Abstract
Objectives
Dipyridamole stress cardiac magnetic resonance (CMR) evaluates the key phases (perfusion and wall motion) of the ischemic cascade. We sought to determine the prognostic value of dipyridamole stress-CMR in consecutive patients symptomatic for chest pain.
Methods
Seven hundred and ninety-three consecutive patients symptomatic for chest pain underwent dipyridamole stress-CMR and were followed up for 810 ± 665 days. Patients were classified in group 1 (no- reversible ischemia), group 2 (stress perfusion defect alone), and group 3 [stress perfusion defect plus abnormal wall motion (AWM)]. End points were "all cardiac events" (myocardial infarction, cardiac death and revascularization) and "hard cardiac events" (all cardiac events excluding revascularization).
Results
One hundred and ninety-five (24 %) all cardiac events and 53 (7 %) hard cardiac events were observed. All and hard cardiac event rates in groups 1, 2, and 3 were 11 %, 49 %, 69 % and 4 %, 8 %, 21 %, respectively, with a higher rate in group 2 vs. group 1 (p<0.01) and group 3 vs. groups 1 and 2 (p<0.01). Multivariate analysis showed the presence of late gadolinium enhancement and stress perfusion defect plus AWM as independent predictors of all and hard cardiac events.
Conclusions
Dipyridamole stress-CMR improves prognostic stratification of patients through differentiation between the different components of the ischemic cascade.
Key Points
• Dipyridamole stress cardiac magnetic resonance helps to assess coronary artery disease.
• Novel technique to study the key phases of myocardial ischemia.
• Combined assessment of perfusion and motion defects.
• Dipyridamole stress imaging has additional value for predicting cardiac events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is a major cause of mortality and morbidity. Thus, identification of patients at high risk for adverse events is crucial to identify those who may receive the greatest benefit from revascularization [1]. However, even though several non-invasive imaging modalities, such as cardiac computed tomography, exercise electrocardiogram (ECG), stress echocardiography, and nuclear stress test are suggested as gatekeepers to invasive coronary angiography (ICA), the diagnostic yield of elective ICA remains low [2]. Stress cardiovascular magnetic resonance (CMR) has been recently recognized as a reliable technique for providing myocardial perfusion and wall motion evaluation without ionizing radiation with a diagnostic accuracy at least comparable to nuclear stress test [3–5]. However, given the increasing health care costs associated with cardiovascular imaging, the prognostic value of stress CMR needs to be better evaluated [6]. The studies so far published have been performed mainly with adenosine or dobutamine and are affected by the separate evaluation of perfusion and wall motion, small sample size, and short-term follow-up. The aim of the current study is to evaluate the mid-term prognostic value of dypiridamole stress CMR in patients with known or suspected CAD, testing the differential impact of the two main components of the ischemic cascade [e.g. perfusion defect and abnormal wall motion (AWM)] on outcomes.
Materials and methods
Study population
We enrolled 911 consecutive patients symptomatic for chest pain (Fig. 1) referred to perform dipyridamole stress CMR. Exclusion criteria were unstable angina, heart failure, known infiltrative or hypertrophic cardiomyopathy, myocarditis, severe claustrophobia, presence of pacemaker or implantable cardioverter device, estimated glomerular filtration rate ≤30 mL/min, and contraindication to dipyridamol use. To avoid the possibility that a revascularization procedure could be directly influenced by stress-CMR findings, patients with early revascularization (within 60 days after stress CMR) were excluded from the study. Our study complies with the Declaration of Helsinki and was approved by the local ethics committee. All patients gave written informed consent at the time of stress-CMR.
Clinical history
A structured interview was obtained in all patients before stress CMR as previously described [7] with particular emphasis on: a) hypertension (blood pressure >140/90 mmHg or use of antihypertensive agents); b) smoking status; c) hyperlipidemia (low density lipoprotein cholesterol >140 mg/dL); c) diabetes mellitus (fasting glucose level >110 mg/dL or need of insulin or oral antidiabetic drugs); e) family history of CAD in first-degree relatives; f) home use of antianginal drugs; g) history of previous revascularization with percutaneous coronary intervention and/or coronary artery bypass graft.
Stress CMR imaging protocol
All patients were evaluated in a 1.5-T scanner (Discovery MR450, GE Healthcare, Milwaukee WI, USA) according to the recommendations of the Society of Cardiovascular Magnetic Resonance (SCMR) [8]. Patients were asked to refrain from smoking, caffeine, theophylline, and beta-blockers for 24 h and to maintain fasting for 6 h. Steady-state free precession cine acquisitions were then acquired at rest during held expiration in multiple short axis and three additional long-axis views (two-, three-, and four-chambers) of the left ventricle. Vasodilatation was induced with dipyridamole injected at 0.84 mg/kg over 6 min. At the end of dipyridamole infusion, 0.1 mmol/kg of Gadolinium-BOPTA (Multihence, Bracco, Milan Italy) was injected intravenously at 4 mL/s followed by saline solution with concomitant acquisition of three short-axis views (using the same geometry of rest imaging) of the left ventricle with first-pass perfusion technique using saturation-prepared T1-weighted fast gradient-echo sequence. Steady-state free precession cine acquisitions were then acquired at stress with the same geometry used at rest. Theophylline was intravenously injected (240 mg i.v.) to null the effect of dypiridamole at the end of the stress test. Ten minutes after contrast injection, breath-hold contrast-enhanced segmented T1-weighted inversion-recovery gradient-echo sequence was acquired with the same prescriptions for cine images to detect late gadolinium enhancement (LGE) as previously described [9]. The inversion time was individually adjusted to null normal myocardium.
CMR post-processing data
CMR datasets were transferred to a dedicated workstation and analyzed with a cardiac software (Report Card 4.0 GE Healthcare, Milwaukee WI, USA) by two expert readers (G.P. and P.G.M.) blinded to the clinical history of the patients using the 17-segment model for the myocardium. For any disagreement in data analysis between the two readers, consensus agreement was achieved involving a third expert reader (D.A.). The following indexes were evaluated according to the recommendations of the SCMR [8]:
-
End-diastolic and end-systolic left ventricle volumes, ejection fraction, and left ventricle mass
-
AWM at rest: each myocardial segment was classified as normal (score 0), hypokinetic (score 1), akinetic (score 2), or diskinetic (score 3). AWM score and number of segments with AWM were defined as the sum of each segment score and as number of segments showing score between 1 and 3, respectively.
-
Stress perfusion defect: a perfusion defect was defined as persistent delay of enhancement during first pass of the contrast agent for >3 heart beats at maximum signal intensity in the cavity of the left ventricle evaluated visually and with segmental location that conformed to coronary territories according to the American Heart Association/American College of Cardiology classification [10]. Accordingly, each myocardial segment was classified as normal (score 0), with subendocardial defect involving ≤50 % of left ventricle thickness (score 1), or as transmural defect involving >50 % of the left ventricle thickness (score 2). Perfusion defect score and number of segments with perfusion defects were defined as the sum of each segment score and as number of segments showing score between 1 and 2, respectively.
-
AWM at stress: AWM score and number of segments with AWM were measured as previously described for rest condition.
-
LGE was defined as a myocardial segment with an increase of signal intensity >2 standard deviation (SD) above the mean signal intensity of remote myocardium [9]. The absolute number and percentage of patients with LGE, the number of myocardial segments showing LGE, and the absolute value and percentage of myocardial mass showing LGE were measured.
-
Stress CMR classification: based on CMR findings, patients were classified in Group 1 (no evidence of ischemia), Group 2 (evidence of reversible stress perfusion defect in at least one myocardial segment), and Group 3 (evidence of reversible stress perfusion defect in at least one myocardial segment plus worsening of stress AWM in comparison with rest). Stress CMR was considered normal for group 1 and abnormal for groups 2 and 3.
Follow-up
Patient follow-up was performed by checking the medical records or by phone interview by researchers unaware of the patients’ CMR results. Events were defined as follows: revascularization defined as elective procedure 60 days after CMR, non-fatal myocardial infarction defined as typical chest pain with elevated cardiac enzyme levels and typical ST-segment changes on the electrocardiogram (7), and cardiac death defined as death caused by acute myocardial infarction, ventricular arrhythmias, or refractory heart failure. We defined hard cardiac events as a combined end point of non-fatal myocardial infarction and cardiac death. All cardiac events were defined as hard cardiac events plus revascularization. In cases of multiple events in a given patient, the first event was included in the analysis.
Statistical analysis
Categorical baseline characteristics were expressed as numbers and percentages, whereas continuous variables with or without normal distribution were expressed as mean ± SD or as median and inter-quartile interval, respectively. To identify the association between CMR variables and outcomes, Cox regression analysis was used. First, univariate analysis of clinical characteristics and CMR was performed to identify potential predictors. Hazard ratios were calculated with 95 % confidence intervals as an estimate of the risk associated with a particular variable. To determine independent predictors of the composite end points, multivariate analysis of variables with p<0.05 in univariate analysis was performed and corrected for the following baseline characteristics: male sex, age, diabetes, hypercholesterolemia, hypertension, family history of CAD, and smoking. Cumulative event rates for each group were obtained with the Kaplan-Meier method for all cardiac events and hard cardiac events and compared with the Wilcoxon log-rank test. Statistical analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA) and the SAS software version 6.12 (SAS Institute Inc., Cary, NC, USA). A p value <0.05 was considered significant.
Results
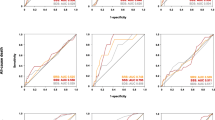
Of 911 patients screened, 98 underwent early elective revascularization and, therefore, were excluded from the analysis, while 20 additional patients were lost during follow-up. Thus, the study population included in the analysis consisted of 793 patients (mean age 63.9 ± 10.9 years; 657 men). The clinical characteristics and CMR findings of the study patients are given in Table 1 and Table 2, respectively. According to stress CMR classification, 570 (72 %), 132 (17 %), and 91 (11 %) patients were categorized in groups 1, 2, and 3, respectively (Fig. 1). Mean follow-up duration was 810 ± 655 days with a median of 622 days (interquartile range 425–963 days). During follow-up, all cardiac event and hard cardiac event end points were reached in 195 (24 %) and 53 (7 %) of patients, with a median event-free survival of 109 days (21–345) and 329 days (196–725), respectively (Table 3). All cardiac events and hard cardiac events in normal and abnormal stress CMR were 67 (12 %) and 23 (4 %) vs. 128 (57 %) and 30 (13 %), respectively, (p<0.0001) with a mean annual rate of 5.4 % and 1.8 % vs. 25 % and 5.8 %, respectively (p<0.0001) (Table 3). Moreover, abnormal stress CMR findings identify a shorter event-free period vs. normal stress CMR for all cardiac events (p<0.0001) and hard cardiac events (p< 0.001). The prevalence of all cardiac events and hard cardiac events in Groups 1, 2, and 3 were 11 %, 49 %, 69 % and 4 %, 8 %, 21 %, respectively, with a stepwise increase according to the severity of stress CMR findings (p<0.01) (Fig. 2). Table 4 summarizes the univariate and multivariate analyses of the clinical characteristics and CMR results that were used for event prediction. In the multivariate analysis, the presence of LGE and a stress CMR showing both perfusion defect and AWM were the most robust and independent predictors of all cardiac events and hard cardiac events. Kaplan-Meier curves showed that stress CMR provides additional prognostic stratification if a perfusion defect is described with or without associated AWM regardless of the history of previous revascularization (Figs. 3 and 4). Figure 5 shows the case of a 62-year-old male patient with angina and evidence of a transmural left ventricle anterior wall perfusion defect in presence of viable myocardium.
Adjusted survival curves without all cardiac events (left panel) and hard cardiac events (right panel) in non-revascularized patients (upper panels) and revascularized patients (lower panels) in patients with normal stress CMR (Group 1), patients with perfusion defect alone (Group 2) and patients with perfusion defect+AWM (Group 3). AWM: abnormal wall motion
Clinical case of a 62-year-old male patient with a history of hypertension and recent onset of chest pain. Stress CMR with dipyridamole showed anterior interventricular septum akinesia (Panel A, arrow) at rest and transmural perfusion defect of the anterior wall of left ventricle (Panel B , arrow) with preserved thickening (Panel C, dotted line). The LGE study showed the presence of a scar at the level of the anterior interventricular septum (Panel D, arrow) without evidence of fibrosis of the left ventricle anterior wall. These findings suggest the presence of inducible ischemia at the level of the anterior wall of the left ventricle indicating myocardial viability. CMR: cardiac magnetic resonance; LGE: late gadolinium enhancement
In a subanalysis of patients referred for early revascularization and excluded form our study we found that the baseline characteristics of this subgroup were comparable to the general study population (age: 64.1 ± 9.2 vs. 63.9 ± 10.9 years, male 85 %). Stress-CMR was negative, positive for perfusion defect alone or positive for perfusion defect plus AWM in 1 (1 %), 10 (10 %), and 88 (88 %), respectively. In all these patients, obstructive CAD was found at ICA, and the revascularization procedure was performed in agreement. Including this subgroup of patients in the overall population, the rate of all cardiac events as compared to the study population does not significantly change for patients with normal stress-CMR and with perfusion defect alone (11 % and 52 % vs. 11 % and 49 %, respectively), while there is an increase for the patients with concomitant perfusion and abnormal wall motion (84 % vs. 69 %, p<0.001).
Discussion
The main findings of this study are: 1) the presence of a positive dipyridamole stress CMR predicts a higher rate of cardiovascular events; 2) the two components of the ischemic cascade (abnormal perfusion and reduced wall motion) have incremental prognostic value over conventionally assessed cardiovascular risk factors; 3) a negative stress test is associated with a low rate of hard cardiac events with a warranty period of at least 1 year.
The accuracy of stress CMR for detecting obstructive CAD has been extensively proved in multiple studies [3–5, 11, 12]. However, the prognostic value of stress CMR has not been fully evaluated in patients with suspected or unknown CAD.
In a recent meta-analysis, Lipinski et al. [6] showed that the annualized event rates were 0.8 % for a negative study and 4.9 % for a positive study, and they observed that evidence of late gadolinium enhancement was significantly associated with a worse prognosis. However, the studies included in the meta-analysis were performed mainly with adenosine or dobutamine and evaluated the two components of the ischemic cascade separately.
Macwar et al. [13] found an annual event rate for hard events of 0.6 %, 1.7 %, and 1.5 % in 564 patients with angina and no previous revascularization who showed an adenosine stress CMR normal, positive for LGE or positive for reversible perfusion defects, respectively. Similarly, Buckert et al. [14] showed a hazard ratio of 3.2 associated with reversible perfusion defect in a larger population (1152 patients) in a long-term (4.2 years) follow-up. These previous data corroborate the view that adenosine stress CMR has a strong ability of prognostic stratification, which seems to be preserved regardless of patient’s gender [15].
Regarding dobutamine studies, Kell et al. [16] evaluated a large cohort (1369 patients) with dobutamine stress CMR and found that the annual cardiac event rate of a negative stress test was 1.1 %, while the hazard ratio associated with a positive dobutamine stress test was 3.3. Similarly, Wallace et al. [17] studied 221 consecutive women with known or suspected CAD and showed that the presence of inducible AWM is associated with a hazard ratio of 2.7 for future hard cardiac events. Of note, women without inducible AWM experienced a favorable prognosis in the 5 following years with an annual event rate of 1.2 %.
Few studies only tested the usefulness of dypiridamol stress CMR for predicting clinical events [18, 19]. Bodi et al. [18] found that the prognostic value of perfusion defects was weaker than AWM under stress, suggesting that wall motion evaluation outweigths perfusion assessment and it is the CMR index most closely related to outcome. However, in this study the rate of events based on a separate analysis of perfusion and wall motion was not evaluated. On the contrary, this was assessed in a following study performed in 601 consecutive patients by the same authors [19] who found an annual hard event rate of 2.9 %, 11.7 %, and 14.1 % at a mean follow-up of 640 days in the three categories described above, respectively. The progressive increase of hard event rate in these three categories may be explained by the observation that the perfusion defect extent is larger in patients with concomitant AWM [20]. Of note, the relationship between perfusion and AWM seems to be lost if the two components of the ischemic cascade are evaluated separately. Indeed, Elhendy et al. [20] compared dobutamine stress test vs. stress perfusion using single-photon emission computed tomography (SPECT) and did not find a direct relationship between the extent of the perfusion defect and wall motion during stress.
Our results demonstrate that patients with a negative stress CMR have a low annual hard event rate (1.8 %) in comparison with patients with a positive stress CMR for perfusion defect alone (3.6 %) or patients with perfusion defect plus AWM (9.4 %). Of note, to improve the classification of patients having or not concomitant kinesis abnormalities, we have preferred to use a semi-quantitative approach employing the AWM score as previously described rather than visual estimation. Indeed, even though AWM score alone has not be found as a predictor of cardiac events, the concomitant presence of a perfusion defect plus kinesis abnormalities based on AWM score is the more robust independent predictor for major cardiac events. In addition, the findings of stress CMR seem to predict adverse outcome beyond the amount of LGE suggesting an additional prognostic value. These data support the potential role of stress CMR in identifying patients at low, middle, and high risk for future cardiac events. It is noteworthy that the annual hard event rate in our patients with stress CMR without inducible ischemia is higher than that reported in previous studies. This result may be explained by the definition used in this study for a normal stress CMR. Indeed, stress CMR was considered normal in case of LGE without inducible ischemia in order to assess specifically the additional value of reversible ischemia. On the contrary, abnormal stress CMR was defined as absence of LGE and reversible ischemia in previous studies. Therefore, considering that LGE is an independent predictor of hard events, it may increase the annual hard cardiac event rate in normal stress CMR. Moreover, the fact that 56 % of our study patients underwent prior myocardial revascularization indicates that they were a higher risk population in comparison with patients enrolled in previous studies. As compared to the other studies testing the prognostic value of stress CMR, the novelty of our study is the use of dipyridamole that, even though it is an underused stressor, showed the capability to perform a grading of risk for events. These findings agree with a few publications available on the prognostic stratification of dipyridamole stress CMR. However, as compared to previous reports, our study has a larger sample size and a longer follow-up. Moreover, we showed for the first time that patients with perfusion defect only had at 1 year a similar outcome to that of patients with normal stress CMR suggesting that an optimal medical therapy could be useful in this setting allowing to postpone revascularization until abnormal kinesis is detected. This new approach could be in agreement with the literature trend that suggests to address the patients to revascularization therapy only in case of at least moderate ischemia. Finally, in our study the rate of revascularized patients is higher as compared to previous studies. This allowed to evaluate the prognostic value of stress CMR analyzing separately patients without and with a previous history of revascularization. Our results showed how the prognostic value of stress CMR is even more robust in revascularized patients. Considering the emerging prognostic value of cardiac computed tomography in native coronary artery evaluation [21] and, on the contrary, its weakness in revascularized patients, the evidence of the prognostic value of stress CMR in this specific subset is an important step forward in the functional assessment in this kind of patients.
Our results have several clinical implications. First, even though stress CMR is similar to echocardiography and nuclear tests for assessing ischemic burden based on wall motion and perfusion abnormalities, it provides also additional information such as left ventricle function at rest, presence of LGE and combined perfusion and wall motion evaluation. Second, this imaging modality is capable of diagnosing other causes of chest pain such as pericarditis and myocarditis. Third, with the increased awareness of cumulative radiation exposure risk associated with nuclear stress tests, stress CMR is a safer alternative for management of patients with suspected or known CAD, particularly in those undergoing repeated tests.
Study limitations
Our study presents several limitations. First, there were a limited number of hard events due to the stable condition of our population. Second, the study lacks information regarding medical therapy after stress CMR. Third, even though a cut-off of 60 days was considered acceptable to define early revascularization, we cannot exclude that some revascularizations beyond this cut-off could have been stress-CMR driven. This may have caused overestimation of the prognostic prediction of all cardiac events by stress CMR. However, while the presence of a positive stress CMR may influence the decision to refer patients to ICA, the decision to perform revascularization was taken at the time of catheterization and was based on the results of invasive findings. Fourth, we did not adjudicate the appropriateness of revascularization. Indeed, in case of stress-CMR driven revascularization procedure of an intermediate coronary artery lesion, we cannot exclude that stress-CMR could be associated with a false positive finding and intermediate coronary stenoses could be a collateral finding at ICA. This scenario could be avoided by using invasive fractional flow reserve measurement as a gatekeeper to PCI as suggested by guidelines. However, revascularizations were not considered in the hard cardiac event analysis to overcome this limitation. Fifth, perfusion was evaluated visually while a quantitative approach has been proved to be more reliable.
Conclusions
Dipyridamol stress CMR provides strong prognostic stratification of patients with suspected or known CAD and allows identification of three categories of patients with low, intermediate and high risk for future cardiac events. Accordingly, it appears an attractive diagnostic strategy to avoid radiation exposure in the management of these patients. Additional studies are warranted to asses if this type of stratification may be useful for choosing between optimal medical therapy and revascularization, reserving the latter option only to patients with the higher risk for cardiac events.
Abbreviations
- AWM:
-
abnormal wall motion
- CAD:
-
coronary artery disease
- CMR:
-
cardiac magnetic resonance
- ECG:
-
electrocardiogram
- ICA:
-
invasive coronary angiography
- LGE:
-
late gadolinium enhancement
- SD:
-
standard deviation
- SPECT:
-
single photon emission computed tomography
References
Shaw LJ, Berman DS, Maron DJ, Mancini GBJ, Hayes SW, Hartigan PM et al (2008) COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 117:1283–1291
Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV et al (2010) Low diagnostic yield of elective coronary angiography. N Engl J Med 362:886–895
Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H et al (2008) MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 29:480–489
Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J et al (2012) Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 379:453–460
Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K et al (2013) MR-IMPACT. Investigators. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J 34:775–781
Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M (2013) Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol 62:826–838
Pontone G, Andreini D, Bartorelli AL, Bertella E, Cortinovis S, Mushtaq et al (2013) A long-term prognostic value of CT angiography and exercise ECG in patients with suspected CAD. JACC Cardiovasc Imaging 6:641–650
Kramer CM, Barkhausen J, Flamm SD, Raymond JK, Nagel E (2008) Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 10:35
Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S et al (2006) Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 113:2733–2743
Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L et al (2008) Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson 10:18
Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC (2007) Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol 50:1343–1353
Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E et al (1999) Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 99:763–770
Macwar RR, Williams BA, Shirani J (2013) Prognostic value of adenosine cardiac magnetic resonance imaging in patients presenting with chest pain. Am J Cardiol 112:46–50
Buckert D, Dewes P, Walcher T, Rottbauer W, Bernhardt P (2013) Intermediate-term Prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: a prospective follow-up study in a consecutive patient population. JACC Cardiovasc Imaging 6:56–63
Coelho-Filho OR, Seabra LF, Mongeon FP, Abdullah SM, Francis SA, Blankstein R et al (2011) Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient's sex. JACC Cardiovasc Imaging 4:850–861
Kelle S, Chiribiri A, Vierecke J, Egnell C, Hamdan A, Jahnke C et al (2011) Long-term prognostic value of dobutamine stress CMR. JACC Cardiovasc Imaging 4:161–172
Wallace EL, Morgan TM, Walsh TF, Dall’Armellina E, Ntim W, Hamilton CA et al (2009) Dobutamine cardiac magnetic resonance results predict cardiac prognosis in women with known or suspected ischemic heart disease. JACC Cardiovasc Imaging 2:299–307
Bodi V, Sanchis J, Lopez-Lereu MP, Nunez J, Mainar L, Monmeneu JV et al (2007) Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J Am Coll Cardiol 50:1174–1179
Bodí V, Rumiz E, Merlos P, Nunez J, Lopez-Lereu MP, Monmeneu JV et al (2011) One-week and 6-month cardiovascular magnetic resonance outcome of the pharmacoinvasive strategy and primary angioplasty for the reperfusion of ST-segment elevation myocardial infarction. Rev Esp Cardiol 64:111–120
Elhendy A, Geleijnse ML, Roelandt JR, van Domburg RT, TenCate FJ, Comel JH et al (1996) Dobutamine-induced hypoperfusion without transient wall motion abnormalities: less severe ischemia or less severe stress? J Am Coll Cardiol 27:323–329
Nasis A, Ko BS, Leung MC, Antonis PR et al (2013) Diagnostic accuracy of combined coronary angiography and adenosine stress myocardial perfusion imaging using 320-detector computed tomography: pilot study. Eur Radiol 23:1812–1821
Acknowledgments
The scientific guarantor of this publication is Gianluca Pontone. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Fabrizio Veglia kindly provided statistical advice for this manuscript.
One of the authors has significant statistical expertise. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: retrospective, diagnostic and prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pontone, G., Andreini, D., Bertella, E. et al. Prognostic value of dipyridamole stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a mid-term follow-up study. Eur Radiol 26, 2155–2165 (2016). https://doi.org/10.1007/s00330-015-4064-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4064-x