Abstract
Objective
The aim of this study was to establish and evaluate (colour Doppler-) high-resolution-ultrasound (hrUS) and bench-top magnetic resonance imaging (btMRI) as new methods to monitor experimental colitis.
Materials and methods
hrUS, btMRI and endoscopy were performed in mice without colitis (n = 15), in mice with acute colitis (n = 14) and in mice with acute colitis and simultaneous treatment with infliximab (n = 19).
Results
Determination of colon wall thickness using hrUS (32 MHz) and measurement of the cross-sectional colonic areas by btMRI allowed discrimination between the treatment groups (mean a vs. b vs. c – btMRI: 922 vs. 2051 vs. 1472 pixel, hrUS: 0.26 vs. 0.45 vs. 0.31 mm). btMRI, endoscopy, hrUS and colour Doppler-hrUS correlated to histological scoring (p < 0.05), while endoscopy and btMRI correlated to post-mortem colon length (p < 0.05).
Conclusions
The innovative in vivo techniques btMRI and hrUS are safe and technically feasible. They differentiate between distinct grades of colitis in an experimental setting, and correlate with established post-mortem parameters. In addition to endoscopic procedures, these techniques provide information regarding colon wall thickness and perfusion. Depending on the availability of these techniques, their application increases the value of in vivo monitoring in experimental acute colitis in small rodents.
Key points
• Improved in vivo monitoring might balance interindividual differences in murine colitis.
• In monitoring murine colitis, btMRI and hrUS are safe and technically feasible.
• Very short examination times underline the usefulness especially of hrUS.
• Results of btMRI and hrUS correlate with endoscopic and post-mortem findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a frequent chronic disease with substantial impact on health and quality of life [1]. However, despite a substantial gain in knowledge over the last decade, the aetiology of IBD is not completely understood [2, 3]. Since reliable in vitro models for IBD are lacking, testing aetiological concepts and developing new therapeutic agents require animal colitis models. Several models of experimental colitis are well established in mice, using toxic agents or genetic modifications to induce specific types of colitis [4, 5]. To evaluate the inflammatory activity of murine colitis in vivo, typically weight loss, stool consistency and rectal bleeding are monitored [6, 7]. Other parameters are determined after sacrificing the animal: standard post-mortem parameters are colon length, colon thickness and weight, and histology.
To balance frequently observed interindividual differences in the course of experimental colitis due to model variability and thus to reduce the number of laboratory animals, it is desirable to use and further develop imaging techniques to follow up colitis in small animals in vivo [8]. The ideal monitoring instrument should provide a high availability with respect to environmental and economic aspects, as well as a high reliability and relevance of the respective imaging results.
Examination techniques like colonoscopy, ultrasound (US) and magnetic resonance imaging (MRI) are routinely used in clinical diagnosis and allow assessment of the severity and extent of colitis in humans, but are not routinely applied in animal models of IBD [9]. Improving imaging capabilities in modern US imaging, especially regarding the high US resolution capacity, might make this technique more attractive for the examination of small animals. While a considerable amount of data and qualified protocols exist regarding colonoscopy in mice [10–12], only very limited experience has been reported in performing US [13], computed tomography [14] and MRI [4, 15–17] in small animals. To our knowledge, a direct comparison of these vivo techniques has not been undertaken so far.
Major disadvantages of conventional MRI are the high technical and environmental requirements, which prevent a broader application of this technique; therefore the use of a smaller MRI device might be advantageous. We report here our first experiences with a prototype bench-top MRI (btMRI) in imaging acute murine colitis.
To improve the usefulness of colour Doppler high-resolution (hr) US, which, to our knowledge, has not been evaluated in follow up colitis in mice so far, a semi-quantitative score was adopted from clinical routine and evaluated to assess the vascularisation of the inflamed intestinal wall. Finally, we aimed at comparing and evaluating colonoscopy, the prototype of btMRI and (colour Doppler-) hrUS with respect to their potential as routine tools to follow up experimental acute murine colitis in vivo.
Materials and methods
Animals
Fifty-one specific pathogen-free male balb/c mice were obtained from Charles River (London, GB) at the age of 2–3 months. The animals were kept in standard laboratory cages in groups of three or four per cage. To avoid potential interfering infections, mice were kept isolated and were fed with pathogen-free food. All care and experimental procedures were performed in accordance with the German national and regional legislation on animal protection.
Induction and treatment of acute colitis
To induce acute colitis, 36 mice were treated for 9 days with 4 % (w/v) reagent grade dextran sulfate sodium solution (DSS; MW 36.000–50.000, MP Biomedicals, Newport Beach, CA, USA) added to the drinking water with free access at all times [18]. Fifteen mice served as a control group and were not treated with DSS.
To examine the ability of the methods to discriminate between acute DSS colitis and DSS colitis under anti-inflammatory treatment, 19 DSS-treated animals were additionally treated once with 40 mg/kg infliximab intraperitoneally before starting the induction of colitis [19, 20].
For daily assessment of colitis activity, intestinal bleeding and stool consistency were documented, using a score described by Wirtz et al. [6].
Endoscopic assessment
Colonoscopy was performed 3 days before inducing colitis and 8 days thereafter using a small animal endoscope (Image1®, Karl Storz, Tuttlingen, Germany). The forward directed charge-coupled device (CCD) camera with an analogue/digital (A/D) converter was placed in the instrument channel of the 18-cm long multipurpose endoscope (2.7 mm diameter). During endoscopy, mice were anaesthetized with isoflurane (Forane®, Abbott, Germany; 2–4 % in oxygen 4 L/min) and placed in a prone position.
To assess the intraluminal status of the intestinal mucosa, the murine endoscopic index of colitis severity (MEICS; ranging from 0 to 15) according to Wirtz et al. [6, 21] was applied.
Bench-top MRI
To ensure the examination of a well defined colon section, 10 μl of an iron oxide-ink-solution (iron oxide Sicovit® black 85 E 172, BASF, Ludwigshafen, Germany; ink 4001® brilliant-black, Pelikan, Hannover, Germany) were injected endoscopically into the colon wall at the splenic flexure via the colonoscopic injection cannula (24G) during endoscopic assessment on day eight (Fig. 1g).
Sequential benchtop MR (btMR) images (from caudal to cranial, a–d) displaying the bentonit-contrasted colon lumen (arrows) in a healthy mouse. (e) and (f) show the measurement of a colonic cross-sectional area using the software ImageJ. The arrows in (g) mark the hypointense signal of the iron oxide-ink-labelling next to the bentonit-contrasted colon lumen. In (f), distortion artefacts of the (ferromagnetic) iron oxide-ink agent are shown
Nine days after the induction of colitis, colon sections were studied using the btMRI MARAN DRX2® (prototype by Oxford Instruments, Molecular Biotools, Witney, UK) with a 0.55 T permanent magnetic system (23 MHz). A T1-weighted signal with a spin echo time of 9.8 ms was set and a 25 × 25 mm field of view was measured in five 3-mm slices immediately caudal of the iron-oxide labelling with a slice separation of 3.3 mm to acquire 64 × 64 pixel images. To reduce the total imaging time to 176 s per examination, only 16 averages with a repetition time of 172 ms were acquired. To improve the imaging of the colonic wall in vivo, a contrast agent (Bentonit-SF®, SERVA 14515) was applied rectally over a 7 F plastic catheter before the btMRI examination (Fig. 1a–d). During the imaging procedure, mice were fixed on a glass slide within the 23 mm diameter gantry of the btMRI and anaesthetized by isoflurane as described above using an inhalation mask installed in the btMRI device (Dräger Vapour System, Dräger, Germany).
To ensure the most similar anatomical locations of the MR-evaluation in different animals, the caudal section of the endoscopically applied iron labelling of the colon wall (at the splenic flexure) was selected for determination of colonic cross-sectional areas (in pixels) by a blinded investigator, using the Java image processing program, ImageJ [22] (Fig. 1e–f).
Ultrasound assessment
Nine days after the induction of colitis, colon wall thickness was measured by US B-Mode-examination using the VEVO 2100 Imaging System (Visual Sonics, Toronto, Canada) with a linear microscan transducer (MS-550D, Visual Sonics, Canada) set to 32 MHz. Before the examination, the mice were anaesthetized as described above and fixed on a heated pad (37 °C). Depilation was achieved using a depilatory cream. An adjustable US transducer was coupled with pre-warmed US gel. The colon was examined at six predefined positions (supplemental Fig. 1). In addition, colour Doppler images were taken (for representative examples see Fig. 2) and subsequently evaluated using a newly developed semi-quantitative score according to the Limberg score in humans [23, 24]. This Doppler-Score was adapted to the examination of mice by replacing the reference colon thickness in humans with the mean thickness of a normal murine colon (0.26 mm) plus one standard deviation of normal murine colon thickness (0.04 mm) as found in our experiments (Table 1).
For all animals the mean thickness (mm) and mean Doppler-score were calculated from the six distinct measurements by a blinded investigator and used for further analysis.
Measurement of post-mortem colonic morphology
Nine days after induction of colitis, mice were sacrificed by cervical dislocation under anaesthesia as described above. The colon including the caecum was excised at autopsy, and length, thickness and weight were measured. Three tissue samples of the colon (distal caecum, splenic flexure and distal rectum) were obtained, fixed with formalin and processed for paraffin embedding.
Histological examinations
Paraffin-embedded colon samples were cut in 5-μm cross-sections. For histological scoring according to Wirtz et al. [6] (score range 0–4), sections were stained with haematoxylin and eosin. Azan stain was used to determine colon wall thickness. By using the digital image analyzer software Lucia G (version 4.80, Laboratory Imaging Ltd), the colon wall thickness of the distal colon was measured in three cross-sections (from outer lamina serosa to inner lamina mucosa, samples taken from the distal caecum, splenic flexure and distal rectum) by a blinded investigator. From the results of the three distinct colon samples the mean score was calculated for an overall histological score.
Statistical analysis
All statistical analyses were performed with SPSS (IBM, version 19) for windows. Significance was confirmed by Student’s t-test and correlations were analysed using Pearson’s correlation coefficient (Pearson Product Moment Correlation). P-values below 0.05 were considered as significant differences.
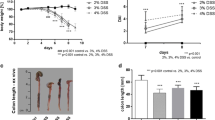
Results
After 9 days, standard parameters like weight loss, colitis activity score and post-mortem parameters were significantly different comparing results of animals without DSS-treatment and animals with DSS-induced acute colitis (n = 14 and n = 15, respectively). As expected for this model of experimental colitis, a significant weight loss was observed in mice with DSS-colitis (23.4 ± 2.4 g vs. 18.4 ± 3.1 g, p < 0.001). The colitis activity score after 9 days was significantly higher in mice with DSS-colitis compared to controls without DSS-colitis (2.6 ± 0.5 vs. 0.08 ± 0.2, p < 0.001). Colon length was 90.2 ± 4 mm in healthy mice and only 59.1 ± 10.2 mm (p < 0.001) in mice with DSS-colitis. Detailed results of the endoscopic, US and btMRI procedures are given in Table 3, and representative exemplary results of the imaging procedures are depicted in Fig. 3.
Exemplary representative images gained by high-resolution ultrasound (hrUS), benchtop MRI (btMRI), colonoscopy and histological assessment of a normal colon and moderate and severe colonic inflammation, illustrating the results of Table 3. In btMRI, the colonic area as analysed by the image processing software ImageJ is indicated by black lines
The duration of the procedures did not differ significantly between the in vivo monitoring methods: from the beginning of anaesthesia to the end of the endoscopic examination, the procedure lasted 5–7 min. The duration of the US examination was between 7 and 12 min (including the depilation procedure and colour Doppler-hrUS). From the beginning of anaesthesia to the end of imaging, btMRI took between 9 and 12 min per animal. Two mice died following btMRI, probably due to anaesthetic-related complications.
Comparison of in vivo monitoring using (colour Doppler-)hrUS, btMRI and colonoscopy
The results of the in vivo imaging procedures were correlated with the respective in vivo colitis activity score and post-mortem assessments (for details see Table 2). Briefly, the endoscopic MEICS-score showed a strong and highly significant correlation with the intestinal bleeding score of Wirtz, as well as with the parameters weight loss, colon length and histological scoring determined post-mortem. btMRI showed a strong and significant correlation with histological scoring and colon length measurement. The results of the US examination and the Limberg score strongly correlated with the histological score, but not with weight loss, colon length or intestinal bleeding.
Through the in vivo imaging methods used, mice with DSS-colitis (n = 14) could be clearly discriminated from healthy mice (n = 15) and from mice with DSS-colitis under anti-inflammatory treatment (n = 19) through significant differences in all diagnostic modalities (for details, see Table 3). In particular, hrUS turned out to be a very useful in vivo diagnostic tool compared to the reference methods (MEICS, post-mortem colon length).
Discussion
In humans, endoscopic visualization of the mucosa is indispensable in diagnosing IBD [25]. Furthermore, several protocols have been established for this endoscopic procedure in mice [11, 26, 27], reflecting its relatively widespread use in experimental colitis.
In our study, endoscopic assessment of the mucosa in the distal murine colon could readily be integrated into the in vivo follow-up procedures, and we could demonstrate a strong and significant correlation of endoscopic findings (MEICS-score) in vivo with post-mortem parameters, as expected from previous findings [10, 11]. While narrow-band imaging (NBI) and chromoendoscopy have been suggested to improve the diagnostic precision of endoscopy [26, 28], the results of our study might not necessarily call for more complex endoscopic procedures to follow up acute murine colitis. Instead, it might be more informative to assess further aspects of colitis, in addition to the mucosal appearance, for instance changes in the thickness or perfusion of the colonic wall, as assessed by hrUS or btMRI, might be of interest.
US and colour Doppler-US allow rapid, non-invasive, sequential assessment of the colon and are routinely applied in patients with IBD [23, 29], but are rarely used to assess experimental colitis in small rodents, maybe due to technical limitations. However, with the increasing resolution of modern US equipment, a re-evaluation of this technique is reasonable. Recently a pilot study [13] was published demonstrating the high practicability and reliability of US for monitoring TNBS- and DSS-colitis in small rodents using a linear array transducer (14 MHz). In the present study, we extend these findings by including patterns of colonic hypervascularisation of the colon as an indicator of inflammation: We demonstrate that the determination of colon wall thickness as well as the modified Limberg score can clearly discriminate between mice with DSS-colitis and animals without colitis and animals with colitis treated with infliximab. Moreover, the significant correlation of US findings with the histological colitis score demonstrates its potential as a routinely applied method to follow up acute experimental colitis in vivo. More complex applications of US in assessing colitis, for example targeted, contrast-enhanced US [30, 31] or US elasticity imaging [32], might be interesting as investigational add-ons to routine US. However, due to the higher complexity of these procedures, they might be reserved for more specific scientific questions, while hrUS as well as colour Doppler-hrUS seem appropriate to monitor the activity of acute colitis routinely in mice. In fact, in our study, the examination of the colon by US could be performed mostly in less than 10 min, making it especially useful in the setting of repeated evaluations.
The measurement of areas of colon sections using btMRI also turned out to be feasible, safe and, moreover, significantly correlated with the histological colitis score and post-mortem colon length. To improve the safety of repeated MRI-examinations, the examination time was kept extremely short to a total scan time of 176 s, while other protocols in small rodents require roughly double that time for one imaging series and more than 20 min for the complete MRI [4, 33]. In other experiments, information on the duration of the MRI procedure is not given [8, 34, 35], making it difficult to assess the value of the respective methods for routine application in the monitoring of experimental colitis. These protocols, mostly including high-resolution MRI and the application of intravenous contrast agents, clearly represent more complex methods to follow up colitis – which might not necessarily be required to monitor acute experimental colitis in vivo; according to our observations, colon wall thickness alone – determined at a predefined and labelled position – seems to be a reliable parameter for assessing acute DSS-colitis. Neverthelessl it is important to point out that the price of the small bench-top design and short examination times in MRI is a relatively low image resolution, which might not allow a reliable detection of details, such as small amounts of ascites surrounding the inflamed colon. Also, it was not possible to discriminate between inflamed and normal colon by measurement of the T1 signal intensity in our study – a limitiation of MRI in murine DSS-colitis that has been observed previously [34].
A novelty in the ultrasonographic examination of murine colitis is the introduction of the modified Limberg score, which to our knowledge has not been used or evaluated in a similar setting so far. The Limberg score is well established for US examinations of the small bowel and colon in humans and is used routinely in a clinical setting. However, especially considering the relatively low number of mice examined in this study, the results should be interpreted with caution and further evaluation of this score in experimental murine coltits is necessary.
It has to be considered that other models of experimental colitis (such as the T-cell transfer model of colitis or TNBS-induced colitis) might display a different pattern of inflammation in the colon. While in DSS-colitis the disease severity is at its maximum in the distal colon, other models have a different disease pattern; however, being aware of different patterns of colitis should make it readily feasible to adapt monitoring by btMRI and hrUS accordingly. Endoscopic labelling of a specific section of the colon through the injection of iron oxide, as presented in our experiments, might be additionally useful for locating the region of interest for btMRI.
btMRI combined with intraluminal contrast enhancement offers a reasonably cost-effective method: in agreement with similar obervations by Mäder et al. [16, 17], btMRI seems to be advantegeous for following up on colonic disease in vivo compared to higher developed MRI-techniques, especially with regard to economic aspects. As a possible disadvantage of btMRI, it has to be considered that only few transversel sections of the colon are assessed, in contrast to hrUS in vivo or the post-mortem analysis of the murine colon, which readily allow examination of the complete colon. However, while hrUS might not reach the same correlation with post-mortem parameters as btMRI in our study, clearly hrUS devices are more readily available even in comparison to the relatively small btMRI used in this study. From our results it is not possible to determine whether the combined use of hrUS and btMRI might further improve in vivo monitoring of murine colitis. Also, considering the low number of animals under examination, it does not seem appropriate to decide, based on our study, whether btMRI or hrUS should be favoured in monitoring experimental colitis in mice.
In conclusion, we demonstrated that in vivo imaging of murine experimental colitis is technically feasible and safe using hrUS, endoscopy or btMRI. Endoscopy has the highest correlation with post-mortem assessments in murine DSS-colitis. hrUS as well as btMRI provide additional aspects for assessing acute colitis, and significantly correlate with the histological colitis score. All methods under investigation were able to discriminate between untreated mice with DSS colitis and mice with DSS-colitis under anti-inflammatory treatment with infliximab and healthy mice.
Introducing the evaluation of colonic vascularisation by colour Doppler-hrUS increases the reliability of hrUS and allows assessment of the inflammation in a more complex approach without requiring a substantial increase in examination time. Depending on availability, hrUS as well as btMRI are valuable methods for complementing endoscopy with regard to in vivo monitoring in experimental murine DSS-colitis.
References
Loftus EV (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126:1504–1517
Baumgart DC, Sandborn WJ (2012) Crohn’s disease. Lancet 380:1590–1605
Brand S (2013) Moving the genetics of inflammatory bowel diseases from bench to bedside: first steps towards personalised medicine. Gut 62:1531–1533
Pohlmann A, Tilling LC, Robinson A et al (2009) Progression and variability of TNBS colitis-associated inflammation in rats assessed by contrast-enhanced and T2-weighted MRI. Inflamm Bowel Dis 15:534–545
Melgar S, Karlsson L, Rehnström E et al (2008) Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol 8:836–844
Wirtz S, Neufert C, Weigmann B et al (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546
Larsson AE, Melgar S, Rehnström E et al (2006) Magnetic resonance imaging of experimental mouse colitis and association with inflammatory activity. Inflamm Bowel Dis 12:478–485
Michael S, Keubler LM, Smoczek A et al (2013) Quantitative phenotyping of inflammatory bowel disease in the IL-10-deficient mouse by use of noninvasive magnetic resonance imaging. Inflamm Bowel Dis 19:185–193
Jelicks LA (2010) Imaging the gastrointestinal tract of small animals. J Neuroparasitol 1, N100504
Huang EH, Carter JJ, Whelan RL et al (2002) Colonoscopy in mice. Surg Endosc 16:22–24
Becker C, Fantini MC, Neurath MF (2006) High resolution colonoscopy in live mice. Nat Protoc 1:2900–2904
Scharl M, Leucht K, Frey-Wagner I et al (2011) Knock-out of β-glucosidase 2 has no influence on dextran sulfate sodium-induced colitis. Digestion 84:156–167
Lied GA, Milde AM, Nylund K et al (2012) Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol 5:195–201
Durkee BY, Weichert JP, Halberg RB (2010) Small animal micro-CT colonography. Methods 50:36–41
Melgar S, Gillberg P-G, Hockings PD et al (2007) High-throughput magnetic resonance imaging in murine colonic inflammation. Biochem Biophys Res Commun 355:1102–1107
Caysa H, Metz H, Mäder K et al (2011) Application of Benchtop-magnetic resonance imaging in a nude mouse tumor model. J Exp Clin Cancer Res 30:69
Besheer A, Caysa H, Metz H et al (2011) Benchtop-MRI for in vivo imaging using a macromolecular contrast agent based on hydroxyethyl starch (HES). Int J Pharm 417:196–203
Whittem CG, Williams AD, Williams CS (2010) Murine colitis modeling using dextran sulfate sodium (DSS). J Vis Exp 1652
Qiu W, Wu B, Wang X et al (2011) PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest 121:1722–1732
Shanmugam NKN, Ellenbogen S, Trebicka E et al (2012) Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS One 7:e38136
Wirtz S, Becker C, Blumberg R et al (2002) Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol 168:411–420
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Neye H, Voderholzer W, Rickes S (2004) Evaluation of criteria for the activity of Crohn’s disease by power Doppler sonography. Digestive 67–72
Limberg B (1999) Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol 37:495–508
Hommes DW, van Deventer SJ (2004) Endoscopy in inflammatory bowel diseases. Gastroenterology 126:1561–1573
Waldner MJ, Wirtz S, Neufert C et al (2011) Confocal laser endomicroscopy and narrow-band imaging-aided endoscopy for in vivo imaging of colitis and colon cancer in mice. Nat Protoc 6:1471–1481
Olson TJP, Halberg RB (2011) in Exp Small Anim Colonoscopy (Miskovitz, P.) (InTech)
Neurath MF, Wittkopf N, Wlodarski A et al (2010) Assessment of tumor development and wound healing using endoscopic techniques in mice. Gastroenterology 139:1837–1843.e1
Hoffmann JC, Preiss JC, Autschbach F et al (2008) Clinical practice guideline on diagnosis and treatment of Crohn’s disease. Z Gastroenterol 46:1094–1146
Bachmann C, Klibanov AL, Olson TS et al (2006) Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 130:8–16
Wang H, Machtaler S, Bettinger T et al (2013) Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin-targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology 3:818–829
Stidham RW, Xu J, Johnson LA et al (2011) Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 141:819–826.e1
Frericks BB, Wacker F, Loddenkemper C et al (2009) Magnetic resonance imaging of experimental inflammatory bowel disease: quantitative and qualitative analyses with histopathologic correlation in a rat model using the ultrasmall iron oxide SHU 555 C. Invest Radiol 44:23–30
Mustafi D, Fan X, Dougherty U et al (2010) High-resolution magnetic resonance colonography and dynamic contrast-enhanced magnetic resonance imaging in a murine model of colitis. Magn Reson Med 63:922–929
Charpentier C, Marion-Letellier R, Savoye G et al (2012) Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm Bowel Dis 18:1940–1949
Acknowledgments
Jens Walldorf and Martin Hermann contributed equally to this research.
The scientific guarantor of this publication is Jens Walldorf. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was not required because no studies on humans were performed. Approval from the institutional animal care committee was obtained. Methodology: experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Predefined positions of ultrasound examination of the colon (GIF 44 kb)
Rights and permissions
About this article
Cite this article
Walldorf, J., Hermann, M., Porzner, M. et al. In-vivo monitoring of acute DSS-Colitis using Colonoscopy, high resolution Ultrasound and bench-top Magnetic Resonance Imaging in Mice. Eur Radiol 25, 2984–2991 (2015). https://doi.org/10.1007/s00330-015-3714-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3714-3