Abstract
Imaging plays a key role in the comprehensive assessment of bowel structural damage. Radiology is fundamental to confirm the initial diagnosis and the extent of disease along the digestive tract. Moreover, imaging is a critical tool in the monitoring of the response to drug therapy. In this chapter, technique, indications and limitations of the major imaging methods used in the assessment of CD (ultrasound, computed tomography, magnetic resonance imaging) are reviewed. A special focus is dedicated to the detection and quantification of bowel fibrosis and bowel wall damage with CT and MRI. Future perspectives are finally reviewed according to the latest developments in imaging technology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
7.1 Introduction
Crohn’s disease (CD) is a destructive, progressive, and disabling inflammatory bowel disease (IBD), potentially involving the gastrointestinal tract from the mouth to anus. Some segments are more involved than others, like ileum and the proximal colonic tract [1]. It is a lifelong condition, progressive but with a typical intermittent activity, characterized by prolonged remission intervals and sudden aggressive recurrences. Despite a purely inflammatory onset of the disease, over 30% of patients develop bowel fibrogenesis (11–44% already at the time of diagnosis) [2], due to the poorly controlled healing process triggered by chronic transmural inflammation. The exact biological mechanisms of fibrosis deposition are still under investigation, but latest evidences confirmed the synergic role of TGF-beta, VEGF, PDGF-alpha, PKC, RAS, RAF, and ERK proteins drawing a complex interplay of genetic, microbial, and immunological factors. Abnormal deposition of extracellular matrix (ECM) is the end product of these molecular cascades, resulting in strictures, scar formation, and tissue distortion. Unfortunately, since bowel fibrosis is irreversible and not responsive to available medications, it usually requires surgical resection with a cumulative anatomical and structural damage. Like fibrotic strictures, fistulae are also common complication of the chronic inflammatory pattern of CD resulting in a permanent bowel damage that usually requires surgery which removes complications but causes further structural damage [3]. Disease eventually recurs in 17–55% of patients at 5 years and 72–73% of patients at 20 years after surgery, leading to new surgical resections in up to 11–32% of cases at 5 years and 46–55% of cases at 20 years [4], with consequent increase in the cumulative bowel damage, loss of quality of life, and disability [5].
The CD phenotypes are classified as non-stricturing non-penetrating [B1], stricturing [B2], and penetrating disease [B3], according to the Montreal classification [6]. Longitudinal follow-up studies have shown that only 40% of patients classified in the B1 group won’t evolve in the stricturing or penetrating group [7, 8]. Prevention of organ damage through an early effective therapy represents a crucial endpoint beyond long-term clinical remission that can impact on the long-term evolution of the disease [9, 10].
7.2 Structural Damage
Imaging plays a key role in the comprehensive assessment of bowel structural damage. Firstly, it is fundamental to confirm the initial diagnosis (f.e. to distinguish Crohn’s disease from ulcerous colitis) and the extent of disease along the digestive tract. Secondly, cross-sectional imaging techniques allow to assess and track the progression of extraintestinal CD manifestations. Moreover, imaging assesses disease activity in CD patients with symptomatic recurrence and represents an important tool in the monitoring of the response to drug therapy. Given its chronic nature, CD needs careful lifelong monitoring to successfully prevent complications and offer the best treatment for the patient. Imaging plays a major role in this continuous assessment, providing an accurate anatomic description of the location and length of CD lesions along the digestive tract, and recently also functional features of the disease have been collected, thanks to the achievement of functional MRI sequences. With many drug therapies for CD being investigated and introduced in the clinical practice, imaging has established as a tool to monitor the response to CD therapy and the progression of bowel damage [11,12,13]. Routine medical imaging for CD includes high-resolution ultrasound, CT-enterography, and MRI-enterography. Endoscopy is also a valuable technique to describe the bowel mucosa but provides just the mucosal assessment with a limited access to the small bowel. Similarly, video-capsule endoscopy does visualize the entire small bowel but cannot provide tissue sampling, and it is contraindicated in obstructed patients. All these intrinsic limitations of the endoscopic assessment make transmural examinations like CT and MRI the two fundamental imaging techniques to yield a rigorous and comprehensive assessment of CD.
Bowel damage in Crohn’s disease (CD) is referred as a spectrum of heterogeneous lesions involving all intestinal layers, ranging from irreversible fibrotic strictures causing luminal narrowing with prestenotic dilatation to abscess, fistulas, aphthous, and deep ulcers [14, 15]. Moreover, active inflammation can coexist with irreversible fibrotic or penetrating lesion [16, 17]. Taking into consideration the Montreal classification, bowel damage is defined as the progression from B1 to B2 and B3, which occurs in the 30–60% of cases in the long term [18]. Bowel damage can be also defined as the presence of strictures, fistulas, or abscesses [14]. Measuring cumulative bowel damage is critical to understand the progression of the disease and to draw an effective treatment plan to prevent it. CD typically starts with a non-stricturing non-penetrating pattern (B1) and evolves to stricturing and/or penetrating during the disease course (B2–B3) [8]. However, stricturing or penetrating complications are present in up to 20–50% at onset, suggesting that early diagnosis and early treatment may be crucial to prevent from structural damage and disease progression. Moreover, as stated by Fiorino et al. [14], the presence of CD-related complications detected by cross-sectional imaging at diagnosis is associated with higher risk of surgery and hospitalizations compared to those without complications. Cross-sectional imaging techniques are the best modalities to depict bowel damage. In particular, US and MRI seem to have the best reproducibility and high sensitivity and specificity in detecting intestinal and extraintestinal damage especially in proximal small bowel, without the risk related to ionizing radiations [19]. On the other hand, if MRI and CT can confidently describe extra-luminal complications like abscesses, fistulas, or the perianal disease [20, 21], endoscopy is complementary to imaging in the detection and evaluation of intraluminal complications, like fibrotic intestinal strictures [22, 23]. The International Program to develop New Indexes in Crohn’s disease (IPNIC) group has worked in the last decade on the integration of MRI findings with endoscopy and clinical history of previous surgery. These efforts lead to the development of the active measure of CD bowel damage, the Lémann index [24], a classification index-based endoscopy, imaging findings (CT and MRI), and surgical history. The Lémann index takes into account strictures, penetration by ulcers, fistulas, abscess, and surgical resection of bowel in each segment for the four CD locations (upper digestive tract, small bowel, colon/rectum, perianal/anal). One major advantage of the Lémann score with regard to other classification systems (e.g., the Montreal classification) is the possibility to quantify the severity of the structural bowel damage. This is particularly useful when measuring bowel damage progression with repeated assessments over time.
7.3 MRI Technique and Assessment of Bowel Damage
Bowel damage is the result of both active and chronic phases of the disease. The active phase is characterized by exacerbation of clinical symptoms laboratory and markers indicative of active inflammation. When assessing bowel damage, MRI plays the fundamental role. 1.5 T or 3 T MR enterography provides both morphological and functional information through conventional MR sequences and dynamic contrast-enhanced (DCE) MRI with diffusion-weighted imaging (DWI). An MRE-based score (MR index) has been developed that has proven to be useful in measuring the activity and severity of CD, alongside the currently used validated endoscopic scores [25]. The MR index has a high accuracy for the detection of disease activity and the detection of ulcerative lesions (Fig. 7.1).
In the acute inflammatory state, MRI shows wall thickening >3 mm (due to acute inflammation or fibrosis), ulcerations, fistulas, and mural and peripheral edema all characterized by hyperintensity in T2-W fat-suppressed/fluid-sensitive sequences, surrounded by a halo of T2-W intermediate signal (Fig. 7.2).
The acute edematous wall can cause stricturing even in the acute phase of the disease, mimicking a chronic fibrostenotic stricture.
Common findings of chronic CD are the fibro-fatty changes of the mesentery, fibrotic strictures, and fistulas, which are better displayed with fast spin echo or contrast-enhanced T1-W sequences. The degree of inflammation correlates well with the hyperintensity on T2-W due to edema, the presence of ulcerations, and blurred margins. By acquiring contrast-enhanced CT or MRI, an early submucosal and serosal hyperenhancement together with the edematous submucosa make the bowel wall appear thickened and markedly layered, with a target appearance. These characteristics of active inflammation are positive predictors of response to anti-TNF agents. Moreover, a significant association exists between T2 hyperintensity, ulceration, inner wall hyperenhancement, blurred margin, wall thickness, and degree of histological inflammation. Since CD is a transmural pathology, a diffuse enhancement may be seen extending toward the mesentery during the active phase. Finally, deep ulcers appear as a high-signal T2-W serpiginous images that alternate with thickened mucosal folds (the so-called cobblestone appearance) [26, 27].
Parameters like the presence of edema, ulcers, pseudopolyps, lymph node enlargement, mural thickness, T1-W bowel wall ratio, T2-W bowel wall ratio, DCE MRI maximum enhancement (ME), initial slope of increase (ISI), time to peak (TTP), and apparent diffusion coefficient (ADC) on DWI allow for a comprehensive evaluation of CD-related damage [27]. A moderate and significant correlation was found among mural thickness, T1 ratio, T2 mural/CSF ratio, ME, ISI, and inflammation [27]. Moreover, mural thickness, T1 ratio, T2 mural/CSF ratio, ME, ISI, and ADC values also showed significant differences between grades of fibrostenosis. T2 mural/CSF ratio can be used to discriminate between inflammation and fibrosis. Interestingly, mural thickness and T1 ratio correlated with both AIS and FS in the study by Tielbeek et al. [27]. These findings support the hypothesis that inflammation and fibrosis are not excluding processes [28]), even in the same bowel segment [29, 30]. Also DCE MRI correlated well with the histopathological specimens in assessing CD activity, since ME and ISI correlated well with a histopathology-based reference standard. DCE MRI confirms to be of additional value to the conventional MRI protocol in order to facilitate better grading of Crohn’s disease activity.
Finally, accurate detection and grading of bowel fibrosis are pivotal to optimize patient’s selection for potential responsiveness to antifibrotic agents that are now under clinical validation [31,32,33]. CT and MRI gave similar results for the detection of bowel wall thickening >3 mm. MRI easily detects signs of bowel wall edema in T2W and has been shown to be as sensitive as CT in the evaluation of extraintestinal abdominal signs of CD, such as involvement of perivisceral fat and abdominal adenopathies. In the evaluation of enteroenteric fistulas, CT accuracy is similar to MRI, although for both techniques sensitivity, specificity, and accuracy have been described as less than 50%.
Finally, neither CT nor MR is sufficiently sensitive and specific for the detection of purely mucosal lesions, and there is still not enough evidence supporting their alternative role to endoscopy in assessing mucosal healing.
7.3.1 Technical Notes for MRI in CD
MRI, with a 91% of specificity and sensitivity, can be considered as the reference imaging technology for assessment of CD. Two techniques are traditionally available: MRI-enterography and MRI-enteroclysis. The latter consists in the administration of oral contrast through a nasojejunal tube but has been replaced by the more common MRI-enterography.
MRI-enterography is the most common MRI technique and is performed after the ingestion for 1–2 L of hyperosmolar oral contrast solution, like 2.5% mannitol solution, barium sulfate, or polyethylene glycol (PEG), with the patient fasting for at least 6 h. The oral contrast medium appears typically low on T1-W and high in signal on T2-W. Glucagon can be used as a spasmolytic drug, and a rectal enema may be useful to increase the visualization of the terminal ileum. Study protocol includes the acquisition of coronal and axial images using a phased array coil and a high-field (1.5–3.0 T) magnet. True fast imaging with steady-state precession and HASTE sequences with and without fat suppression are usually obtained. Fast-suppressed coronal and axial (or three-dimensional) T1-W breath-hold/VIBE gradient echo of the abdomen and pelvis are finally required, before and 70s and 7 minutes after intravenous contrast administration, to evaluate the presence of fibrosis. Moreover, DWI assessment has been increasingly inserted in the CD MRI protocol. Being the DWI inversely related to the cell density, an acutely inflamed area will appear as an area of restricted diffusion (hyperintense) with decreased ADC values. Despite the discomfort of a large amount of liquid, the high costs of the examination, the difficulty to hold the breath intermittently, and the long MR acquisition times, MRI provides excellent details of ulcers, mural penetration, wall thickening, hyperemia, and peri-intestinal reactive structures without exposing the patients to the risks of ionizing radiations.
7.4 Detection of Fibrosis
Fibrosis is the result of a chronic inflammation and is a cause of major complications like bowel obstruction. Among the many classifications proposed to quantify fibrosis in CD, the Montreal classification [17] is the most common. CD patients that develop fibrosis are labeled as Montreal class B2 inflammatory phenotype.
Fibrosis deposition occurs predominantly in deep layers of the bowel. Limited edema, a compact tissue, and a reduced number and diameter of the vessels compared with the normal mucosa are typical findings inside the fibrotic areas.
Since treatment options are formulated on the discrimination between active inflammatory and fibrotic-predominant lesions, a major challenge for medical imaging is to differentiate lesions that can still respond to medical treatment (inflammatory-related) from the ones that benefit only from endoscopic balloon dilatation or surgical resection (fibrotic-related bowel thickening) [3, 34, 35]. At this regard, both MRI and CT can detect fibrosis with direct and indirect signs, but MR enteroclysis is significantly more sensitive than CT (with and without contrast administration) to detect intestinal stricture (sensitivity 57% vs 42%, specificity 82% vs 68%). Postoperative recurrence of fibrostenosis has been investigated with CT virtual colonoscopy, but its diagnostic performance has not overpassed colonoscopy yet.
In order to maximize the visibility of the strictures, enterography technique (CT or MRI), better if performed after enteroclysis, is the best choice [36]. Pseudosacculations in the antimesenteric side of the bowel wall indicate presence of fibrosis [37]. Mural thickness, edema, and stratification have been found to correlate with fibrosis, but a global agreement has not been met yet.
The intestinal stenotic tract appears thickened, proportionally to the histological degree of fibrosis, and suffers usually a luminal narrowing. Decrease of signal intensity of the thickened bowel wall or low bowel-wall signal intensity, fat-saturated T2-weighted MRI images, and a reduction in bowel-wall early enhancement or absent or minimal transmural enhancement on gadolinium contrast MR are usually associated with intestinal fibrosis. MRI can therefore potentially distinguish between patients with fibrosis and superimposed inflammation and those with chronic fibrosis [38].
However, signal intensity is not always decreased in T2W within the fibrotic areas. Higher T2 mural/CSF ratios have been significantly associated with more inflammation as well as mild fibrostenosis, whereas low T2 mural/CSF ratio is significantly associated with low inflammation scores and with severe fibrostenosis. Decrease in ADC values also correlated significantly with fibrostenosis [27]. Even if fibrosis is better evaluated with MRI, it still can be detected on CT due to indirect signs like bowel sacculations, dilatations, and mural thickness.
DCE MRI represents an added value in the assessment of the CD activity. Even if an early contrast enhancement is not found in the fibrotic areas, later, contrast-enhanced phase shows a homogenous pattern of enhancement that progresses over time along with the natural history of the disease (Fig. 7.3).
Rimola et al. described different patterns of enhancement at 70s and at 7 min, representing the two phases with the higher predicting value for CD activity [39]. At 70 s, three patterns are known: enhancement of superficial (mucosal) layer, homogeneous enhancement (all bowel wall enhancing equally), and layered enhancement (both mucosa-submucosa and serosa enhancing, with an in-between band of poor enhancement). Even if at 70 s the more superficial layers show a slightly increased contrast enhancement, at 7 min, each layer (deep and superficial) shows a similar hyperenhanced appearance. A homogenous pattern of enhancement at 70 s, an increased signal intensity in T2-W, and a progressive contrast enhancement >25% between 70 s and 7 min have been demonstrated to be a possible indicator of fibrosis [39], although these findings need to be validated in further studies (Fig. 7.4).
7.5 Ultrasound
Ultrasound (US) is a routine imaging technique for the assessment of CD patients. Some current applications of US and its implementations like color Doppler and CEUS are monitoring the activity of the disease, especially in pediatric patients or when a good-quality MRI scan may be complicated to achieve. A proper US examination requires a high-frequency probe and a multiplanar image acquisition of the whole abdomen. A pre-FAST scan is recommended, and a full bladder provides an acoustic window for the assessment of the pelvic bowel loops. CEUS can provide information about the microvascular density of the thickened bowel wall since US contrast agents like polyethylene glycol solution do not have the interstitial phase, drawing a direct correlation between vascularization and the contrast enhancement [2, 40,41,42]. Ultrasound elastography is also showing some potential in evaluating bowel wall fibrosis and in discriminating between active or chronic inflammation. Moreover, wall stiffness measurement seems to be an emerging tool to detect the presence of severe fibrosis in a stenotic intestinal tract [43]. Strain differences in the pathological bowel tracts have been directly associated with increased muscular fibers and collagen deposition and allow to discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens [44].
Common findings of CD in ultrasound are bowel wall thickening (large bowel wall >3 mm, small bowel wall >2.5 mm), strictures associated with prestenotic dilatation, a decrease of normal peristalsis, duct-like structures representing fistulas, and fibro-fatty, echogenic, and hyperemic changes of the mesentery associated with local lymphadenopathies. A scoring system, the sonographic lesion index for CD (SLIC), has been recently proposed by Calabrese et al., based on the small intestine contrast ultrasonography (SICUS), which unfortunately lacks of good reproducibility due to its complexity, thus limiting its wide adoption [45, 46].
7.6 Computed Tomography
Computed tomography (CT) is a widely available, time-sparing, cross-sectional imaging method that allows diagnosis and monitoring of CD.
In the active phase of the disease, CT provides visualization of mural stratification and thickening, submucosal fat deposition, fat stranding around the bowel wall, and the presence of lymphadenopathies. Since the late 1990s, contrast-enhanced CT has entered in clinical practice for the evaluation of CD. After injection of iodized intravenous contrast medium, wall hyperemia can be quantified, and typical finding of active inflammation like the “comb sign” due to the distended vasa recta of the intestinal arcades in the mesentery can be found. Deep mural ulcers can be better visualized with MRI than with CT. On the contrary, acute emergencies like pneumoperitoneum due to visceral perforation, toxic megacolon with its classic appearance of loss of haustral folds, mural thinning, and dilation >6 cm are optimally displayed by CT intestinal and extraintestinal complications of CD like abscess, perforation, fistula, or bowel obstruction which can easily be described with contrast enhanced CT.
In the chronic phase, fat proliferation can be found in the mesentery as well as submucosal fat deposition in the affected bowel tracts. Despite the good diagnostic performance and the relatively inexpensive costs when compared to MRI, CT still presents the disadvantage of ionizing radiation exposure (reduced from to 15 mSv to 1–5 mSv when using specific iterative dose reduction techniques in CT scans) and the discomfort of ingesting a considerable amount of contrast liquid. Recently, dual-energy CT scan has been used in the evaluation of CD, with slightly better diagnostic accuracy than conventional CT [47]. Despite these technical improvements, recent studies have demonstrated [48].
Nevertheless, CT still provides higher sensitivity in detecting lymphadenopathies and in assessing the extent of abscesses and to plan their percutaneous drainages. Despite the overall accuracy of detecting active inflammation is comparable to MRIs, CT is no more considered as the reference imaging method in the initial diagnosis or to rule out CD complications. The rationale of CT is still to be found, however, in its favorable ratio between rapidity and diagnostic accuracy and in its wide availability, which makes it the best option in the emergency setting [49].
7.7 Nuclear Medicine
In severe CD cases, a 18FDG-PET/CT hybrid imaging may be useful for the evaluation of CD as it is highly sensitive in identifying acute inflammation, even more than MRI in some recent published studies [32, 50]. FDG-PET/CT combined with ultrasound resulted in a 100% detection rate of strictures in a recent work by Lenze et al. [32, 50]. Also, a hybrid approach has been found useful in discriminating between purely fibrotic, acute inflammatory, and mixed strictures. Imaging biomarkers extracted from PET/MRI images like SUVmax, signal intensity on T2-weighted images × SUVmax and ADC × SUVmax [51]. In particular, ADC × SUVmax > 3000 is the indicated threshold to differentiate purely fibrotic strictures from inflammatory or mixed ones.
7.8 Future Prospects
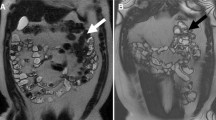
State-of-the-art CD imaging allows to noninvasively monitor disease activity and treatment response (Fig. 7.5).
Same patient of Fig. 7.1 (a) T2-W sequence in coronal and axial plane (b) shows resolution of the strictures and of the inflammatory parameters after therapy. T1-W FS sequence after Gd administration shows only mild wall enhancement
Latest efforts aim to provide functional information about disease activity in order to achieve an earlier diagnosis. MRI-based investigations are focusing on interesting technologies like the MR diffusion-weighted imaging and MR magnetization transfer imaging. DW imaging promises to detect early inflammatory changes or early treatment response (Fig. 7.6).
DWI hyperintensity correlated well with endoscopic inflammation in CD and may play a critical role in the assessment of patients who cannot bear IV contrast administration. MR magnetization transfer imaging rose excitement about its potential ability to discriminate fibrotic scarring from acute inflammation by quantifying the mean magnetization transfer ratio, which is lower in tissues with active inflammation [38]. Fast cinematic balanced steady-state free precession sequences are another frontier that needs to be further investigated. In addition to this traditional protocol, newer MRI techniques have been introduced in the study of CD, like the automated motility mapping analysis and magnetization transfer MR. As peristalsis functionality is compromised in CD, its evaluation through particular MRI sequences is useful to quantify its involvement. Sequences like fast T2-W SSFP or echo planar with serial acquisition of images every 300–1000 ms during a normal breath-hold period can assess visually and quantitatively if the normal peristalsis is still preserved. One last point has to be dedicated to hybrid imaging. As a raising imaging modality, MRI can be coupled with a simultaneous positron-emission tomography acquisition. The PET/MRI scan combines an excellent morphologic portrait of the pathology with the pharmacokinetic functional information of PET. No strong evidence have been presented yet, but the growing interest in the hybrid approach will provide soon more insights [52]. Ultrasound elastography imaging (UEI) is an emerging imaging technique which has already showed promising results [53] in detecting bowel fibrosis in advanced CD. Further validation is needed to guide UEI into the daily clinical practice.
Endoscopy is not well accepted by most patients, and an alternative, noninvasive method to assess mucosal healing would be appreciated. MRI has shown promising results with the introduction of the magnetic resonance index of activity (MaRIA), which correlated well with endoscopic findings [54]. Novel MR biomarkers could represent the logical next step toward accurate assessment of bowel mucosa.
In conclusion, the efforts of modern imaging aim to provide a meticulous monitoring program of the disease progression and of the early treatment response, in order to improve the long-term outcome and to prevent irreversible structural damage.
References
Freeman HJ. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J Clin Gastroenterol. 2003;37:216–9.
Sasaki T, Kunisaki R, Kinoshita H, et al. Doppler ultrasound findings correlate with tissue vascularity and inflammation in surgical pathology specimens from patients with small intestinal Crohn’s disease. BMC Res Notes. 2014;7:363.
Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62.
Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol. 2005;11:3971–9.
Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut. 2012;61:241–7.
Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15.
Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–82.
Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–50.
Molenaar ETH, Voskuyl AE, Dinant HJ, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42.
Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut. 2017;66:2179–87.
Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–82.e1.
Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lémann index is reversible on anti-TNF therapy for Crohn’s disease. J Crohns Colitis. 2015;9:633–9.
Bodini G, Giannini EG, De Maria C, et al. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease. A study performed using the Lémann index. Dig Liver Dis. 2017;49:175–80.
Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis. 2017;11:274–80.
Peyrin-Biroulet L, Loftus EV, Colombel J-F, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–7.
Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Preventing collateral damage in Crohn’s disease: the Lémann index. J Crohns Colitis. 2016;10:495–500.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A.
Vester-Andersen MK, Prosberg MV, Jess T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705–14.
Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–45.
Pariente B, Peyrin-Biroulet L, Cohen L, et al. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol. 2011;197:42–9.
Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17:1073–80.
Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–12.
Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–9.
Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148:52–63.e3.
Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–20.
Eder P, Michalak M, Katulska K, et al. Magnetic resonance enterographic predictors of one-year outcome in ileal and ileocolonic Crohn’s disease treated with anti-tumor necrosis factor antibodies. Sci Rep. 2015;5:10223.
Tielbeek JAW, Ziech MLW, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24:619–29.
Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252:712–20.
Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis. 2011;17:984–93.
Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis. 2012;18:849–56.
Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–35.
Lenze F, Wessling J, Bremer J, et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis. 2012;18:2252–60.
Latella G, Sferra R, Speca S, et al. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013;17:1283–304.
Lahat A, Chowers Y. The patient with recurrent (sub) obstruction due to Crohn’s disease. Best Pract Res Clin Gastroenterol. 2007;21:427–44.
Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55–60.
Ram R, Sarver D, Pandey T, et al. Magnetic resonance enterography: a stepwise interpretation approach and role of imaging in management of adult Crohn’s disease. Indian J Radiol Imaging. 2016;26:173–84.
Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53.
Fiorino G, Bonifacio C, Malesci A, et al. MRI in Crohn’s disease—current and future clinical applications. Nat Rev Gastroenterol Hepatol. 2011;9:23–31.
Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–40.
Ripollés T, Rausell N, Paredes JM, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohns Colitis. 2013;7:120–8.
Wilkens R, Peters DA, Nielsen AH, et al. Dynamic contrast-enhanced magnetic resonance enterography and dynamic contrast-enhanced ultrasonography in Crohn’s disease: an observational comparison study. Ultrasound Int Open. 2017;3:E13–24.
Morimoto K, Watanabe K, Noguchi A, et al. Clinical impact of ultrathin colonoscopy for Crohn’s disease patients with strictures. J Gastroenterol Hepatol. 2015;30(Suppl 1):66–70.
Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis. 2015;21:2605–12.
Dillman JR, Stidham RW, Higgins PDR, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014;33:2115–23.
Calabrese E, Zorzi F, Zuzzi S, et al. Development of a numerical index quantitating small bowel damage as detected by ultrasonography in Crohn’s disease. J Crohns Colitis. 2012;6:852–60.
Calabrese E, Zorzi F, Pallone F. Ultrasound of the small bowel in Crohn’s disease. Int J Inflam. 2012;2012:964720.
Peng JC, Feng Q, Zhu J, et al. Usefulness of spectral computed tomography for evaluation of intestinal activity and severity in ileocolonic Crohn’s disease. Therap Adv Gastroenterol. 2016;9:795–805.
Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–85.
Haas K, Rubesova E, Bass D. Role of imaging in the evaluation of inflammatory bowel disease: how much is too much? World J Radiol. 2016;8:124–31.
Holtmann MH, Uenzen M, Helisch A, et al. 18F-Fluorodeoxyglucose positron-emission tomography (PET) can be used to assess inflammation non-invasively in Crohn’s disease. Dig Dis Sci. 2012;57:2658–68.
Catalano OA, et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in crohn disease. Radiology. 2016;278:792–800. https://doi.org/10.1148/radiol.2015150566.
Stanley E, Moriarty HK, Cronin CG. Advanced multimodality imaging of inflammatory bowel disease in 2015: an update. World J Radiol. 2016;8:571–80.
Giannetti A, Matergi M, Biscontri M, et al. Real-time elastography in Crohn’s disease: feasibility in daily clinical practice. J Ultrasound. 2017;20:147–55.
Buisson A, Pereira B, Goutte M, et al. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig Liver Dis. 2017;49:1211–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bonifacio, C., Gennaro, N., Fiorino, G. (2019). Role of Imaging in Detecting Bowel Fibrosis and Bowel Damage. In: Rimola, J. (eds) Cross-Sectional Imaging in Crohn’s Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-96586-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-96586-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96585-7
Online ISBN: 978-3-319-96586-4
eBook Packages: MedicineMedicine (R0)