Abstract
Due to climate change and human activities, Antarctic shag populations are experiencing shifts in their distribution range, habitat, and population size. To assess their health, we collected hematological and biochemical of male and female South Georgia shags (Phalacrocorax georgianus) during breeding on Laurie Island, South Orkney Island, Antarctica. Leukocyte profile, heterophil/lymphocyte ratio, hematocrit, and concentrations of glucose, total proteins, cholesterol, and triglycerides were measured. None of the measured metrics showed signs of clinical pathology or disease. Overall, the parameters measured were consistent with those previously reported for other cormorant species. Males had higher protein and cholesterol concentrations, indicating differences in nutritional status between the sexes during reproduction. This study is the first report on blood parameters of South Georgia shags in Antarctica and may be useful for future meta-analyzes comparing blood parameters of different species and geographic areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The South Georgia shag (SSG, Phalacrocorax georgianus) is a colonial seabird distributed along the South Orkney Islands in Antarctica and the sub-Antarctic South Sandwich and South Georgia Islands (Orta et al. 2021). This species is part of a large group of Southern Hemisphere blue-eyed shags (Watson 1975; Siegel-Causey 1988) that includes Antarctic shags (Phalacrocorax bransfieldensis). To date, debate continues as to whether South Georgia and Antarctic shags are distinct species or subspecies (Orta et al. 2021). For this work, South Georgia shag is considered a separate species from Antarctic shag. Based on historical data published from various locations and years, Dunn et al. 2022 estimate the population size of SGS to be between 5349 and 10,849 breeding pairs, of which between 17 and 38% are in South Orkney (Dunn et al. 2022). Several studies have reported changes in the population size of South Georgia shags in South Orkney. On Signy Island, Dunn et al. (2022) found an overall decline in nesting pairs of 40.9% (− 1.3% per year), with a fluctuating decline from the 1990s to 2020/2021. South Georgia and Antarctic shags are the only flying birds in Antarctica that feed primarily on benthic demersal fishes (Casaux and Barrera-Oro 2006), therefore, their population trends could be reflecting changes in coastal fish populations (Casaux and Barrera-Oro 1993).
Antarctica is considered a pristine zone; however, it is subject to various factors of environmental change. In this regard, it is one of the regions most affected by climate change, with air temperatures increasing by 0.61 ± 0.34 °C per decade (Turner et al. 2020). At the same time, human activities have increased significantly mainly due to fishing, the tourism industry, and scientific research (Tejedo et al. 2011). In this context, Casaux and Barrera-Oro (2016) suggested that the decreasing abundance of demersal fish prey caused by fishing activities is a possible cause of the decline of Antarctic shag populations they had studied. On the other hand, Casanovas et al. (2015) suggested that climate change is likely responsible for the population change of cormorants, consistent with the effects of climate change on other seabirds in the region. Therefore, monitoring the populations of SSGs in multiple locations is necessary to understand the factors regulating their population trends and assess the marine ecosystem’s overall status.
Physiological data have become an important tool for monitoring the health of wildlife species in the face of rapidly changing environments (Barbosa et al. 2013; D’Amico et al. 2016) because they can alert us to changes in the status of individuals, allowing early detection of problems and potentially providing an opportunity to mitigate them. Environmental changes can affect the health of wild vertebrate populations in a variety of ways (Harvell et al. 2002; Acevedo-Whitehouse and Duffus 2009). For example, changes in food availability due to fisheries or ecological changes may be reflected in the nutritional status of individuals (e.g., suboptimal protein levels, and depletion of fat reserves). In addition, tourism increases the possibility of contact between wildlife and humans, which can induce physiological stress in animals and impair immunocompetence (Ellenberg et al. 2007), while increasing the risk of exposure to new pathogens (Walton 2012). Also, pollutants can impair immune defenses (Barbosa et al. 2013).
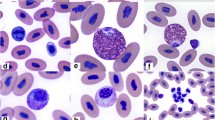
The leukocyte profile provides information about immune function by determining the total leukocyte count and the five leukocyte types, including heterophils, eosinophils, basophils, lymphocytes, and monocytes (Roitt et al. 2001). Heterophils and lymphocytes are the most abundant immune cells in birds (Campbell 1995). Heterophils respond to infection, inflammation, or poor nutrition (Maxwell and Robertson 1998), whereas lymphocytes contribute to cell-mediated and humoral adaptive immunity (Campbell 1995). Both types of leukocytes respond to stressors such as sudden environmental changes and/or changes in food availability (Davis et al. 2008). As a result, these changes can increase the number of heterophils and decrease the number of lymphocytes in the circulating blood. Therefore, the H/L ratio is often used as a measure of stress (Davis et al. 2008). Monocytes are involved in the phagocytosis of tissue debris and pathogens such as fungi and protozoa (Kerr 2002). The least abundant leukocytes in birds are eosinophils and basophils. While eosinophils are associated with parasitic infections (Bertellotti et al. 2016), basophils are involved in acute inflammatory responses (Maxwell and Robertson 1998). Along with the hematological immune parameters, the birds’ health status can be evaluated through biochemistry parameters. Numerous studies in birds have demonstrated the relationship between nutritional status, body condition, and plasma metabolites, including total proteins, glucose, and lipids (cholesterol and triglycerides) (Artacho et al. 2007).
The physiological responses of the organisms to environmental changes vary according to the ecology and sensitivity of each species. Therefore, knowledge of species-specific responses is of great value as an indicator of ecosystem change and physiological data can provide valuable baseline information for ecological studies and comparisons across geographic areas, species, and periods. As part of a broader research project on the reproductive and trophic ecology of SGS, we examined the health status of adult SGS breeding on South Orkney Island. The objective of the study was to determine the hematology and biochemistry associated with the body condition and immune function of SGS during the breeding season. This study represents the first compilation of this information and establishes a baseline for the endangered free-ranging South Georgia Shags.
Methods
The study was conducted on Laurie Island, South Orkney Islands, Antarctica. In this work, SGS were sampled on two small offshore islands in Brown Bay (60° 41’ S, 044° 38’ W) and Jessie Bay (60° 41’ S 044° 42’ W) in January 2018. Sampling occurred during late chick rearing (chicks approximately four to six weeks of age).
We sampled individuals captured with a landing net (a small net located at the end of a long stick) on the nest. Blood (2 to 3 ml) was collected from the brachial vein using heparinized syringes (3 ml) with a sterilized needle (23G). Each bird was weighed using a 5000 g dynamometer and examined for external signs of disease or injury. Blood was stored in Eppendorf tubes and kept refrigerated until brought to the laboratory. To measure hematocrit, 75 µl of blood was placed in microcapillary tubes and centrifuged at 12,000 g for 12 min. Leukocytes were analyzed on blood smears prepared with one drop of blood, fixed with ethanol for 10 min, air-dried, and stained with Tinction 15 (Biopur). The smears were examined under a microscope (400 X) and all leukocytes were counted in ten consecutive fields to determine the white blood cell (WBC) count (D’Amico et al. 2010). The relative percentage of each leukocyte type in a total of 100 leukocytes was obtained under 1000X microscopic magnification (Campbell 1995). The physiological stress index H/L was calculated from the heterophil and lymphocyte values (Davis et al. 2008). Blood stored in Eppendorf tubes was centrifuged to remove the plasma fraction for biochemical determinations. Plasma was analyzed with a spectrophotometer (METROLAB 1600 Plus, UV–Vis) to determine the concentration of total proteins (g/dl), cholesterol (mg/dl), triglycerides (mg/dl), and glucose (mg/dl), indicating the nutritional status of the subjects.
Basic descriptive statistics were obtained for each parameter. Normality and homogeneity of variance were tested using Shapiro-Wilk and Levene’s tests. The T-test was used to examine differences between sexes in biochemical parameters. Because the hematologic variables did not meet the normality assumption, a nonparametric Mann-Whitney U test was used to examine sex differences. Statistical significance was set at p ˂ 0.05. For statistical analyses and a graphical representation, we used R software, version 2.12.1.
Results and discussion
We captured 11 females and 12 males during the breeding season. No abnormalities or signs of disease were observed in any of the birds during handling. Mean body weights were 2.54 and 2.61 kg for females and males, respectively, and did not differ significantly between sexes (T-test = 39, p = 0.693). The values of physiological parameters obtained for female and male SGS during breeding are shown in Table 1. Overall, most values of parameters reported in this study were within the range previously described for other seabirds (Newman et al. 1997; Ferrer et al. 2017).
Hematocrit values for SGS were 54% in females and 55% in males. In general, reference ranges for hematocrit in birds are between 35 and 55% (Campbell 1995). The values observed in SGS may be attributed to a high respiratory function of a unit of blood volume required to maintain a high level of metabolic energy under extremely cold weather conditions (Myrcha and Kostelecka-Myrcha 1980).
In both sexes, heterophils were the most abundant leukocyte type, followed by lymphocytes (Table 1). This observation differs from what is generally reported in birds, where lymphocytes are usually the most abundant leukocyte type (Campbell 1995; Newman et al. 1997). Our results are consistent with those reported for juvenile Double-crested (Kuiken and Danesik 1999) and Pelagic cormorants (Phalacrocorax pelagicus) (Newman et al. 1997), whereas lymphocytes predominated in Black-faced cormorants (Phalacrocorax fuscescens) and Great cormorants (Phalacrocorax carbo) (Melrose and Nicol 1992; Minias et al. 2013). Consequently, there is no consistent pattern in the percent distribution of leukocytes in different cormorant species. D’Amico et al. (2014) suggested that the prevalence of heterophils in Gentoo penguins (Pygoscelis papua) is related to greater parasitic diversity. Because the other leukocyte types found in SGS were in low proportions and within the ranges reported for birds (Campbell and Ellis 2007), we suspect that the highest percentage of heterophils found here is probably not clinically significant and may be due to other factors such as a stressful environment, stage of the annual cycle, and others (Davis et al. 2008). Males, on the other hand, had higher numbers of eosinophils (Table 1), which is mainly related to gastrointestinal parasite infections (Thrall et al. 2012).
Glucose and total protein concentrations in our study were similar to previous values reported for other species such as Imperial cormorant (P. atriceps) (Gallo et al. 2013), Pelagic cormorant (P. pelagicus) (Newman et al. 1997), Double-crested cormorant (P. auratus) (Kuiken and Danesik 1999), Crested cormorant (P. carbo sinensis) (Minias et al. 2013), and Flightless cormorant (P. harrisi) (Travis et al. 2006). However, triglyceride levels were higher than those observed in Imperial cormorant (Gallo et al. 2013) and Double-crested cormorant (Kuiken and Danesik 1999). The higher triglyceride levels found in this study could be related to recent food intakes as it was reported by Jenni and Schwilch (2001) for the pond warbler (Acrocephalus scirpaceus). During mid- and late-chick rearing, mates forage several times per day and take turns in the nest, resulting in males and females from different nests foraging at the same time (Casaux and Bertolin 2018), so, likely, adults were freshly fed at the time of sampling. In contrast, SGS had lower cholesterol levels than those reported for Imperial and Crested cormorants (Kuiken and Danesik 1999; Gallo et al. 2013), but similar levels to those reported for Pelagic (Newman et al. 1997) and Flightless cormorants (Travis et al. 2006). Cholesterol concentrations have been reported to be influenced by the qualitative composition of the diet and regulated by lipid metabolism (Duncan et al. 1994). Therefore, the lower levels observed in our study could be related to the low-fat fish of the Notothenidae consumed by cormorants in the study area (Bertolin and Casaux 2019). Significant differences were found for cholesterol and total proteins, which were both higher in males (Table 1). These results could indicate a better individual condition of males during reproduction or that reproduction is more energetically demanding for females. Nevertheless, previous studies of reproduction in this species suggest biparental care, with similar time and energy budgets for both sexes of SGS (Bernstein and Maxson 1984, 1985). Conversely, Casaux et al. (2001) provided evidence of sex segregation in foraging areas and diets of Antarctic shag from South Shetland Island, suggesting that other factors may influence the condition of males and females of SGS.
The physiological parameters measured in this study represent a range of hematological and biochemical findings in apparently healthy shags on South Orkney Island, Antarctica, during the breeding season. Yearly environmental changes could also impact the physical and physiological condition of cormorants. Although the sample size is limited, the values in this study are the first physiological values presented for this species. Therefore, our results can be used for comparisons with future health monitoring, including pathogenic surveillance of this species in the study area and elsewhere, as well as with related species.
Data availability
The datasets generated and analyzed during the current study are not publicly available because some of the data are still under analysis as part of a doctoral thesis that is being carried out by a researcher from the Argentine Antarctic Institute. However, some of these data are available from the corresponding author on reasonable request.
References
Acevedo-Whitehouse K, Duffus ALJ (2009) Effects of environmental change on wildlife health. Philos Trans R Soc B Biol Sci 364:3429–3438. https://doi.org/10.1098/rstb.2009.0128
Artacho P, Soto-Gamboa M, Verdugo C, Nespolo RF (2007) Blood biochemistry reveals malnutrition in black-necked swans (Cygnus melanocoryphus) living in a conservation priority area. Comp Biochem Physiol - Mol Integr Physiol 146:283–290. https://doi.org/10.1016/j.cbpa.2006.10.031
Barbosa A, De Mas E, Benzal J et al (2013) Pollution and physiological variability in Gentoo penguins at two rookeries with different levels of human visitation. Antarct Sci 25:329–338. https://doi.org/10.1017/S0954102012000739
Bernstein NP, Maxson SJ (1984) Sexually distinct daily activity patterns of Blue-Eyed shags in Antarctica. Condor 86:151. https://doi.org/10.2307/1367031
Bernstein NP, Maxson SJ (1985) Reproductive energetics of blue-eyed shags in Antarctica. Wilson Bull 97:450–462
Bertellotti M, D’Amico VL, Palacios MG et al (2016) Effects of antihelminthic treatment on cell-mediated immunity in Gentoo penguin chicks. Polar Biol 39:1207–1212. https://doi.org/10.1007/s00300-015-1839-0
Bertolin ML, Casaux R (2019) Diet overlap among top predators at the South Orkney Islands, Antarctica. Polar Biol 42:371–383. https://doi.org/10.1007/s00300-018-2428-9
Campbell TW (1995) Avian hematology and cytology, 2nd edn. Iowa State University, Iowa
Campbell TW, Ellis CK (2007) Hematology of birds. In Avian and Exotic animal hematology and cytology, p 3–50
Casanovas P, Naveen R, Forrest S, Poncet J, Lynch HJ (2015) A comprehensive coastal seabird survey maps out the front lines of ecological change on the western Antarctic Peninsula. Polar Biol 38:927–940. https://doi.org/10.1007/s00300-015-1651-x
Casaux RJ, Barrera-Oro ER (1993) The diet of the blue-eyed shag, Phalacrocorax atriceps bransfieldensis feeding in the Bransfield Strait. Antarct Sci 5:335–338. https://doi.org/10.1017/S0954102093000458
Casaux R, Barrera-Oro E (2006) Shags in Antarctica: their feeding behavior and ecological role in the marine food web. Antarct Sci 18:3–14. https://doi.org/10.1017/S0954102006000010
Casaux R, Barrera-Oro E (2016) Linking population trends of Antarctic shag (Phalacrocorax bransfieldensis) and fish at Nelson Island, South Shetland Islands (Antarctica). Polar Biol 39:1491–1497
Casaux R, Bertolin ML (2018) Foraging patterns of the antarctic shag Phalacrocorax bransfieldensis at harmony point. Antarctica Mar Ornithol 46:169–175
Casaux R, Favero M, Silva P, Baroni A (2001) Sex differences in diving depths and Diet of Antarctic Shags at the South Shetland Islands. J F Ornithol 72:22–29. https://doi.org/10.1648/0273-8570-72.1.22
D’Amico VL, Bertellotti M, Baker AJ, González PM (2010) Hematological and plasma biochemistry values for endangered red knots (Calidris canutus rufa) at wintering and migratory sites in Argentina. J Wildl Dis 46:644–648
D’Amico VL, Coria N, Palacios MG et al (2016) Physiological differences between two overlapped breeding Antarctic penguins in a global change perspective. Polar Biol 39:57–64. https://doi.org/10.1007/s00300-014-1604-9
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. https://doi.org/10.1111/j.1365-2435.2008.01467.x
Duncan RJ, Prasse KW, Mahaffey EA (1994) Veterinary laboratory medicine: clinical pathology. Iowa State University, Ames
Dunn MJ (2022) Long-term population size and trends of South Georgia shags (Leucocarbo [atriceps] georgianus) at Signy Island, South Orkney Islands and Bird Island, South Georgia. Polar Biol 45:177–189. https://doi.org/10.1007/s00300-021-02978-2
Ellenberg U, Setiawan AN, Cree A et al (2007) Elevated hormonal stress response and reduced reproductive output in yellow-eyed penguins exposed to unregulated tourism. Gen Comp Endocrinol 152:54–63. https://doi.org/10.1016/j.ygcen.2007.02.022
Ferrer M, Morandini V, Perry L, Bechard M (2017) Factors affecting plasma chemistry values of the black-browed albatross Thalassarche melanophrys. Polar Biol 40:1537–1544. https://doi.org/10.1007/s00300-017-2075-6
Gallo L, Quintana F, Svagelj W, Uhart M (2013) Hematology and Blood Chemistry values in Free-Living Imperial cormorants (Phalacrocorax atriceps). Avian Dis 57:737–743. https://doi.org/10.1637/10521-022713-reg.1
Harvell CD, Mitchell CE, Ward JR et al (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296:2158–2162. https://doi.org/10.1126/science.1063699
Jenni L, Schwilch R (2001) Plasma metabolite levels indicate a change in body mass in reed warblers Acrocephalus scirpaceus. Avian Sci 11:55–65
Kerr MG (2002) Veterinary laboratory medicine. Clinical biochemistry and Haematology, 2nd edn. Blackwell Science Ltd
Kuiken T, Danesik KL (1999) Hematology and serum chemistry of captive juvenile double-crested cormorants (Phalacrocorax auritus). Can Vet J 40:493–496
Maxwell MH, Robertson GW (1998) The avian heterophil leucocyte: a review. Worlds Poult Sci J 54:155–178. https://doi.org/10.1079/wps19980012
Melrose WD, Nicol SC (1992) Haematology, red cell metabolism and blood chemistry of the black-faced cormorant Leucocarbo fuscescens. Comp Biochem Physiol - Part Physiol 102:67–70. https://doi.org/10.1016/0300-9629(92)90013-G
Minias P, Kaczmarek K, Janiszewski T, Markowski J (2013) Hematology and plasma biochemistry values of great cormorant (Phalacrocorax carbo sinensis) nestlings. J Wildl Dis 49:194–196. https://doi.org/10.7589/2012-02-055
Myrcha A, Kostelecka-Myrcha A (1980) Hematological studies on Antarctic birds. I: hematological indices in some species of the birds studied during Australian summer. Pol Polar Res 1:169–173
Newman SH, Piatt JF, White J (1997) Hematological and plasma biochemical reference range of alaskan seabirds: the ecological significance and clinical importance. Colon Waterbirds 20:492–504
Orta J, Garcia EFJ, Christie DA et al (2021) Antarctic Shag (Leucocarbo bransfieldensis). In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) Birds of the World. Cornell Lab of Ornithology, Ithaca, New York
Roitt I, Brostoff J, Male D (2001) Immunology. Mosby, London
Siegel-Causey D (1988) Phylogeny of the Phalacrocoracidae. Condor 90:885–905
Thrall MA, Weiser G, Allison R, Campbell T (2012) Veterinary hematology and clinical chemistry. Wiley-Blackwell
Tejedo P, Pertierra L, Benayas J, Boada M (2011) Equilibrios Sobre El Hielo: una breve (pero completa) revisión del conocimiento sobre El Impacto humano en la Antártida. Ecosistemas 20:69–86. https://doi.org/10.7818/re.2014.20-1.00
Travis EK, Vargas FH, Merkel J et al (2006) Hematology, plasma chemistry, and serology of the flightless cormorant (Phalacrocorax harrisi) in the Galápagos Islands, Ecuador. J Wildl Dis 42:133–141. https://doi.org/10.7589/0090-3558-42.1.133
Turner J, Marshall GJ, Clem K et al (2020) Antarctic temperature variability and change from station data. Int J Climatol 40:2986–3007. https://doi.org/10.1002/joc.6378
Walton D (2012) Keeping the aliens out. Antarct Sci 24. https://doi.org/10.1017/S0954102012000594
Watson E (1975) Birds of the Antarctic and sub-antarctic. American Geophysical Union, Washington DC
Acknowledgements
We would like to express our appreciation to members of Orcadas Station for their logistic help and field assistance and to the Argentine Antarctic Institute, in particular to Dr. Ricardo Casux for their support of this project.
Funding
This research was funded by the Instituto Antartico Argentino and the Project “Interacciones predador-presa” (PICTA 2010-03). Marianela Beltran was supported by a doctoral fellowship funded by the Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.B., and M.B.; methodology, M.B., and V.D.; formal analysis, M.B.; resources, M.B.; investigation, M.B., V.D. C.F., and M.B.; writing—original draft preparation, M.B., V.D., M.B., and C.F; writing—review and editing, V.D., and M.B.; visualization, M.B..; supervision, M.B.. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The protocol for capturing and sampling birds were ethically reviewed and approved for the Departamento de Gestion Ambiental de la Direccion Nacional del Antartico: Exp 2017-FEAMB- CT- GA -35 218. This study was carried out considering the Nuremberg Code, the Helsinki Declaration, and its amendments. This study followed the guidelines established in the Conduct Code of the Antarctic Search Scientific Committee for the Use of Animals for Scientific Purposes in Antarctica.
Competing interests
The authors declare no competing interests.
Disclaimer/Publisher’s note
The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of Polar Biology and/or the editor(s). Polar Biology and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beltran, M., D’Amico, V., Carla, F. et al. Physiological parameters of South Georgia Shag (Phalacrocorax georgianus) during breeding in South Orkney Island, Antarctica. Polar Biol 47, 309–313 (2024). https://doi.org/10.1007/s00300-024-03236-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03236-x