Abstract
Objective
To assess radiation dose and image quality in body CT-angiography (CTA) with automated attenuation-based tube voltage selection (ATVS) on a 192-slice dual-source CT (DSCT).
Methods
Forty patients (69.5 ± 9.6 years) who had undergone body CTA with ATVS (ref.kVp 100, ref.mAs 90) using a 2x192-slice CT in single-source mode were retrospectively included. All patients had undergone prior CTA with a 2x128-slice CT and ATVS with identical imaging and contrast media protocols, serving for comparison. Images were reconstructed with iterative reconstruction at similar strength levels. Radiation dose was determined. Image quality was assessed semi-quantitatively (1:excellent, 5:non-diagnostic), aortic attenuation, noise and CNR were determined.
Results
As compared to 128-slice DSCT, 192-slice DSCT selected tube voltages were lower in 30 patients (75 %), higher in 3 (7.5 %), and similar in 7 patients (17.5 %). CTDIvol was lower with 192-slice DSCT (4.7 ± 1.9 mGy vs. 5.8 ± 2.1 mGy; p < 0.001). Subjective image quality, mean aortic attenuation (342 ± 67HU vs. 268 ± 67HU) and CNR (9.8 ± 2.5 vs. 8.2 ± 2.9) were higher with 192-slice DSCT (all p < 0.01), all datasets being diagnostic.
Conclusion
Our study suggests that ATVS of 192-slice DSCT for body CTA is associated with an improved image quality and further radiation dose reduction of 19 % compared to 128-slice DSCT.
Key Points
• 192-slice DSCT allows imaging from 70 kVp to 150 kVp at 10 kVp increments.
• 192-slice DSCT allows for radiation-dose reduction in body-CTA with ATVS.
• Subjective and objective image quality increase compared to 128-slice DSCT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
CT angiography (CTA) represents a well-established imaging method to accurately and noninvasively assess vessel anatomy and disease [1]. CTA has widely replaced conventional angiography for diagnostic vascular imaging. Today it represents the imaging standard for planning and surveillance of endovascular and surgical treatment of vascular disease. For example, patients with aortic disease require lifetime monitoring to exclude complications such as device migration, endoleak formation, or disease progression [2]. The relatively high cumulative radiation dose associated with repeated CT imaging necessitates the development of radiation dose reducing strategies while maintaining the diagnostic yield of the examination. Several technologies are currently available for radiation dose reduction including automated attenuation-based tube current modulation, use of low X-ray tube voltage, and iterative image reconstruction [3].
Imaging at lower tube voltage not only allows for a reduction in radiation dose, but due to imaging closer to the k-edge of iodine (33,2 keV), has demonstrated an increase in the attenuation of iodine, consequently resulting in a higher vessel contrast in CTA [4, 5]. A recently introduced automated attenuation-based tube voltage selection algorithm is characterized by a task- and patient-based selection of the tube voltage and leads to both an optimized image quality and a reduction in radiation dose in body CTA [6–9].
A new 192-slice DSCT system, equipped with X-ray tubes of 120 kW each, allows for imaging at tube voltages ranging from 70 kVp to 150 kVp at 10 kVp increments. In contrast, previous 128-slice DSCT, equipped with X-ray tubes of 100 kW each, allowed for imaging at 70 kVp and from 80 kVp to 140 kVp at 20 kVp increments. A recent phantom study showed that the higher tube power of 192-slice DSCT allows for low kilovoltage imaging in obese patients without a relevant increase in image noise, because the higher tube power allows for an adequate increase in tube current also at low tube potentials [10].
To the best of our knowledge, no study so far has evaluated the performance of the automated attenuation-based tube voltage selection algorithm with 192-slice DSCT in vivo. Our study hypothesis was that 192-slice DSCT, owing to more flexible options for individual selection of tube voltage levels in combination with the higher tube power, would result in a further reduction of selected tube voltage levels and hence, radiation dose, as well as in an improved image quality. Thus, the purpose of this study was to determine the performance of automated attenuation-based tube voltage selection with 192-slice DSCT in terms of radiation dose and image quality in body CTA in comparison with 128-slice DSCT at similar protocol and contrast media settings in the same patients.
Subjects and methods
Patient population
Forty consecutive patients who were referred for follow-up CTA between November and December 2013, and who had undergone prior CT imaging performed with automated attenuation-based tube voltage selection, were retrospectively included in this study. Mean body mass index (BMI) of each patient was recorded from the electronic patient file. The study had institutional review board approval. Due to the retrospective nature of the study, the local ethics committee waived written informed consent.
CT data acquisition
CTA studies were performed on a 192-slice DSCT system (SOMATOM Force, Siemens Healthcare, Germany) equipped with an integrated circuit detector (Stellar Infinity, Siemens). This CT machine allows for tube voltage levels from 70-150 kVp at 10 kVp steps. The scanner is equipped with a dynamic collimator to minimize overranging. Studies were performed with automated attenuation-based tube voltage selection (CAREkV) and automated tube current modulation (CAREDose4D) with a reference kVp of 100 and reference mAs of 90. The contrast media injection protocols consisted of a tailored biphasic injection with a total bolus of 80 mL of iopromide (300 mg/mL; Ultravist 300, Bayer Schering Pharma). The first 35 mL were injected at 5 mL/s directly followed by 45 mL injected at 2.5 mL/s and a 20 mL saline flush injected at 2.5 mL/s [11].
A bolus-tracking technique was used by placing a region of interest (ROI) in the descending aorta and using an attenuation threshold of 100 HU at 120 kVp to determine the time point of scan initiation. The CTA scan range extended from the apex of the lungs to below the symphysis pubis in all patients. The CT was operated in the single-source mode, using the following parameters: detector collimation 96 × 0.6 mm, slice collimation 192 × 0.6 mm by means of a z-flying focal spot, pitch 1.2, and tube rotation time 0.5 seconds.
For comparison, we used prior studies from the same patients that were acquired between May 2012 and December 2013, which were performed in single-source mode with automated attenuation-based tube voltage selection and automated tube current modulation on a 128-slice DSCT, also equipped with a high resolution detector and a dynamic collimator to minimize overranging (SOMATOM Definition Flash, Siemens Healthcare, Germany). All prior imaging was performed with identical scans (reference kVp 100, reference mAs of 90) and contrast media protocol settings (80 mL of iopromide, first 35 mL injected at 5 mL/s, following 45 mL injected at 2.5 mL/s, and 20 mL saline flush injected at 2.5 mL/s).
All images were reconstructed with a slice thickness of 2 mm and an increment of 1.6 mm at a field-of-view (FoV) of 450 × 450 mm and using iterative reconstructions (192-slice DSCT: advanced model-based iterative reconstruction (ADMIRE), strength level 3 [12]; and 128-slice DSCT: sinogram-affirmed IR (SAFIRE), strength level 3 [13].
Radiation dose estimation
Radiation doses were estimated by retrieving the electronically logged CTDIvol and dose-length product (DLP) values from the patient protocols. The effective dose was calculated by multiplying the DLP with a mean conversion coefficient for the chest (0.014 mSv / (mGy cm)), abdomen (0.015 mSv / (mGy cm)), and pelvis (0.015 mSv / (mGy cm)) [14, 15]. Additionally size-specific dose estimates (SSDEs) were calculated by using the conversion factors for effective diameters from the American Association of Physicists in Medicine Report 204 [16] with SSDE = conversion factor eff dia * CTDI vol .
Automated attenuation-based tube voltage selection
The automated attenuation-based tube voltage selection algorithm consists of a software algorithm (CAREkV, Siemens) which optimizes both tube voltage and tube current for each patient to reach a user-defined image quality [17]. Based on the patient attenuation along the z-axis, measured in the CT topogram, the software calculates patient-specific mAs curves for all kV levels, based on the scan range, patients’ constitution, and selected scan type. Optimal dose efficiency is then calculated based on these kV-specific mAs curves [6, 9].
Iterative reconstructions and phantom experiment
ADMIRE represents the successor of SAFIRE and contains three main modifications in comparison with SAFIRE. For one, ADMIRE uses a weighted filtered back projection loop, which accelerates the removal of geometrical imperfections. Advanced models of the scanner geometry components are integrated into the iterative cycles. Second, the ADMIRE reconstruction begins with iterations in the raw data domain, followed by iterations in the image domain until a target noise reduction level is reached, depending on the selected ADMIRE strength level of five available levels. Noise is reduced due to interaction between statistical modeling in raw data and image domains, the raw data being translated to an image domain (“master 3D volume”), resulting in higher effective iteration speed. Furthermore, a local signal-to-noise ratio analysis is performed in order to separate data into “information” and “noise”. While in SAFIRE only the nearest data is incorporated in the analysis, ADMIRE uses data from a larger neighbourhood [18].
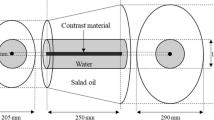
To compare the effect of the iterative reconstruction algorithm on image noise, a phantom study was performed using an abdominal anthropomorphic phantom (Quality Assurance in Radiology and Medicine (QRM), Moehrendorf, Germany), serial number QSA-453) with two different extension rings simulating a medium (350 × 250 mm) and a large (400 × 300 mm) body size. The phantoms were imaged with identical CT protocol parameters as patients, and the datasets were reconstructed with the same slice thickness and increment, as well as FoV, with both SAFIRE and ADMIRE at a strength level of 3, similar to the patient study. Image noise was measured by placing a total of 9 ROIs in each phantom - one ROI in liver, one in the background tissue and one in fat on three consecutive slices.
CT data analysis
Qualitative analysis
Two independent radiologists (EL; DBH, with 1 and 10 years of experience in radiology) assessed overall image quality on the 2-mm axial CT images using a 5-point Likert scale regarding visibility and sharpness of small vascular detail (1, excellent image quality; 2, good; 3, moderate; 4, poor, diagnostic confidence significantly reduced; and 5, unacceptable, non-diagnostic) [19]. Before beginning the assessment, readers were instructed on criteria of image grading and rated five cases together. These five cases were not included in the final study sample. Adjustment of window centre and width level settings for optimal subjective image quality was allowed.
Quantitative analysis
Quantitative analysis was done by a reader not involved with qualitative image rating (AW, with 5 years of experience in radiology). On the same axial images the attenuation of the aorto-iliac system was measured using a circular ROI in the centre of the arteries at four different locations: the ascending aorta at the level of the right pulmonary artery (ROI 1), the descending aorta at the level of the right pulmonary artery (ROI 2), the abdominal aorta at the level of the origin of the superior mesenteric artery (ROI 3), and the left common iliac artery (ROI 4), as previously done [19]. Calcifications and thrombus in or adjacent to the vessel walls were avoided. If stents were present in the common iliac artery preventing adequate measurements, ROI 4 was placed more distally in the external iliac artery. The mean aorto-iliac attenuation (ROI mean) was calculated using the previously obtained attenuation values (ROIs 1-4) for each patient. Image noise, defined as the standard deviation of attenuation, was measured in ROI 3. Furthermore, attenuation of the right psoas muscle (ROI pm) was measured at the height of the lower pole of the right kidney. Contrast-to-noise ratio (CNR) was calculated (CNR = (ROI mean – ROI pm)/ Noise) [6]. For comparing patient size between the two CT scans at different time points, the lateral (LAT) and anteroposterior (AP) diameter of each patient was measured at the level of the origin of the superior mesenteric artery. Effective diameters were calculated as \( effective\ diameter=\sqrt{AP\ast LAT} \).
Statistical analysis
Continuous variables are expressed as means ± standard deviation, categorical variables as medians and as frequencies or percentages, respectively.
Interreader agreement concerning subjective image quality was assessed by using κ statistics. According to Landis and Koch, values were considered as: 0.41–0.60, moderate agreement; 0.61– 0.80, substantial agreement; and 0.81– 1.00, almost perfect agreement. Radiation dose and image quality were compared using the Wilcoxon signed rank test. Patients’ sizes, attenuation, image noise and CNR were compared using t-test for paired samples or Wilcoxon signed rank test as appropriate (following normality testing using the Shapiro-Wilk test).
A commercially available software was use to perform all statistical analysis (SPSS, release 22 for Windows, SPSS Inc., Chicago, Il, USA). A two sided p-value below 0.05 was considered to indicate statistical significance.
Results
The 40 included patients (35 men, 5 women) had a mean age of 69.5 years ± 9.6 years, ranging from 47 - 87 years. Patients were referred for clinically indicated thoraco-abdominal CTA of the aorta after endovascular repair and/or vascular surgery (n = 34), after conservative treatment (n = 6) of aortic aneurysm (n = 31), or aortic dissection (n = 9). Mean time interval between the two CT scans was 6.25 ± 4.4 months (range 0.5-18 months). Mean BMI was 26.8 ± 4.8 kg/m2 (range 21.1 - 39.7 kg/m2). There was no significant difference in patients’ sizes between the two CT scans (mean lateral diameter, 33.6 ± 2.9 cm vs. 33.3 ± 2.9 cm, p = 0.662; mean anteroposterior diameter, 25.6 ± 3.8 cm vs. 25.8 ± 3.9 cm, p = 0.957; mean effective diameter 30.5 ± 3.1 vs. 30.3 ± 3.1, p = 0.095).
Tube voltage selection and radiation dose
With 192-slice DSCT, selected tube voltage decreased in 30 (75 %) patients (by 10 kVp, n = 27; by 20 kVp, n = 1; by 30 kVp, n = 2), increased in 3 (7.5 %) patients (by 10 kVp), and remained the same in 7 (17.5 %) patients compared to 128-slice DSCT (Fig. 1). The resulting effective tube current was significantly higher for 192-slice DSCT scans than for 128-slice DSCT scans (135 ± 35 mAs compared to 151 ± 50 mAs, p < 0.01).
CTDIvol was lower with 192-slice DSCT (4.7 ± 1.9 mGy vs. 5.8 ± 2.1 mGy, p < 0.001). At a similar scan range in the z-axis (derived from table position, 68.9 cm for 128-slice DSCT vs. 70.3 cm for 192-slice DSCT , p = 0.238), the mean effective radiation dose with 192-slice DSCT was reduced by an average of 19.2 % (1 mSv) from 5.9 ± 1.9 mSv to 4.9 ± 2.1 mSv with 128-slice DSCT (p < 0.001) (Fig. 2). SSDE was lower with 192-slice DSCT (5.5 ± 1.6 mGy vs. 7.0 ± 1.7 mGy, p < 0.001).
Qualitative analysis
Interreader agreement for subjective image quality was fair for 128-slice DSCT (κ = 0.38) and substantial for 192-slice DSCT (κ = 0.66). Image quality significantly increased with 192-slice DSCT (median score, 2; range 2-3, both readers) compared to 128-slice DSCT (median score, 3; range 2-3, both readers) (p < 0.01, both readers). All images were of diagnostic image quality, none had a score of 4 or 5 (4 = poor, diagnostic confidence significantly reduced; 5 = unacceptable, non-diagnostic) (Figs. 3 and 4).
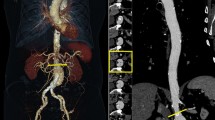
A 49-year old man with a history of type B-dissection of the aorta imaged for follow- up after conservative treatment. Axial images (W/L 360/70) of a body CTA obtained with 128-slice DSCT at 100 kVp (A) and with 192-slice DSCT at 90 kVp (B). Note good image quality of both CT scans and higher attenuation of the aorta obtained with 192-slice DSCT. Radiation dose was lower with 192-slice DSCT and 90 kVp (CTDIvol 3.2 mGy vs. 4.7 mGy).
A 65-year old man with a history of aortoiliac Y-graft implantation. Axial images (W/L 360/70) of a body CTA acquired with 128-slice DSCT at 100 kVp (A) and with 192-slice DSCT at 90 kVp (B). Note higher attenuation and thus better subjective image quality with 192-slice DSCT. The CTDIvol was lower with 192-slice DSCT and 90 kVp (CTDIvol 3.7 mGy vs. 4.7 mGy)
Quantitative analysis
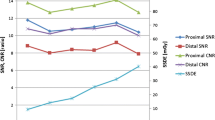
Aortoiliac attenuation increased in all locations (ROI1-4) with 192-slice DSCT compared to 128-slice DSCT (Fig. 5a). Mean attenuation was significantly higher with 192-slice DSCT (342 ± 67 HU vs. 268 ± 67 HU, p < 0.001), while noise also increased with 192-slice DSCT (31 ± 5 HU vs. 28 ± 6 HU, p < 0.05) (Fig. 5b). Mean CNR increased by 19.5 % with 192-slice DSCT compared to 128-slice DSCT (9.8 ± 2.5 vs. 8.2 ± 2.9, p < 0.01) (Fig. 5c, Table 1). Post hoc power analysis for CNR revealed a power of 0.72.
Box- and-whisker plots demonstrating lower aortoiliac attenuation at all four locations (a) on previous 128-slice DSCT images (A) compared to 192-slice DSCT images (B) (p < 0.001). ROI1, ascending aorta at level of the right pulmonary artery; ROI2, descending aorta at the level of the right pulmonary artery; ROI3, abdominal aorta at the level of the origin of the superior mesenteric artery; ROI4, the left common iliac artery. Mean attenuation (a) and mean CNR (c) were significantly higher (p < 0.001; p < 0.01) on 192-slice (B) compared to previous 128-slice DSCT images (A), while image noise also increased on 192-slice DSCT (b) (p < 0.05)
The phantom experiment indicated similar noise levels without significant differences on images reconstructed with SAFIRE and with ADMIRE at the same strength levels in both phantom sizes (M: SAFIRE 24.0 ± 3.1, ADMIRE 25.0 ± 3.4, p = 0.90. L: SAFIRE 24.2 ± 2.9, ADMIRE 24.3 ± 3.9, p = 0.87).
Discussion
The aim of this study was to determine the performance of automated attenuation-based tube voltage selection with 192-slice DSCT in terms of radiation dose and image quality in body CTA, given the more flexible options for tube voltage selection and the higher tube power of the CT scanner. Thus, we performed an intra-individual comparison of these parameters in the same patients who underwent body CTA with both 192- and 128-slice DSCT with identical scan protocol and contrast media protocol settings. By doing so, we could demonstrate that with 192-slice DSCT the algorithm resulted in lower tube voltages in most patients (75 %), and consequently was associated with a reduction of radiation dose by 19 %. Interestingly, the diagnostic yield of the examinations was maintained in all patients, while subjective and objective parameters of image quality were improved.
In most patients tube voltage decreased from 100 kVp to 90 kVp. In only one patient tube voltage decreased from 100 kVp to 80 kVp. This highlights the advantage of the 10 kVp increments in tube voltage leveling in 192-slice DSCT compared to the 20 kVp increments in the predecessor 128-slice DSCT. The smaller increments allowed for a more gradual change in tube voltage, with more patients benefitting from a lower radiation dose. Previous studies assessing radiation dose in CTA with CAREkV compared to a protocol with fixed tube voltage achieved a dose reduction ranging from 16-56 % [6, 8, 9], due to lower tube voltage. For example, Goetti et al., showed that CAREkV lead to a tube voltage reduction to 80 kVp in 2.9 % and to 100 kVp in 51.4 %. Our study adds to the existing literature that the further refinement of the automated attenuation-based tube voltage selection algorithm with 192-slice DSCT, enabling incremental 10 kVp steps, has led to a further reduction of tube voltages and hence, of radiation dose of 19.2 %. The CTDIvol was chosen as the main parameter to compare radiation between the two CT systems. The number of detector rows is different between the two CT systems, but given that both scanners are equipped with a dynamic collimator, the effect of overranging should be negligible. Most importantly, overranging does influence the DLP but not the CTDIvol, which was the major radiation dose parameter used for comparison in our study.
Simultaneously with the decreasing tube voltage on 192-slice DSCT, the tube current increased (from the 135 effective mAs to the average151 effective mAs) to maintain the user-defined image quality of the examination. However, because radiation dose is proportional to the tube voltage change to an exponential power ranging from 2.5-3.1 (depending on patient size) but is proportional to the tube current [20] in a linear manner, the effect of the lower tube voltage on radiation dose was predominant. In general, CT systems have certain limits in increasing the tube current (when lowering the tube voltage) because of restrictions in tube power. Our study indicates that 192-slice DSCT, which is equipped with a generator power of 120 kW for each tube - compared to 100 kW per tube in 128-slice DSCT- allowing for low tube voltage imaging in more patients, since tube current could be increased further.
Due to imaging at lower mean tube voltage, the attenuation of the aorta and iliac arteries in our patients was higher compared to the prior exam with otherwise identical contrast injection- and imaging-parameters. This effect is a consequence of imaging closer to the k-edge of iodine which was demonstrated also in several previous studies using fixed lower tube voltage [5, 21]. This implicates the potential to decrease the amount of intravenous contrast as an additional advantage of low tube voltage imaging. Further studies have to be performed to evaluate the lowest amounts of intravenous contrast that are needed for each tube voltage in order to fully exploit the advantages of low kV-imaging. Especially because patients with abdominal aortic aneurysms often have coexisting renal dysfunction [22], a reduced amount of intravenous iodinated contrast would be desirable.
It is important to note that the current study was not aimed at an optimization of CT protocols for aorto-iliac angiography with 192-slice DSCT in regard to the lowest possible radiation and contrast media dose. Given the fact that all CTA studies were of diagnostic image quality, also those acquired with 128-slice DSCT, the increase in CNR could have been also invested in a reduction of radiation dose (through, e.g., a reduction of the reference tube current), for obtaining CTA examinations with a maintained - though not improved - image quality.
We have to acknowledge the following study limitations. First, as mentioned above, this study did not investigate the full potential of 192-slice DSCT regarding radiation dose reduction of aorto-iliac CTA. In contrast, we kept all protocol and contrast media parameters constant among studies for exploiting the performance of automated attenuation-based tube voltage selection with the new scanner in combination with the higher tube power. Certainly, many parameters such as tube current, pitch, and contrast media amount could be changed in future studies to further benefit from technical advances and to achieve further radiation dose reduction. Second, median time interval between the two CTs was 6 months, and possible changes of cardiac output could influence attenuation, however, the major outcome parameter for this study was radiation dose, which is comparable since patient size did not change. Furthermore, as mentioned above, two different advanced IR algorithms were used. However, the automated tube voltage selection occurs prior to image reconstruction and, thus, is not influenced by the reconstruction algorithm. Attenuation values are not influenced by the reconstruction algorithm, and we demonstrated in the phantom experiment that image noise reduction with SAFIRE and ADMIRE are similar at the same strength level, thus not affecting quantitative results such as the CNR. Finally, we did not evaluate the accuracy of the CTA examinations, since no gold standard method was available.
In conclusion, our study suggests that automated attenuation-based tube voltage selection of 192-slice DSCT for body CTA is associated with an improved subjective and objective image quality as compared to previous 128-slice DSCT at identical scan and contrast media protocols, and results in a further radiation dose reduction of 19 %. Future studies should aim at the separation of the effect of the ATVS algorithm from other potentially relevant technical parameters such as the detector size and the IR algorithm.
References
Foley WD, Karcaaltincaba M (2003) Computed tomography angiography: principles and clinical applications. J Comput Assist Tomogr 27:S23–S30
Chaikof EL, Brewster DC, Dalman RL et al (2009) The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 50:S2–S49
Lee TY, Chhem RK (2010) Impact of new technologies on dose reduction in CT. Eur J Radiol 76:28–35
Wintersperger B, Jakobs T, Herzog P et al (2005) Aorto-iliac multidetector-row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol 15:334–341
Kalva SP, Sahani DV, Hahn PF, Saini S (2006) Using the K-edge to improve contrast conspicuity and to lower radiation dose with a 16-MDCT: a phantom and human study. J Comput Assist Tomogr 30:391–397
Goetti R, Winklehner A, Gordic S et al (2012) Automated attenuation-based kilovoltage selection: preliminary observations in patients after endovascular aneurysm repair of the abdominal aorta. AJR Am J Roentgenol 199:W380–W385
Schwarz F, Grandl K, Arnoldi A et al (2013) Lowering radiation exposure in CT angiography using automated tube potential selection and optimized iodine delivery rate. AJR Am J Roentgenol 200:W628–W634
Siegel MJ, Ramirez-Giraldo JC, Hildebolt C, Bradley D, Schmidt B (2013) Automated low-kilovoltage selection in pediatric computed tomography angiography: phantom study evaluating effects on radiation dose and image quality. Investig Radiol 48:584–589
Winklehner A, Goetti R, Baumueller S et al (2011) Automated attenuation-based tube potential selection for thoracoabdominal computed tomography angiography: improved dose effectiveness. Investig Radiol 46:767–773
Meinel FG, Canstein C, Schoepf UJ et al (2014) Image quality and radiation dose of low tube voltage 3rd generation dual-source coronary CT angiography in obese patients: a phantom study. Eur Radiol 24:1643–1650
Fleischmann D, Rubin GD, Bankier AA, Hittmair K (2000) Improved uniformity of aortic enhancement with customized contrast medium injection protocols at CT angiography. Radiology 214:363–371
Gassenmaier T, Petri N, Allmendinger T et al (2014) Next generation coronary CT angiography: in vitro evaluation of 27 coronary stents. Eur Radiol 24:2953–2961
Winklehner A, Karlo C, Puippe G et al (2011) Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol 21:2521–2526
Macari M, Chandarana H, Schmidt B, Lee J, Lamparello P, Babb J (2006) Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology 241:908–914
Medicine AAoPi (2008) The Measurement, Reporting, and Management of Radiation Dose in CT. Report of AAPM Task Group 23 of the Diagnostic Imaging Concil CT Committee. Available via www.aapm.org/pubs/reports/rpt_96.pdf. Accessed 15 Jan 2014
(2011) American Association of Physicists in Medicine. Size Specific Dose Estimates (SSDE) in Pediatric and Adult CT Examinations. Report of AAPM Task Group 204. Available via http://www.aapm.org/pubs/reports/rpt_204.pdf. Accessed 15 Jan 2014
Krazinski AW, Meinel FG, Schoepf UJ et al (2014) Reduced radiation dose and improved image quality at cardiovascular CT angiography by automated attenuation-based tube voltage selection: intra-individual comparison. Eur Radiol 24:2677–2684
Gordic S, Desbiolles L, Stolzmann P et al (2014) Advanced modelled iterative reconstruction for abdominal CT: qualitative and quantitative evaluation. Clin Radiol 69:e497–504
Schindera ST, Graca P, Patak MA et al (2009) Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Investig Radiol 44:650–655
Maldjian PD, Goldman AR (2013) Reducing radiation dose in body CT: a primer on dose metrics and key CT technical parameters. AJR Am J Roentgenol 200:741–747
Kanematsu M, Goshima S, Miyoshi T et al (2014) Whole-body CT angiography with low tube voltage and low-concentration contrast material to reduce radiation dose and iodine load. AJR Am J Roentgenol 202:W106–W116
Chun KC, Teng KY, Chavez LA et al (2014) Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional veterans affairs health care system. Ann Vasc Surg 28:87–92
Acknowledgments
The scientific guarantor of this publication is Hatem Alkadhi. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained.
Written informed consent was waived by the Institutional Review Board. No study subjects or cohorts have been previously reported. Methodology: retrospective, case-control study, performed at one institution.
Conflict of interest
None of the authors have any conflicts of interest or disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winklehner, A., Gordic, S., Lauk, E. et al. Automated attenuation-based tube voltage selection for body CTA: Performance evaluation of 192-slice dual-source CT. Eur Radiol 25, 2346–2353 (2015). https://doi.org/10.1007/s00330-015-3632-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3632-4