Abstract
Objective
To evaluate the accuracy of two different sonographic median nerve measurement calculations in predicting carpal tunnel syndrome (CTS) severity in a study population with clinically and electrophysiologically confirmed CTS.
Methods
643 wrists of 427 patients (325 females and 102 males, age range: 17–90 years, mean ± SD: 57.9 ± 14.7) were included with CTS diagnosis based on clinical and nerve conduction studies (NCS). Cross-sectional area (CSA) measurement of the median nerve was performed at the carpal tunnel level (CSAc) and at the pronator quadratus muscle level (CSAp). Two parameters were calculated: delta (∆-CSA), which is the difference between proximal and distal measurements, and ratio (R-CSA), calculated by dividing distal over proximal measurements.
Results
Patients were classified into mild, moderate and severe CTS based upon NCS. The mean ∆-CSA (4.2 ± 2.6, 6.95 ± 2.2 and 10.7 ± 4.9 mm2) and mean R-CSA (1.5 ± 0.4, 1.95 ± 0.4 and 2.4 ± 0.7) values were significantly different between all groups (p < 0.001). Optimal cut-off values for ∆-CSA and R-CSA were 6 mm2 and 1.7, respectively, to distinguish mild from moderate disease, and 9 mm2 and 2.2, respectively, to distinguish moderate from severe disease.
Conclusion
Threshold values for the calculated sonographic parameters ∆-CSA and R-CSA are useful in predicting CTS severity compared to NCS.
Key Points
• Two proposed parameters were calculated (∆-CSA, R-CSA) and compared to NCS.
• A defined sonoanatomical proximal landmark was used for the calculation.
• Both parameters showed ability to detect CTS severity comparable to NCS.
• Cut-off values could be determined for both parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) is the most common peripheral entrapment neuropathy, caused by compression of the median nerve within the wrist area [1, 2]. A minority of studies suggest that the prevalence of CTS may be as high as 3 % or 4 % [3]; however, most publications report a prevalence of approximately 2 % of the adult population in the USA, affecting 4–10 million patients [4, 5].
An emerging role for ultrasound (US) in the diagnosis of CTS has been described in the past few years [6–10].
Cross-sectional area (CSA) measurement of the median nerve is the most important and commonly used US parameter for CTS diagnosis. Measurements obtained at different points along the carpal tunnel (inlet and outlet) compared with CSA measurements at the mid forearm may further improve the diagnostic utility of US [11–16].
Several studies have calculated a ratio between a proximal measurement of the median nerve at the mid forearm and a distal measurement of the median nerve at the carpal tunnel inlet (R-CSA) [9, 10]. Other studies have suggested using the CSA difference between a proximal median nerve measurement at the level of the pronator quadratus and a measurement within the carpal tunnel, resulting in a delta CSA (∆-CSA). Although these two recently proposed parameters (R-CSA, ∆-CSA) demonstrate better discriminating performance than CSA measurement alone, no correlation was found with NCS severity, and threshold values for NCS grades were not defined [17–19].
The goal of this study was to evaluate the role of US in the prediction of CTS severity by correlating two recently proposed parameters (∆-CSA and R-CSA) to nerve conduction studies (NCS) in order to establish threshold values for sonographic grading of CTS.
Materials and methods
This study was approved by the university ethics committee. Informed written and verbal consents were obtained from all patients. The study was conducted on 643 wrists in 427 consecutive patients (325 females and 102 males) with an age range from 17–90 years (mean ± SD: 57.93 ± 14.73) in the period from January 2010 to December 2013. All patients showed characteristic clinical symptoms of CTS and the diagnosis was confirmed by NCS performed 1 week prior to US examination. CTS severity was classified on the basis of the electrophysiological results as mild, moderate and severe according to the modified scoring system of Padua et al. [20] (Table 1). In the current study we limited our analysis to three grades of disease severity (mild, moderate and severe), and did not include a category for extremely severe CTS since both severe and extremely severe CTS are treated with the same therapeutic option.
The examining radiologist was blinded to patient symptoms and to NCS results, and did not ask the patients for any clinical information.
Any participant with a history of a systemic disease such as rheumatoid arthritis, diabetes mellitus or thyroid dysfunction was excluded from the study. Pregnancy, history of distal radius fracture, provisional diagnosis of cervical radiculopathy as well as previous history of treatment for CTS (surgery or corticosteroid injection) were additional exclusion criteria.
Our study aimed to assess the ability of high resolution US measurements of the median nerve to classify the severity of CTS; therefore a healthy control group was not included.
A musculoskeletal radiologist with 15 years’ experience in musculoskeletal US performed the examination with a 15-6 MHz linear array transducer (LOGIQ E9, GE Healthcare, USA) or an 18-5 MHz linear array transducer (HI VISION Prerius, Aloka Medical, Tokyo, Japan). Subjects were seated facing the examiner with their arms extended, their wrists resting on a flat surface, their forearms supine and their fingers semi-extended. Real-time axial US imaging of the median nerve was performed from the distal forearm to the carpal tunnel outlet. Two measurements of the median nerve CSA were obtained as previously described [17]: the distal CSA measurement was obtained at the point of maximal median nerve change in shape within the carpal tunnel between its inlet and exit points. The largest CSA obtained between the scaphoid-pisiform level and the trapezium-hamate level – the inlet and exit levels of carpal tunnel – was registered as CSAc. A second measurement obtained more proximally at the level of the proximal third of the pronator quadratus muscle in the distal forearm, was defined as the proximal CSA (CSAp). The median nerve was depicted between the flexor pollicis longus and the flexor digitorum superficialis tendons at the level of the pronator quadratus muscle. Transducer pressure was minimized at this site during median nerve examination and transducer angle was maintained perpendicular to the nerve course in order to improve the reproducibility of our sonographic measurements. It must be stressed that a perpendicular orientation of the transducer relative to the nerve is mandatory during the entire examination.
Measurement of the CSA was performed by tracing a continuous caliper around the margin of the median nerve. The trace was performed along the inner border of the perineural echogenic rim, corresponding to the perineurium around the hypoechoic median nerve. Measurements at each level (distal and proximal) were repeated three consecutive times with the mean used for the statistical calculations.
Two parameters (∆-CSA and R-CSA) were calculated for each wrist using the two previously described parameters: The difference (∆-CSA) was calculated by subtracting CSAp from CSAc, the ratio (R-CSA) was calculated by dividing CSAc by CSAp (CSAc/CSAp).
Statistical analysis
Statistical analysis was performed with Stata 12.1 (College Station, TX, USA). The unit of analysis for this study was the individual wrist. Each wrist was evaluated independently.
For the tabulation of CSAc, ∆-CSA and R-CSA, the analysis was stratified by severity of disease in each wrist on the basis of NCS. Histograms and box plots of ∆-CSA and R-CSA were plotted at each level of disease severity to allow a visual demonstration of the distribution of ∆-CSA and R-CSA scores as a function of NCS. Mean and standard deviation (SD) were calculated for CSAc, ∆-CSA and R-CSA in each severity group. One-way analysis of variance (ANOVA) was applied to determine whether each CSAc, ∆-CSA and R-CSA varied significantly with severity of disease. A significant result in the ANOVA was followed by unpaired t-tests to document significant differences for each of these three parameters in mild versus moderate disease, and in moderate versus severe disease. A p-value <0.05 was considered significant for these comparisons.
Paired receiver operating characteristic (ROC) analysis was performed to compare the diagnostic accuracy of ∆-CSA to that of R-CSA for the classification of CTS severity based upon the area under the ROC curves (AUC). As the two wrists on an individual patient are paired and may not represent statistically independent observations, the statistical comparison of ROC AUC was repeated independently for the subsets of right wrists and left wrists to verify the presence of statistically significant results. The optimal cut-off points for distinguishing mild from moderate disease and for distinguishing moderate from severe disease were determined by Youden’s index to maximize the sum of sensitivity and specificity.
Results
Each wrist was classified into one of three main groups according to CTS severity based on the results of NCS; group 1 represented mild CTS (n = 272 wrists), group 2 represented moderate CTS (n = 152 wrists) and group 3 represented severe CTS (n = 219 wrists).
The mean ± SD CSAc was 12.52 ± 2.74 mm2 in group 1 patients, 14.66 ± 2.50 mm2 in group 2 patients, and 18.81 ± 5.31 mm2 in group 3 patients. ANOVA and follow-up t-tests demonstrated a significant difference in CSAc among patients with mild, moderate and severe CTS (p < 0.001).
Histograms and box plots of ∆-CSA and R-CSA are presented at each level of disease severity (Figs. 1 and 2). These graphs clearly demonstrate that although there is an overlap in values of ∆-CSA and R-CSA at different levels of disease severity, there is a clear tendency for higher values of ∆-CSA and R-CSA with greater severity of disease. Furthermore, the box plots demonstrate little overlap among the interquartile ranges for these parameters at the different levels of disease severity.
Box plots of ultrasound parameters, stratified on the basis of NLG score. The interquartile range is shown by the shaded gray box. Outlier scores are represented by the points outside the error bars. Note that there is little overlap among the interquartile scores. a Box plot for ∆-CSA; b Box plot for R-CSA
The mean ∆-CSA was 4.21 ± 2.56 mm2 for group 1, 6.95 ± 2.18 mm2 for group 2 and 10.68 ± 4.92 mm2 for group 3 patients (Figs. 5, 6 and 7). ANOVA and follow-up t-tests demonstrated a significant difference in ∆-CSA among patients with mild, moderate and severe CTS (p < 0.001).
The mean ± SD R-CSA was 1.53 ± 0.35 in group 1, 1.95 ± 0.39 in group 2 and 2.38 ± 0.67 in group 3 patients (Figs. 5, 6 and 7). ANOVA and follow-up t-tests demonstrated a significant difference in R-CSA among patients with mild, moderate and severe CTS (p < 0.001).
ROC analysis revealed good discriminating ability with the use of ∆-CSA and R-CSA (AUC = 0.859 for ∆-CSA and AUC = 0.841 for R-CSA in the differentiation between mild and moderate disease severity). A good ROC discriminating ability was noted for distinguishing moderate from severe disease with AUC = 0.783 for ∆-CSA and AUC = 0.710 for R-CSA in the differentiation between group 2 and 3 patients (moderate and severe groups). Discrimination between mild and severe disease was even better, with AUC = 0.934 for ∆-CSA, and AUC = 0.915 for R-CSA (Figs. 3 and 4).
Comparisons of the AUC between ∆-CSA and R-CSA demonstrated no significant difference for distinguishing mild from moderate disease in the overall group (p = 0.07) or among the subgroup of right-sided wrists, although there was a statistically significant difference among the left-sided wrists (AUC = 0.83 for ∆-CSA vs. 0.79 for R-CSA; p = 0.02). The ∆-CSA parameter was significantly superior for distinguishing moderate from severe disease in the overall group (p < 0.001) as well as among the subgroups of right-sided wrists (AUC = 0.79 for ∆-CSA vs. 0.72 for R-CSA; p < 0.001) and left-sided wrists. (AUC = 0.77 for ∆-CSA vs. 0.71 for R-CSA; p = 0.018). Finally, the ∆-CSA parameter was superior for distinguishing mild from severe disease in the overall group (p = 0.003) as well as in the subgroup for left-sided wrists (AUC = 0.92 for ∆-CSA vs. 0.90 for R-CSA; p < 0.02), although the difference in AUC failed to meet our test for significance for right-sided wrists (AUC = 0.94 for ∆-CSA vs. 0.93 for R-CSA; p = 0.06). Taking these results together, both ∆-CSA and R-CSA demonstrated good discriminating severity for disease severity with a tendency for ∆-CSA to outperform R-CSA by a small margin (Figs. 5, 6, and 7).
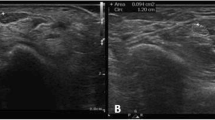
a Transverse ultrasound image of the median nerve (outlined) at the proximal level (level of the pronator quadratus muscle) in a 53-year-old female patient with mild CTS, the CSAp is 7 mm2. R = radius, PQ = pronator quadratus muscle. b Transverse ultrasound image of the median nerve (outlined) at the distal level (level of the carpal tunnel) showing a CSAc of 12 mm2. The ∆-CSA therefore is 5 mm2, whereas the R-CSA is 1,71 consistent with the grading of a mild CTS sonographically. FCR = flexor carpi radialis tendon
a Transverse ultrasound image of the median nerve (outlined) at the proximal level (level of the pronator quadratus muscle) in a 42-year-old male patient with moderate CTS, the CSAp is 6 mm2. R = radius, PQ = pronator quadratus muscle. b Transverse ultrasound image of the median nerve (outlined) at the distal level (level of the carpal tunnel) showing the CSAc is 13 mm2. The ∆-CSA therefore is 7 mm2, whereas the R-CSA is 2,16 consistent with the grading of a moderate CTS sonographically. In addition the nerve shows loss of echogenicity because of oedema. FCR = flexor carpi radialis tendon; arrows indicate flexor retinaculum
a Transverse ultrasound image of the median nerve (outlined) at the proximal level (level of the pronator quadratus muscle) in a 65-year-old female patient with severe CTS, the CSAp is 7 mm2. PQ = pronator quadratus muscle. b Transverse ultrasound image of the median nerve (outlined) at the distal level (level of the carpal tunnel) showing a CSAc of 18 mm2. The ∆-CSA therefore is 11 mm2, whereas the R-CSA is 2,57 consistent with the grading of a severe CTS sonographically. In addition the nerve shows loss of echogenicity because of oedema and loss of the fascicular pattern. FCR = flexor carpi radialis tendon, arrows indicate flexor retinaculum
The sensitivities and specificities of US-measured ∆-CSA with threshold values of 5, 6, 7 and 8 mm2 for the differentiation between group 1 and 2 patients and threshold values of 7, 8, 9 and 10 mm2 for the differentation between group 2 and 3 patients are presented in Table 4. The optimal Youden’s index was obtained using a ∆-CSA threshold of 6 mm2 to differentiate mild from moderate CTS, with which 224 of 272 patients with mild CTS (group 1) and 121 of 152 patients with moderate CTS (group 2) were correctly diagnosed. The optimal Youden’s index to differentiate moderate from severe CTS was obtained by using a ∆-CSA threshold value of 9 mm2, with which 134 of 152 patients with moderate CTS (group 2) and 138 of 219 patients with severe CTS (group 3) were correctly diagnosed (Tables 2 and 3)
The sensitivities and specificities of US-measured R-CSA with threshold values of 1.5, 1.6, 1.7 and 1.8 for the differentiation between group 1 and 2 patients and threshold values of 1.8, 2, 2.2 and 2.4 for the differentation between group 2 and 3 patients are presented in Table 4. The optimal Youden’s index was obtained by using a R-CSA threshold of 1.7 to distinguish mild from moderate CTS, with which 215 of 272 patients with mild CTS (group 1) and 117 of 152 patients with moderate CTS (group 2) were correctly diagnosed. The optimal Youden’s index was obtained using a R-CSA threshold of 2.2 to distinguish mild from moderate CTS with which 128 of 152 patients with moderate CTS (group 2) and 116 of 219 patients with severe CTS (group 3) were correctly diagnosed (Tables 2 and 4).
Discussion
Multiple US parameters have been studied for the diagnosis of CTS, including retinacular bowing, retinacular thickness, echogenicity of the median nerve, flattening ratio of the median nerve and measurement of CSA of the median nerve [16, 21–23]. Among these parameters, CSA of the median nerve is the most popular parameter for CTS detection, although there is lack of consensus regarding diagnostic cutoff values for CTS. In order to improve the diagnosis of CTS based upon CSA, other more specific parameters have been described, including the ratio between CSA of the median nerve at the carpal tunnel (distal) and at the mid forearm (proximal), as well as the ratio between the median nerve CSA and the carpal tunnel CSA (nerve/tunnel index), and the difference between CSA of the median nerve both distally and proximally [1, 2, 8, 10].
In contrast to earlier studies that utilized the CSA of the median nerve proximally at the mid forearm [2, 9, 10], recent studies [17–19] have defined a landmark for proximal measurement at the level of the pronator quadratus muscle at the distal forearm. This measurement was thought to provide a more reproducible landmark for calculation of the proximal median nerve CSA and to better compensate for the interindividual variability in the CSA of the median nerve [17]. Nonetheless, although calculations based upon this proximal median nerve measurement showed increased values in diseased versus normal subjects, and increased values with CTS severity, these studies could not adequately define CTS severity subgroups.
Several studies have confirmed the feasibility of distal CSA measurement of the median nerve not only in the diagnosis of CTS, but also in the grading of CTS severity [9, 24–27], especially when using the ratio measurement [8–10].
Altinok et al. [8] studied the CSA ratio at the forearm for the diagnosis of CTS in 29 wrists (11 mild and 18 moderate) without including severe cases. Their study did not define a cut-off value to discriminate between mild and moderate groups. Our study was able to discriminate between mild and moderate CTS patient groups with a sensitivity of 77 % and a specificity of 79 % using a cut-off value of 1.7.
Kang et al. [9], in a study of 148 wrists (110 CTS and 38 controls), proposed cut-off values for the wrist forearm ratio of 1.89 in the diagnosis of moderate to severe CTS, and 2.2 for severe CTS. These values are similar to our results, as we found optimal cut-off values of 1.7 and 2.2 for the distinction between mild and moderate CTS, and the distinction between moderate and severe CTS, respectively. Although the studies differ in the included study populations and the level of proximal CSA measurement, they showed marked agreement in the ability of the investigational parameter (ratio) to detect CTS severity and discriminate between different subgroups.
In contrast to our study, Mhoon et al. [10] showed that the ratio obtained from the mid forearm had a high sensitivity of 97 % for detecting electrodiagnostic abnormalities, but a low specificity of about 29 % using clinical examination as a gold standard reference for diagnosis. Their use of a different level of proximal CSA measurement may reduce their specificity due to greater interindividual variablity, as it provides no fixed landmark for measurement.
In a recent study [17], ∆-CSA was proposed as a parameter showing significant differences between normal subjects and patients, and its discriminating ability was significantly superior to distal CSA only of the median nerve. Although both parameters showed a tendency to be higher in wrists with highly positive NCS, that study did not demonstrate a significant difference between patients with mildly positive NCS and highly positive NCS.
In the current study both ∆-CSA and R-CSA showed good discriminating power among wrists with a different severity of CTS based upon threshold cut-off values. ROC analysis demonstrated that ∆-CSA cut-off values proved superior to R-CSA cut-off values, although this difference was marginally significant for the comparison of mild to moderate CTS (p = 0.07) and highly significant for the comparison of moderate to severe CTS (p < 0.001).
To the best of our knowledge our series is the largest reported study in evaluation of the severity of CTS. Furthermore, the use of the proximal CSA measurement at the distal forearm at the level of pronator quadratus as a fixed landmark has not been previously published for the calculation of the R-CSA. The objective of our study was to validate ∆-CSA and R-CSA for the differentiation between CTS patients according to disease severity, with NCS as the reference standard for diagnosis. We found good discriminating power for both the ∆-CSA and R-CSA parameters in assessment of CTS severity, and calculated optimal threshold values for each parameter.
Sonographic evaluation of CTS has numerous advantages. US of the carpal tunnel is widely available, quick and easy to perform, non-invasive and provides a quantitative measurement with validated diagnostic value. As demonstrated in our study, clinical CTS diagnosis and grading can be followed by US for confirmation of the diagnosis and quantitative grading of severity. The application of NCS should be reserved for difficult diagnostic cases, especially those with discordant clinical and sonographic findings. By utilising sonographic evaluation to confirm the clinical diagnosis of CTS, we can reduce the dependence on invasive, time-consuming NCS for the diagnosis and grading of CTS.
Abbreviations
- CTS:
-
Carpal tunnel syndrome
- NCS:
-
Nerve conduction studies
- CSA:
-
Cross-sectional area
- CSAc:
-
Cross-sectional area at the carpal tunnel
- CSAp:
-
Cross-sectional area proximally
- ∆-CSA:
-
Delta cross-sectional area
- R-CSA:
-
Ratio
References
Kim HS, Joo SH, Cho HK, Kim YW (2013) Comparison of proximal and distal cross-sectional areas of the median nerve, carpal tunnel, and nerve/tunnel index in subjects with carpal tunnel syndrome. Arch Phys Med Rehabil 94(11):2151–2156
Hobson-Webb LD, Massey JM, Juel VC, Sanders DB (2008) The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol 119(6):1353–1357
Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I (1999) Prevalence of carpal tunnel syndrome in a general population. JAMA 282(2):153–158
Lawrence RC, Felson DT, Helmick CG et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58(1):26–35
Descatha A, Dale AM, Franzblau A, Coomes J, Evanoff B (2010) Diagnostic strategies using physical examination are minimally useful in defining carpal tunnel syndrome in population-based research studies. Occup Environ Med 67(2):133–135
Klauser AS, Tagliafico A, Allen GM et al (2012) Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol 22(5):1140–1148
Visser LH, Smidt MH, Lee ML (2008) High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurosurg Psychiatry 79(1):63–67
Altinok T, Baysal O, Karakas HM et al (2004) Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol 59(10):916–925
Kang S, Kwon HK, Kim KH, Yun HS (2012) Ultrasonography of median nerve and electrophysiologic severity in carpal tunnel syndrome. Ann Rehabil Med 36(1):72–79
Mhoon JT, Juel VC, Hobson-Webb LD (2012) Median nerve ultrasound as a screening tool in carpal tunnel syndrome: correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle Nerve 46(6):871–878
Sernik RA, Abicalaf CA, Pimentel BF, Braga-Baiak A, Braga L, Cerri GG (2008) Ultrasound features of carpal tunnel syndrome: a prospective case-control study. Skelet Radiol 37(1):49–53
Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO (2006) The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg [Am] 31(5):726–732
Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A (2005) The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol 56(3):365–369
Wong SM, Griffith JF, Hui AC, Lo SK, Fu M, Wong KS (2004) Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology 232(1):93–99
Ziswiler HR, Reichenbach S, Vogelin E, Bachmann LM, Villiger PM, Juni P (2005) Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum 52(1):304–311
Yesildag A, Kutluhan S, Sengul N et al (2004) The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol 59(10):910–915
Klauser AS, Halpern EJ, De ZT et al (2009) Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology 250(1):171–177
Klauser AS, Halpern EJ, Faschingbauer R et al (2011) Bifid median nerve in carpal tunnel syndrome: assessment with US cross-sectional area measurement. Radiology 259(3):808–815
Miyamoto H, Halpern EJ, Kastlunger M (2014) Carpal tunnel syndrome: diagnosis by means of median nerve elasticity: improved diagnostic accuracy of US with sonoelastography. Radiology 270(2):481–486
Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P (1997) Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand 96(4):211–217
Sarria L, Cabada T, Cozcolluela R, Martinez-Berganza T, Garcia S (2000) Carpal tunnel syndrome: usefulness of sonography. Eur Radiol 10(12):1920–1925
Wong SM, Griffith JF, Hui AC, Tang A, Wong KS (2002) Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum 46(7):1914–1921
Buchberger W, Judmaier W, Birbamer G, Lener M, Schmidauer C (1992) Carpal tunnel syndrome: diagnosis with high-resolution sonography. AJR Am J Roentgenol 159(4):793–798
Mohammadi A, Afshar A, Etemadi A, Masoudi S, Baghizadeh A (2010) Diagnostic value of cross-sectional area of median nerve in grading severity of carpal tunnel syndrome. Arch Iran Med 13(6):516–521
Karadag YS, Karadag O, Cicekli E et al (2010) Severity of Carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int 30(6):761–765
Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M (2009) Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound 37(3):125–131
Ajeena IM, Al-Saad RH, Al-Mudhafar A, Hadi NR, Al-Aridhy SH (2013) Ultrasonic assessment of females with carpal tunnel syndrome proved by nerve conduction study. s 2013:754–764
Acknowledgments
The scientific guarantor of this publication is Andrea S. Klauser. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise: Dr. Ethan J. Halpern, Department of Radiology and Urology, Jefferson Prostate Diagnostic Center and Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klauser, A.S., Abd Ellah, M.M.H., Halpern, E.J. et al. Sonographic cross-sectional area measurement in carpal tunnel syndrome patients: can delta and ratio calculations predict severity compared to nerve conduction studies?. Eur Radiol 25, 2419–2427 (2015). https://doi.org/10.1007/s00330-015-3649-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3649-8