-CTP-ASPECTS and baseline CT-ASPECTS (indicating overestimation of ischemic core) in 31.4% and 11.6% of patients, respectively (Fig. 2).
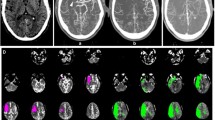
Illustrative case: a patient suffering from left ICA occlusion with a NIHSS of 16. a Baseline caudal-cranial axial CT performed 80 min after symptom onset showed ASPECTS of 10. b RAPID-CTP performed immediately afterwards showed an ischemic core defined by CBF < 30% threshold of 23.4cc, corresponding to RAPID-CTP-ASPECTS of 7. c Magnetic resonance imaging (MRI) diffusion imaging 24 h after TICI 3 thrombectomy showing only scattered micro-infarct and d 24 h CT showing ASPECTS of 10. The 24-h NIHSS was 6
Subgroup Analysis
A subgroup of 43 patient who had CT and RAPID-CTP within 90 min from symptom onset was analyzed, and the CT-ASPECTS was still found to correlate better than RAPID-CTP-ASPECTS with the final infarct extent (rs:0.65, p <0.001 vs. rs:0.31, p =0.043 for CBF < 30% and rs:0.57, p =0.004 for CBV < 34%).
Amongst 38 patients who had TICI 2b revascularization, CT-ASPECTS still correlated better than RAPID-CTP-ASPECTS (CBF < 30%) with the final infarct extent (rs:0.48, p = 0.006 vs. rs:0.22, p =0.23). Amongst the 48 patients who had TICI 3 revascularization, CT-ASPECTS demonstrated the strongest correlation with the final infarct extent and performed better than RAPID-CTP-ASPECTS (CBF < 30%) (rs:0.68, p <0.001 vs. rs:0.20, p = 0.023).
Discussion
The present study of 86 anterior circulation ELVO patients successfully treated with thrombectomy showed that baseline CT-ASPECTS correlated with the extent of ischemic core and predicted the presence or absence of established infarct better than automated CTP calculations using the thresholds of CBF < 30% or CBV< 34% of normal tissue as determined by RAPID. Also, RAPID-CTP overestimated the ischemic core in close to one third of patients. These findings are important as they highlight the potential shortcomings of adopting automated CTP algorithms as the predominant patient selection criteria for thrombectomy and reaffirmed the clinical utility of CT-ASPECTS in treatment decisions for LVO patients.
The evidence in support of CTP for the prediction of final infarct volume was largely based on studies carried out before 2015 where patients were treated predominantly with intravenous thrombolysis and recanalization rates were in the range of 20–45% [5,6,7,8,9]. In these studies, the low CBF area delineated by CTP correlated well with MRI diffusion weighted imaging core as well as clinical outcome. This could be in part due to the relative low recanalization rate and long reperfusion time of intravenous thrombolysis, leading to the eventual infarct expansion in the majority of patients. Bivard et al. demonstrated in a case-control study that the eventual ischemic core of patients treated by thrombectomy is smaller than those treated by intravenous thrombolysis alone, despite comparable occlusion location and baseline perfusion lesion volumes [10]. In the era of thrombectomy when the recanalization rate of large vessel occlusions is 75% or more and the time it takes to achieve recanalization is significantly shorter, the accuracy of using the CBF < 30% threshold for predicting eventual ischemic core may be questioned [11, 12].
In a series of ELVO patients of which 77% achieved TICI 2b/3 reperfusion after thrombectomy, CTP using the Syngo MMWP software package (Siemens, Erlangen, Germany) overestimated the ischemic core volume in 38% of patients [13]. It is important to point out that the Syngo software package used a CBV threshold (CBV < 1.2 ml/100 ml) to define ischemic core. Austein et al. compared the three commercially available automated software packages for CTP (Siemens Syngo, RAPID, Philips Brain CT Perfusion Package). In their study, the RAPID software correlated with the final infarct volume better than its counterparts; however, they also identified that it overestimated the infarct volume in 21% of patients and had a 33.3% rate of false positive malignant mismatch profile [14]. The present study comprised of only successfully reperfused ELVO patients likewise identified a tendency of RAPID-CTP to overestimate ischemic core, especially in patients who had complete (TICI 3) revascularization. Therefore, despite the post hoc analysis of SWIFT-PRIME cohort of 47 patients, which demonstrated that RAPID-CTP with CBF < 30% and CBV < 34% threshold provided accurate assessment of ischemic core[15], our findings caution against using automated CTP-derived ischemic core volume as an absolute exclusion criteria for thrombectomy. In fact, one recently published study found that up to 10% of patients who would be denied thrombectomy based on a large CTP-derived ischemic core achieved good clinical outcome after successful thrombectomy [16].
It was suggested that amongst patients who present early, ischemic changes may not be readily apparent on CT and CTP may add value in early identification of infarcted tissue [17]. This study analyzed the subgroup of patient who had CT and RAPID-CTP within 90 min from symptom onset and found the CT-ASPECTS still correlated better than RAPID-CTP with the final infarct extent.
Limitations
This study has several limitations. The use of MRI diffusion weighted imaging was not routinely performed for post-thrombectomy patients to determine ischemic core volume, which could underestimate micro-infarcts not detectable on CT. Volumetric assessment was not available for infarct core volume determination at follow-up. There are potential selection and recall biases in all retrospective studies including this study. Assessment with ASPECTS can be reader-dependent and affected by the level of training and experience. This study sought to limit these by having two raters (each with more than 5 years of experience) blinded to outcome independently assessing the ASPECTS, and satisfactory inter-rater agreement was obtained. The study examined discrepancies between CT-ASPECTS and CTP-ASPECTS determined by one software package (RAPID) using CBF < 30% and CBV < 34% thresholds. The 24-h CT ASPECTS assessment may be confounded by edema surrounding infracted brain. It is possible that alternative thresholds or other imaging software package may have a stronger association with CT-ASPECTS and ischemic core.
Conclusion
The CT-ASPECTS correlated with ischemic core better than RAPID-CTP-ASPECTS in ELVO patients successfully treated with thrombectomy when using a core threshold parameter of CBF < 30% or CBV < 34%. This CTP-based ischemic core did not correlate with the baseline CT and tended to depict a larger core than the final infarct as assessed by 24-h CT. Thus, the authors caution against overly relying on automated CTP imaging criteria alone for thrombectomy patient selection.
Abbreviations
- ASPECTS:
-
Alberta Stroke Program Early Computed Tomography Score
- ELVO:
-
Emergent large vessel occlusion
References
Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, Mlynash M, Kim S, Hamilton S, Yeatts SD, Palesch Y, Bammer R, Broderick J, Marks MP. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke. 2017;12:896–905.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-18.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG; DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21.
Knoepfli AS, Sekoranja L, Bonvin C, Delavelle J, Kulcsar Z, Rüfenacht D, Yilmaz H, Sztajzel R, Altrichter S, Lövblad KO. Evaluation of perfusion CT and TIBI grade in acute stroke for predicting thrombolysis benefit and clinical outcome. J Neuroradiol. 2009;36:131–7.
Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, Ombelet F, McElduff P, Bivard A, Parsons M, Levi C. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88:2248–53.
Bivard A, Levi C, Krishnamurthy V, McElduff P, Miteff F, Spratt NJ, Bateman G, Donnan G, Davis S, Parsons M. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015;138(Pt 7):1919–31.
Souza LC, Payabvash S, Wang Y, Kamalian S, Schaefer P, Gonzalez RG, Furie KL, Lev MH. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis. 2012;33:8–15.
Campbell BC, Yassi N, Ma H, Sharma G, Salinas S, Churilov L, Meretoja A, Parsons MW, Desmond PM, Lansberg MG, Donnan GA, Davis SM. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int J Stroke. 2015;10:51–4.
Bivard A, Kleinig T, Miteff F, Butcher K, Lin L, Levi C, Parsons M. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol. 2017;82:995–1003.
Lui YW, Tang ER, Allmendinger AM, Spektor V. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010;31:1552–63.
Lum C, Ahmed ME, Patro S, Thornhill R, Hogan M, Iancu D, Lesiuk H, Dos Santos M, Dowlatshahi D; Ottawa Stroke Research Group (OSRG). Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke. 2014;45:2683–8.
Boned S, Padroni M, Rubiera M, Tomasello A, Coscojuela P, Romero N, Muchada M, Rodríguez-Luna D, Flores A, Rodríguez N, Juega J, Pagola J, Alvarez-Sabin J, Molina CA, Ribó M. Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9:66–9.
Austein F, Riedel C, Kerby T, Meyne J, Binder A, Lindner T, Huhndorf M, Wodarg F, Jansen O. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke. 2016;47:2311–7.
Mokin M, Levy EI, Saver JL, Siddiqui AH, Goyal M, Bonafé A, Cognard C, Jahan R, Albers GW; SWIFT PRIME Investigators. Predictive value of RAPID assessed perfusion thresholds on final infarct volume in SWIFT PRIME (solitaire with the intention for thrombectomy as primary endovascular treatment). Stroke. 2017;48:932–8.
Haussen DC, Dehkharghani S, Rangaraju S, Rebello LC, Bouslama M, Grossberg JA, Anderson A, Belagaje S, Frankel M, Nogueira RG. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke. 2016;47:2318–22.
Padroni M, Boned S, Ribó M, Muchada M, Rodriguez-Luna D, Coscojuela P, Tomasello A, Cabero J, Pagola J, Rodriguez-Villatoro N, Juega JM, Sanjuan E, Molina CA, Rubiera M. CBV_ASPECTS improvement over CT_ASPECTS on determining irreversible ischemic lesion decreases over time. Interv Neurol. 2016;5:140–7.
Funding
This work was supported by the Health and Medical Research Fund research fellowship scheme of Hong Kong, grant number 01150027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.C.O. Tsang, S. Lenck, C. Hilditch, P. Nicholson, W. Brinjikji, T. Krings, V.M. Pereira, F.L. Silver and J.D. Schaafsma declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tsang, A.C.O., Lenck, S., Hilditch, C. et al. Automated CT Perfusion Imaging Versus Non-contrast CT for Ischemic Core Assessment in Large Vessel Occlusion. Clin Neuroradiol 30, 109–114 (2020). https://doi.org/10.1007/s00062-018-0745-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0745-6