Abstract
Thecosome pteropods form an important part of marine food webs, especially in polar ecosystems, and are the focus of research on ocean acidification. Although the larval stages of species in the genus Limacina often form major components of zooplankton communities, little is known of their population dynamics. We report high Limacina spp. abundance in March 2000 during surface zooplankton community sampling via a Continuous Plankton Recorder (CPR; 270-µm mesh) in a large area within the seasonal ice zone of the Southern Ocean. Regions with high Limacina spp. abundances extended to 600 nautical miles (ca 1110 km). Annual variability in Limacina spp. abundance and shell size is evaluated using North Pacific standard net (100-µm mesh) data from the same area and sampling periods (March) from 1997 to 2006. Although the relative total abundance of Limacina spp. in 2000 was the highest in the study period, its overall abundance was lower than the mean value for that period. Mean shell size for most years ranged 160–300 µm, while a relatively large mean size (444.7 µm) occurred in 2000. We conclude that a CPR with 270-µm mesh could catch large Limacina individuals that dominated in March 2000. The timing of reproduction and growth of the new generation may influence Limacina abundance throughout the sampling area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pteropods, ubiquitous components of Southern Ocean zooplankton communities, can be extremely abundant regionally among mesozooplankton size fractions (Hunt et al. 2008; Steinberg et al. 2015). It has recently been demonstrated that thecosome pteropods may be severely affected by ocean acidification caused by increased atmospheric CO2 concentrations (Bednaršek et al. 2012a; Manno et al. 2016). They contribute to the carbon cycle through their fast-sinking fecal pellets, and deposition of shells after mass die-offs (Manno et al. 2007, 2010, 2018; Bednaršek et al. 2012b, 2016; Thibodeau et al. 2019). Although the biomass-dominant genus Limacina is widespread throughout the Southern Ocean, aspects of its life cycle remain uncertain (Hunt et al. 2010). Two Southern Ocean species are known: Limacina retroversa australis (Eydoux and Souleyet, 1840) and L. rangii (d′Orbigny, 1835), previously referred to as L. helicina antarctica Woodward, 1854 (Janssen et al. 2019). Of them, L. retroversa is thought to be a sub-Antarctic species, and L. rangii to occur largely south of the Polar Front (PF) to the Antarctic coast (Hunt et al. 2008). Spawning in L. rangii occurs primarily from late summer to autumn (Hunt et al. 2008; Thibodeau et al. 2020), and its larval stages are sometimes major contributors to total zooplankton abundance during autumn, especially in the seasonal ice zone—an ecologically important region in the Southern Ocean (Brierley and Thomas 2002).
The Continuous Plankton Recorder (CPR; 270-µm mesh size) provides an effective and rapid means of monitoring micro- and mesozooplankton distribution patterns within approximately 10 m of the surface to assess the effects of environmental change over large oceanic scales (Hosie et al. 2003; Takahashi and Hosie 2021). Since its launch in 1991, the Southern Ocean CPR (SO-CPR) survey program has produced the largest, most comprehensive and systematic spatial and temporal data set of Antarctic zooplankton. Because data have been collected using a consistent sampling methodology, the mapping of seasonal, inter-annual, long-term, and spatial variation in plankton diversity is possible, as is the identification of select planktons which can act as indicators of environmental change for monitoring the health of the Southern Ocean (Takahashi and Hosie 2021).
While SO-CPR survey data has revealed high-density patches of pteropods (Hunt et al. 2008; Pinkerton et al. 2020), shell damage during collection has hampered accurate species identification, for which reason taxa have been identified to Limacina spp. (Takahashi et al. 2011). Abundances of Limacina spp. collected south of Australia from 1997 to 2006 were typically low over the austral winter, and increased slowly from November to January, peaked in February, and remained relatively high in March, and declined until the end of May (Hunt et al. 2008). Their lifespan is estimated at one year based on the seasonal abundance data integrated using > 200-µm mesh nets. Although the strong seasonal cycle of CPR data has complemented this scenario, these are only surface data and are considered to be an underestimate of the new generation resulting from the large mesh size used. Several polar region studies suggest a range of one or two generations of Limacina occur per year, longevity from 1 to 3 years, continuous or discrete reproduction to occur once or twice a year, and for peak densities to occur from spring to late summer (Gannefors et al. 2005; Hunt et al. 2008; Bednaršek et al. 2012b; Nishizawa et al. 2016; Wang et al. 2017; Thibodeau et al. 2020; Boissonnot et al. 2021). The highly complex nature of data, and differences in its interpretation, limits our ability to generalize from these studies. Therefore, to fully understand the CPR data, the complementary use of the species identification data and shell size of the genus Limacina via the simultaneous use of a finer net is required. In particular, it is important to accumulate data on the abundance and size structure in March just before the abundances decrease.
We focus on CPR data from the seasonal ice zone in 2000, during which time high abundances of Limacina spp. occurred over a large area, and for which abundance peaked in March. As a part of the zooplankton monitoring program of the Japanese Antarctic Research Expedition (JARE), regular sampling using a North Pacific (NORPAC) standard net (100-µm mesh size) from a depth of 150 m to the surface was conducted simultaneously on the CPR transect. The comparative studies by Hunt and Hosie (2003) already demonstrated that despite sampling differences between the CPR and NORPAC nets, the evenness index indicated that all communities had a similar distribution of abundance amongst species. The objectives of this study were, first, to discuss the spatial distribution, abundance, and species composition of zooplankton communities based on the CPR samples in March 2000, with an emphasis on their pteropod composition, and second, to evaluate the annual variability of abundance and shell size of Limacina spp. using NORPAC net data collected in the same area and same sampling periods (March) from 1997 to 2006 (with the exception of 1999 and 2005, during which samples were not collected).
Materials and methods
CPR towing
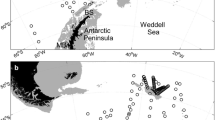
CPR sampling was conducted between 9 and 16 March 2000 aboard the icebreaker Shirase during the 41st JARE cruise (JARE–41), from the Japanese Showa Station, Antarctica, toward Sydney, Australia, along two transects: West to East (WE1), latitude 63° 00′ S, from 133° 00′ E to 149° 22′ S, and South to North (SN1–4), longitude 150° 00′ E, from 62° 59′ S to 47° 40′ S (Fig. 1, Table 1). The CPR (Type II, Mark V) was towed horizontally from the stern at a ship speed of approximately 14 knots, using a cable paid out to 100 m, with the CPR sampling at approximately 10 m depth. The CPR has a square mouth with a 1.27 × 1.27 cm area, expanding into a tunnel 10 × 5 cm, with water passing through a 270-µm silk mesh filter within the CPR. During sampling, zooplankton captured on the silk were preserved in a formaldehyde bath within the CPR. After sampling, zooplankton samples retained between silk meshes were preserved in a 10% v/v neutral buffered formalin solution. In the laboratory, 275 separate silk segments were cut from five tows, each representing 5 nautical miles of filtration. The length of each segment was calculated based on the stop and start positions of each transect and estimated from 1-min interval GPS positions recorded between start and stop times.
The zooplankton on each segment were identified to the lowest taxonomic level possible and counted using a stereomicroscope (SMZ1500, Nikon, Japan). Pteropod shells were usually damaged by the silks; therefore, no attempt was made to differentiate between species of Limacina. Ostracods and most small hyperiid amphipods were not identified to species. Rhincalanus gigas (Copepoda: Calanoida) nauplii were distinguished from other calanoid nauplii by their large size and morphology. Copepodite stages of smaller calanoid copepod adults such as Clausocalanus spp. and Microcalanus pygmaeus are difficult to identify and were grouped as “copepodite indet.” Calyptopis and furcilia stages of euphausiid species were distinguished from adults (post-furcilia). Zooplankton abundance was converted to individuals m−3, with the volume of water filtered assumed to be 100% of the volume calculated from the length of each segment and the mouth opening of the CPR.
Simultaneous measurements of surface temperature, salinity, and in vivo fluorescence intensity were made with an on-board surface monitoring system (Fukuchi and Hattori 1987), pumping seawater from a depth of approximately 8 m. Chlorophyll a concentration was determined by filtering water samples onto glass fiber filters; filters were then soaked immediately in N,N-dimethylformamide (Suzuki and Ishimaru 1990), the pigment extracted, and pigment concentrations determined fluorometrically with a Turner Designs model 10R fluorometer (Parsons et al. 1984). For chlorophyll a calibration, pumped surface water was sampled two or three times daily. In vivo fluorescence data were calibrated as follows:
Nighttime samples were defined as those where photosynthetically active radiation (PAR) was < 100 µmol s−1 m−2. Species richness (r), i.e., number of species, was calculated for each sample.
NORPAC net sampling
NORPAC-net sampling in the seasonal ice zone (defined as south of 59°E in the present study) was regularly carried out in three areas (N1: 140° E, 63–65° S; N2: 150° E, 63–65° S; and N3: 150° E, 59–61° S) on board the icebreaker Shirase during JARE–38 (1997) to JARE–47 (2006) (except JARE–40 (1999) and 46 (2005)) in the Indian sector of the Southern Ocean en route from Syowa Station in March (Fig. 1, Table 2). Additionally, on the SN transect of the CPR towing in JARE–41, regular sampling with a NORPAC net was conducted along 150° 00′ E from 63° 00 to 47° 30′ S (Fig. 1, Table 2). Sampling stations were located at intervals of 3–4 degrees of latitude (N2–N6).
The NORPAC standard net, made of nylon bolting cloth XX13 (100-µm mesh openings), was used at all sampling stations. The net was hauled vertically at a speed ca 1 m s−1, from an approximate depth of 150 m. The maximum depth reached was estimated from the wire angle and length of wire paid out. All samples obtained were immediately preserved onboard in a 5–10% neutral-buffered formalin-seawater solution. Volumes of water filtered through each net were estimated using a flowmeter that was mounted at the center of the mouth ring of each net. While abundances of Limacina may be underestimates because individuals can migrate deeper than 150 m (Bednaršek et al. 2012a; Manno et al. 2016), sampling always occurred during the daytime. Accordingly, the effects of diel vertical migration in analysis of annual variation in abundance using NORPAC sampling data are likely to be minimal.
The type of sampling device and its mesh size affect plankton catchability. A comparative study using both the CPR and a commonly used plankton net fitted with 200-µm mesh revealed differences in catchability, with the underestimate of abundance (Clark et al. 2001; John et al. 2001). Because Hunt and Hosie (2003) reported similar plankton abundance and species composition in 270-µm mesh CPR and NORPAC-net collected samples from along the same sampling track, the CPR design and its sampling method appear to produce less error in estimates of plankton abundance than different-sized meshes do. Our NORPAC samples were collected from vertical hauls (0–150 m) using a net with 100-µm mesh, which has been demonstrated to be suitable for the capture, and estimation of the quantitative abundance and community structure of micro- and mesozooplankton (Makabe et al. 2012).
Analysis of zooplankton and Limacina spp. of NORPAC samples
Zooplankton were identified to the lowest practical taxonomic level, generally to species or genus, using a stereomicroscope (SMZ1500, Nikon, Japan). Total zooplankton and Limacina abundances were converted to individuals m−3. From each sample, 50 Limacina individuals were randomly selected, and their shell diameter and greatest height were measured (Thibodeau et al. 2020). Our measurements of shell diameter ranged 0.14–0.68 µm. Because veligers of the Arctic L. helicina have diameters < 0.3 µm, and juveniles ≥ 0.3 µm (Lalli and Wells 1978), our Limacina likely comprised both veliger and juvenile stages.
Two Limacina species, L. retroversa australis and L. rangii, are major components of Southern Ocean zooplankton communities: the former dominant in sub-Antarctic regions, the latter primarily south of the PF (Hunt et al. 2008). These two species differ in their shell height/diameter ratio, with that of L. retroversa australis being approximately 1.5 and that of L. rangii approximately 0.75 (World Register of Marine Species (WoRMS) database; http://www.marinespecies.org/index.php). Because the shell height/diameter ratio of Limacina collected from the seasonal ice zone south of 59°E was 0.69 ± 0.07 (mean ± SD), these specimens are most likely to be L. rangii. The relationship between shell diameter and shell height is significantly correlated (Fig. 2).
Statistical analysis
Zooplankton abundance from the CPR was further analyzed by cluster analysis, using Bray–Curtis dissimilarity and unweighted pair group average linkage, to compare species/taxon composition between sampling areas, following the procedures described by Field et al. (1982). To compare community structures, data were transformed using the log10 (x + 1) function to reduce biases caused by highly abundant taxa. One-way analysis of variance and Tukey′s tests were used to test the null hypothesis that the abundance levels of a species/taxon did not differ between sample groups. Newman–Keuls multiple-range tests were performed to identify inter-cluster differences in species/taxa abundance levels. Statistical analyses were carried out in Primer Version 6 (Clarke and Gorley 2006) and IBM SPSS Statistics for Windows Version 23 (Arbuckle 2014).
Results
West–East transect CPR samples
Sea surface temperature, salinity, and chlorophyll a concentration were relatively constant through the transect. There were some surface monitoring system errors around 139° 36′ E, and between 139° 58′ E and 140° 52′ E (Fig. 3a). Temperature fluctuated slightly around 1.5 °C (mean ± SD; 1.56 ± 0.18), and salinity was stable around 33.7 PSU (33.7 ± 0.066). Chlorophyll a concentration was also low and stable, ranging 0.47–0.70 mg m−3. Twenty-four species/taxa were identified throughout the transect, and zooplankton abundance (mean ± SD; 88.6 ± 92.9 ind. m−3) was high in the eastern area, especially for Limacina spp. (mean ± SD; 58.5 ± 69.9 ind. m−3). Maximum zooplankton (489.9 ind. m−3) and Limacina spp. (353.8 ind. m−3) abundances occurred at 142° 26′ E (Fig. 3a). Along the transect, Limacina spp. was the predominant taxon, contributing 66% to total zooplankton abundance.
a Sea surface temperature, salinity, chlorophyll a concentration, Limacina spp. abundance, and other zooplankton abundance recorded from the West–East Continuous Plankton Recorder (CPR) transect of the Japanese Antarctic Research Expedition (JARE) 41. b Result of cluster analysis and species composition of the two major clusters
Cluster analysis revealed two major widely distributed groups separated at 141° E, with a 37.6% dissimilarity level (Fig. 3b, Table 3). Cluster A comprised 36 segments located in the western area, whereas Cluster B had 77 segments located in the eastern area. In both clusters, sea surface temperature, salinity, and chlorophyll a concentration were at very similar levels (Table 3). Although species composition was also similar, abundance was significantly high in Cluster B. Limacina spp. was the dominant species/taxon in both clusters, comprising 41.6% of total zooplankton abundance in Cluster A, with a mean abundance of 8.9 ± 4.6 ind. m−3; in Cluster B, Limacina spp. comprised 68.7% of total zooplankton abundance, with a mean of 99.3 ± 71.8 ind. m−3. In both clusters, Limacina spp. was followed by cyclopoid copepod Oithona similis, and copepodite indet. as the most abundant species/taxa. In Cluster B, these three species/taxa had significantly more abundant than in Cluster A (Table 3).
South–North transect CPR samples
Sea surface temperature increased from 1.3 to 10.9 °C toward the north, and salinity also increased gradually from 33.51 to 34.25 PSU (Fig. 4a). We identified the circumpolar frontal position using both sea surface temperature and salinity data. Although the actual circumpolar frontal position can be reliably identified only through deep-oceanographic observations (Orsi et al. 1995), both sea surface temperature and salinity correspond well between regions of steep physical change and mean frontal position (Sokolov and Rintoul 2002). The PF and Sub-Antarctic Front (SAF), determined by sea surface temperature profile, occurred between 58° 50′ S and 58° 00′ S, and 55° 17′ S and 54° 12′ S, respectively, and were associated with an abrupt change in sea surface temperature. South of the SAF, chlorophyll a concentrations were relatively stable between 0.5 and 0.75 mg m−3, rising to 0.92 mg m−3 in the low latitudes. Thirty-three species/taxa were identified through this transect. Zooplankton abundance (mean ± SD; 30.9 ± 43.8 ind. m−3), especially for Limacina spp., changed dramatically on the border of the PF, with high abundances occurring on the southern side. Maximum zooplankton (282.7 ind. m−3) and Limacina spp. (267.4 ind. m−3) abundances occurred at 59° 25′ S (Fig. 4a). Along the transect, Limacina spp. was the dominant taxon, comprising 41.9% of the total zooplankton abundance.
a Sea surface temperature, salinity, chlorophyll a concentration, Limacina spp. abundance, and other zooplankton abundance recorded from the South-North Continuous Plankton Recorder (CPR) transect of the Japanese Antarctic Research Expedition (JARE) 41. b Result of cluster analysis and species composition of Cluster D, which had a high abundance of Limacina spp. PF Polar Front, SAF Sub-Antarctic Front
Cluster analysis of the SN transect identified six clusters and six outliers, with a 51.6% dissimilarity level (Table 4). Cluster A comprised 45 segments from nighttime samples around SAF and south of PF (Fig. 4b). Zooplankton abundance was relatively low at 14.05 ind. m−3, and the small copepods O. similis and copepodite indet. were dominant. Cluster B comprised 68 segments located mainly north of the SAF. Surface water in this region was warm (7.94 °C), and chlorophyll a concentration was highest at 0.79 mg m−3. Species richness was relatively high at 6.53 (Table 4). This cluster was also characterized by the occurrence of the euphausiids Euphausia frigida and E. triacantha, amphipods Hyperiella antarctica and Primno macropa, and ostracoda, none of which occurred in any other cluster. Cluster C comprised the three samples located in the PF (Fig. 4b). Foraminifera abundance levels were significantly higher in Cluster C than in any other cluster (Table 4).
Cluster D comprised 31 segments located south of the PF (Fig. 4b). This cluster had the lowest sea surface temperature (1.52 °C) and highest zooplankton abundance of any of the clusters (Table 4). Total zooplankton abundance had a mean of 94.6 ind. m−3, and included the highest abundance levels of Limacina spp. with 77.3 ind. m−3. Cluster E comprised 12 segments located between the PF and SAF. Zooplankton abundance was relatively low with 9.27 ind. m−3, and the abundance of appendicularians of the genus Oikopleura spp. was the highest of any cluster (Table 4). Cluster F comprised 21 segments located in the vicinity of SAF. This cluster had the lowest zooplankton abundance of any of the clusters.
South-North transect NORPAC samples
Species in NORPAC-net samples from five stations, collected simultaneously with CPR, were very similar, with Limacina spp., O. similis, and copepodite indet. being dominant (Fig. 5a). At northern stations N5 and N6, the abundance of foraminiferans was relatively high (66.5% and 28.0%, respectively). Limacina spp. occurred in all samples, and peaked at 357.8 ind. m−3 (20.2% of total zooplankton abundance) at the southernmost station N2 (Fig. 5b). The shell diameter of Limacina spp. was the largest at station N3 (mean ± SD; 447.2 ± 123.0 µm), followed by N4 (484.4 ± 181.4 µm), and N2 (357.8 ± 118.6 µm). At the northern stations, Limacina spp. abundance decreased by half at N5 (189.6 ± 89.0 µm) and N6 (178.4 ± 42.4 µm).
Seasonal ice zone NORPAC samples
The relative abundance of L. rangii in six samples in the N1 area was stable and low, and contributed 6.4–23.5% to total zooplankton abundance (Fig. 6). The highest zooplankton and L. rangii abundances, L. rangii shell diameter, and chlorophyll a concentration occurred in 2006, and the lowest in 2004. Although the mean shell diameter exceeded 300 µm in 2006, in other years it was below 270 µm (Table 5).
The contribution of L. rangii to total zooplankton abundance in six samples from the N2 area fluctuated from 2.1% in 2006 to 38.7% in 2001 (Fig. 6). The highest total zooplankton and Limacina spp. abundances were found in 2001, 4,771.6 and 1,847.3 ind. m−3, respectively. The largest L. rangii occurred in 2001 (mean 362.6 µm) and 2000 (mean 357.8 µm). The mean shell diameter was < 270 µm for 4 years (1997, 2002, 2004, and 2006) (Table 5).
In eight samples from the N3 area, L. rangii dominated, accounting for 61.5% of the total zooplankton abundance in 2000, and 44.8% in 2001 (Fig. 6). Conversely, total zooplankton abundance was lowest in 2000 at 229.4 ind. m−3. The highest L. rangii abundance was found in 2001, at 2083.3 ind. m−3. Furthermore, large L. rangii occurred in 2002 (mean 540.2 µm) and 2000 (mean 444.7 µm). The mean shell diameter was < 270 µm for 4 years (1997, 1998, 2001, and 2004) (Table 5).
Discussion
High abundance of Limacina spp. in CPR samples
SO-CPR survey program data (> 42,000 samples) have accumulated over 25 years, with samples mostly collected between September and April. Data collected in the seasonal ice zone in March are based on approximately 9000 samples, in which Limacina spp. occurs in 31.3% of them. In the samples collected during the JARE-41 cruise in March 2000, a high abundance of Limacina spp. in a large area within the seasonal ice zone was observed. The maximum abundance of Limacina spp. in the present study was 353.8 ind. m−3, contributing to 72.2% of total zooplankton abundance, while the maximum abundance of Limacina spp. from CPR samples collected south of Australia in February was 479 ind. m−3 (Hunt et al. 2008). Limacina spp. abundance in CPR samples peaked in February, and small hotspots have been reported as typical patchy zooplankton distributions (Hunt and Hosie 2005, 2006; Hosie et al. 2014). Conversely, in almost all segments of the WE transect and 31 segments of the SN transect in this study (Cluster D in Table 4), Limacina spp. was the most dominant in the zooplankton communities. These regions had 120 segments, i.e., they extended to 600 nautical miles (ca 1110 km). In particular, the eastern side of the WE transect (Cluster B in Table 3), and the south of PF in the WE transect (Cluster D in Table 4) were continuously observed to have high abundances, with a mean of 99.3 ind. m−3 and 77.3 ind. m−3, respectively. Although Limacina spp. abundance in our study is similar to abundances reported in other CPR studies, they have not been previously reported over such a large area.
One factors that influences distribution on the surface of Limacina spp. is diel vertical migration. Because L. rangii and L. retroversa have typical diel vertical migration patterns during summer (Lalli and Gilmer 1989; Hunt et al. 2008; Conroy et al. 2020), they are observed in high abundance in the upper layer at night. The CPR is towed horizontally at a constant depth, which means that the diurnal vertical migration of some zooplankton taxa may affect abundance in the observed data on a 24-h scale (Takahashi and Hosie 2021). Along the WE line, eastern zooplankton abundances were higher, especially for Limacina spp. Percentages of O. similis also increased in the east similar to the total zooplankton abundance. The timing of the increase in the abundance synchronized with nighttime around 141° E, but not around 134°E (Fig. 3a). High abundance levels also continued during daytime around 145° E. Therefore, no clear diel change in zooplankton abundance or species composition was apparent. Zooplankton abundances along the SN transect were high on the southern side on the boundary around the PF. The most dominant species, Limacina spp., occurred only in very low numbers north of the PF. Cluster D, which had a high abundance of Limacina spp., contained both day and nighttime samples (Fig. 4b, Table 4). The areas of 60.5°S to 59.5°S, where high abundance levels were observed, included a large proportion of daytime tows. No clear diel changes in Limacina spp. abundance were apparent along the SN transect.
Member species of the gymnosome pteropod genus Clione are monophagous predators that feed exclusively on Limacina (Lalli and Gilmaer 1989). Fluctuations in L. rangii abundance may be driven by predator–prey dynamics with Clione limacina antarctica (Weldrick et al. 2019). While we have no evidence of predator pressure directly reducing Limacina abundance, stable L. rangii populations may support gymnosome abundances (Thibodeau et al. 2019). Unfortunately, because gymnosome species are only rarely collected with the CPR, feeding relationships cannot be evaluated.
As with other factors, food availability is related to abundance and distribution patterns. Thecosome pteropods are predominantly omnivorous, but diatoms and dinoflagellates dominate the gut contents of L. rangii in the Southern Ocean (Hopkins 1987; Thibodeau et al. 2022). Regional and inter-annual variation in primary production is probably the major determinant of spatial and temporal variability in pteropod densities (Seibel and Dierssen 2003). However, chlorophyll a concentrations in the WE transect were stable at low levels, ranging between 0.47 and 0.70 mg m−3. In the south of the SAF in the SN transect, mean chlorophyll a concentrations were also stable between 0.5 and 0.75 mg m−3. Two clusters with high Limacina spp. abundance, Cluster B of the WE transect and Cluster D of the SN transect, had normal chlorophyll a concentration levels with a mean of 0.57 mg m−3and 0.58 mg m−3, respectively (Tables 3, 4). Thus, no relationship was apparent between Limacina spp. abundance and chlorophyll a concentration; factors other than primary production may have influenced pteropod abundance throughout the sampling area during the sampling periods of this rare event of March 2000.
Limacina abundance and shell size in NORPAC samples
Despite the different sampling layers, NORPAC data obtained simultaneously with CPR data for five stations had similar species compositions, except for foraminiferans and Limacina spp. to the north of the SAF (Fig. 5a). Planktonic foraminifera are a large group of protists, and their distribution is highly seasonal and also known to be patchy. They sometimes dominate CPR towing samples, with very high abundances (Takahashi et al. 2010). Recently, finer plankton nets (100 µm) have provided a more realistic view of plankton′s ecological significance in the seasonal ice zone of the Southern Ocean (Ojima et al. 2013, 2015; Takahashi et al. 2017). Therefore, it is thought that high foraminiferan abundance observed only in NORPAC samples was based on the differences in mesh size and timing of sampling.
The abundance of Limacina spp. in samples obtained via the CPR decreased sharply in waters bordering the PF, and Limacina spp. occurred in low numbers on the northern side. Although NORPAC samples also decreased in waters bordering on the PF, Limacina occurred in northern samples with low abundance (Fig. 5b). The same tendency was seen in shell diameter of Limacina spp., and shell size decreased by half in the north of the SAF. Limacina retroversa is considered to be predominantly a sub-Antarctic species, and to be smaller-sized than L. rangii. This species has been reported to occur at low densities or be completely absent south of the PF (Hunt et al. 2008). The station N4 located between PF and SAF had relatively larger mean shell size, but standard deviation was high (Fig. 5b). This may suggest that Limacina abundance shifted from L. rangii to L. retroversa in this region. Because the mean shell size at stations N5 and N6 was < 270 µm, it is clear that the abundance of Limacina spp. collected only via the NORPAC net was a result of differences in mesh size.
In the seasonal ice zone south of 59°E, in which we found a high Limacina spp. abundance in the JARE − 41 CPR samples, we examined the variability of abundance and shell size of Limacina for the 10 years from 1997 to 2006 (Fig. 6). Based on the shell height/diameter ratio, abundant Limacina larvae south of 59°E in our survey were most likely those of L. rangii. This species is typical of Antarctic waters, and its abundance increased towards lower Southern Ocean latitudes (Hunt et al. 2008; Mackey et al. 2012; Bednaršek et al. 2012b). Limacina rangii spawns primarily in late summer to autumn (Hunt et al. 2008), and its larval stages are sometimes a major contributor to the zooplankton community in the seasonal ice zone during autumn (Brierley and Thomas 2002).
In the present study, a mean shell diameter < 270 µm was observed in 13 of 20 samples. Limacina individuals < 300 µm in size are in the veliger stage (Lalli and Gilmer 1989). Thus, it was clear that Limacina veliger abundance was underestimated in CPR samples with mesh size of 270 µm. A comparative study of 76-µm, 202-µm, and 330-µm mesh nets demonstrated Limacina spp. abundance in the 76-µm mesh of 21.96 ind. m−3, while they were completely absent in the 202-µm and 330-µm mesh nets (Hopkins 1971). To evaluate veliger abundance more accurately, it is necessary to use a suitable mesh size for larger microzooplankton (100 − 200 µm).
In another data set for JARE zooplankton monitoring using NORPAC in March, Nishizawa et al. (2016) measured samples collected at 110° E and 64° S from 1987 to 2008. The mean shell diameter of Limacina spp. was 209.2 ± 33.8 µm, and there was no significant fluctuation in the shell size over the 20-year study period. In March 2000, when high abundance was widely observed via CPR, L. rangii abundances were low compared with other years (Fig. 6). However, relatively large-sized individuals were found in N2 (mean 357.8 µm) and N3 (mean 444.7 µm) (Table 5). It is thought that the CPR was able to catch the large-sized individuals that were dominant during CPR towing in March 2000. At each sampling site, there was no association between Limacina abundance, shell size, and environmental factors, such as chlorophyll a concentration and sea surface temperature, at the time of sampling. The timing of reproduction and the growth of the new generation by a year may have influenced Limacina abundance and shell size throughout the sampling area. Long-term observations in the western Antarctic Peninsula have revealed sea ice to be the dominant driver of pteropod abundance (Thibodeau et al. 2020). The model proposed by Thibodeau et al. (2020) indicates that earlier sea-ice retreat in the austral spring leads to rapid pteropod growth, with more open water combining with warmer waters of sea surface temperature. It is also possible that the rare event that we report for March 2000 was because of early sea-ice retreat producing an environment conducive to pteropod growth.
Thecosome pteropods are major components of polar food webs, and they can dominate zooplankton communities in the Southern Ocean seasonal ice zone. Because these pteropods are a major aragonite-producing group, they are vulnerable to the effects of ocean acidification (Orr et al. 2005; Fabry et al. 2008). Accordingly, two Southern Ocean Limacina species have been proposed as indicators of acidification (Manno et al. 2012; Mekkes et al. 2021; Johnston et al. 2022). Early life stages are more susceptible to the effects of climate change than adults (Dupont and Thorndyke 2009; Gardner et al. 2018). The Limacina collected in our study were either veligers or juveniles. Because larval shells form relatively quickly (Bednaršek et al. 2012b; Thibodeau et al. 2020; Weldrick et al. 2021), larval stages may be more sensitive to environmental changes than those of adults. An understanding of population dynamics, and the accumulation of fundamental biological data for these larval stages, is therefore necessary to more fully evaluate recruitment and long-term population viability in response to environmental change such as ocean acidification. Long-term monitoring of Limacina abundance and growth characteristics at a fixed location, and high-resolution annual time series data on population structure at multiple locations, would enable accurate determination of species′ lifespans and responses to environmental change.
Data availability
All raw data on which this study is based are available at the Australian Antarctic Data Centre via the home page of the Southern Ocean Continuous Plankton Recorder (SO-CPR) Survey (http://data.aad.gov.au.aadc/cpr/).
References
Arbuckle JL (2014) Amos 23.0 user′s guide. Chicago, IBM SPSS 1–702
Bednaršek N, Tarling GA, Bakker DCE, Fielding S, Jones EM, Venables HJ, Ward P, Kuzirian A, Lézé B, Feely RA, Murphy EJ (2012a) Extensive dissolution of live pteropods in the Southern Ocean. Nat Geogr 5:881–885. https://doi.org/10.1038/ngeo1635
Bednaršek N, Tarling GA, Fielding S, Bakker DCE (2012b) Population dynamics and biogeochemical significance of Limacina helicina antarctica in the Scotia Sea (Southern Ocean). Deep-Sea Res II 59–60:105–116. https://doi.org/10.1016/j.dsr2.2011.08.003
Bednaršek N, Harvey CJ, Kaplan IC, Feely RA, Možina J (2016) Pteropods on the edge: cumulative effects of ocean acidification, warming, and deoxygenation. Prog Oceanogr 145:1–24. https://doi.org/10.1016/j.pocean.2016.04.002
Boissonnot L, Kohnert P, Ehrenfels B, Søreide JE, Graeve M, Stübner E, Schrödl M, Niehoff B (2021) Year-round population dynamics of Limacina spp. early stages in a high-Arctic fjord (Adventfjorden, Svalbard). Polar Biol 44:1605–1618. https://doi.org/10.1007/s00300-021-02904-6
Brierley AS, Thomas DN (2002) Ecology of Southern Ocean pack ice. In: Southward AJ et al (eds) Advances in marine biology, vol 43. Academic Press, London, pp 171–276
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Clark RA, Frid CLJ, Batten S (2001) A critical comparison of the two long-term zooplankton time series from the central west North Sea. J Plankton Res 23:27–39. https://doi.org/10.1093/plankt/23.1.27
Conroy JA, Steinberg DK, Thibodeau PS, Schofield O (2020) Zooplankton diel vertical migration during Antarctic summer. Deep-Sea Res I 162:103324. https://doi.org/10.1016/j.dsr.2020.103324
Dupont S, Thorndyke MC (2009) Impact of CO2-driven ocean acidification on invertebrate′s early life-history-what we know, what we need to know and what we can do. Biogeosci Discuss 6:3109–3131. https://doi.org/10.5194/bgd-6-3109-2009
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Mar Sci 65:414–432. https://doi.org/10.1093/icesjms/fsn048
Field JG, Clarke KR, Warwick RM (1982) A practical strategy for analysing multispecies distribution patterns. Mar Ecol Prog Ser 8:7–52. https://doi.org/10.3354/meps008037
Fukuchi M, Hattori H (1987) Surface water monitoring system installed on board the icebreaker Shirase. Proc NIPR Symp Polar Biol 1:7–55
Gannefors C, Böer M, Kattner G, Graeve M, Eiane K, Gulliksen B, Hop H, Falk-Petersen S (2005) The Arctic sea butterfly Limacina helicina lipids and life strategy. Mar Biol 147:169–177. https://doi.org/10.1007/s00227-004-1544-y
Gardner J, Manno C, Bakker DCE, Peck VL, Tarling GA (2018) Southern Ocean pteropods at risk from ocean warming and acidification. Mar Biol 165:8. https://doi.org/10.1007/s00227-017-3261-3
Hopkins TL (1971) Zooplankton standing crop in the Pacific sector of the Antarctic. In: Llano GA, Wallen IE (eds) Biology of the Antarctic Seas. American Geophysical Union, Washington, D. C, pp 347–367
Hopkins TL (1987) Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar Biol 96:93–106. https://doi.org/10.1007/BF00394842
Hosie GW, Fukuchi M, Kawaguchi S (2003) Development of the Southern Ocean Continuous Plankton Recorder survey. Prog Oceanogra 58:263–284. https://doi.org/10.1016/j.pocean.2003.08.007
Hosie GW, Mormède S, Kitchener J, Takahashi K, Raymond B (2014) Chapter 10.3. Near-surface zooplankton communities. In: De Broyer C, Koubbi P et al (eds) Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 422–430
Hunt BPV, Hosie GW (2003) The Continuous Plankton Recorder in the Southern Ocean: a comparative analysis of zooplankton communities sampled by the CPR and vertical net hauls along 140°E. J Plankton Res 24:1561–1579. https://doi.org/10.1093/plankt/fbg108
Hunt BPV, Hosie GW (2005) Zonal structure of zooplankton communities in the Southern Ocean south of Australia: results from a 2150 km continuous plankton recorder transect. Deep-Sea Res I 52:1241–1271. https://doi.org/10.1016/j.dsr.2004.11.019
Hunt BPV, Hosie GW (2006) The seasonal succession of zooplankton in the Southern Ocean south of Australia, part I: the seasonal ice zone. Deep-Sea Res I 53:1182–1202. https://doi.org/10.1016/j.dsr.2006.05.001
Hunt BPV, Pakhomov EA, Hosie GW, Siegel V, Ward P, Bernard K (2008) Pteropods in Southern Ocean ecosystems. Prog Oceanogr 78:193–221. https://doi.org/10.1016/j.pocean.2008.06.001
Hunt BPV, Strugnell J, Bednarsek N, Linse K, Nelson RJ, Pakhomov E, Seibel B, Steinke D, Wurzberg L (2010) Poles apart: the ′′Bipolar′′ Pteropod species Limacina helicina is genetically distinct between the Arctic and Antarctic Oceans. PLoS ONE 5:e9835. https://doi.org/10.1371/journal.pone.0009835
Janssen AW, Bush SL, Bednaršek N (2019) The shelled pteropods of the northeast Pacific Ocean (Mollusca: Heterobranchia, Pteropoda). Zoosymposia 13:305–346. https://doi.org/10.11646/zoosymposia.13.1.22
John EH, Batten SD, Harris RP, Hays GC (2001) Comparison between zooplankton data collected by the Continuous Plankton Recorder survey in the English Channel and by WP-2 nets at station L4, Plymouth (UK). J Sea Res 46:223–232. https://doi.org/10.1016/S1385-1101(01)00085-5
Johnston NM, Murphy EJ, Atkinson A, Constable AJ, Cotté C, Cox M, Daly KL, Driscoll R, Flores H, Halfter S, Henschke N, Hill SL, Höfer J, Hunt BPV, Kawaguchi S, Lindsay D, Liszka C, Loeb V, Manno C, Meyer B, Pakhomov EA, Pinkerton MH, Reiss CS, Richerson K, Smith WO Jr, Steinberg DK, Swadling KM, Tarling GA, Thorpe SE, Veytia D, Ward P, Weldrick CK, Yang G (2022) Status, change, and futures of zooplankton in the Southern Ocean. Front Ecol Evol 9:624692. https://doi.org/10.3389/fevo.2021.624692
Lalli CM, Wells FE (1978) Reproduction in genus Limacina (Opisthobranchia Thecosomata). J Zool 186:95–108. https://doi.org/10.1111/j.1469-7998.1978.tb03359.x
Lalli CM, Gilmer RW (1989) Pelagic snails: the biology of holopanktonic gastropod mollusks. Stanford University Press, Palo Alto
Mackey A, Atkinson A, Hill S, Ward P, Cunningham N, Johnston NM, Murphy EJ (2012) Antarctica microzooplankton of the southwest Atlantic sector and Bellingshausen Sea: historical distributions: relationships with food; and implications for ocean warming. Deep-Sea Res II 59–60:130–146. https://doi.org/10.1016/j.dsr2.2011.08.011
Makabe R, Tanimura A, Fukuchi M (2012) Comparison of mesh size effects on mesozooplankton collection efficiency in the Southern Ocean. J Plankton Res 34:432–436. https://doi.org/10.1093/plankt/fbs014
Manno C, Sandrini S, Tositti L, Accornero A (2007) First stages of degradation of Limacina helicina shells observed above the aragonite chemical lysocline in Terra Nova Bay (Antarctica). J Mar Syst 68:91–102. https://doi.org/10.1016/j.jmarsys.2006.11.002
Manno C, Accornero A, Umani SF (2010) Importance of the contribution of Limacina helicina faecal pellets to the carbon pump in Terra Nova Bay (Antarctica). J Plankton Res 32:145–152. https://doi.org/10.1093/plankt/fbp108
Manno C, Morata N, Primicerio R (2012) Limacina retroversa′s response to combined effects of ocean acidification and sea water freshening. Estuar Coast Shelf Sci 113:163–171. https://doi.org/10.1016/j.ecss.2012.07.019
Manno C, Peck VL, Tarling GA (2016) Pteropod eggs released at high pCO2 lack resilience to ocean acidification. Sci Rep 6:25752. https://doi.org/10.1038/srep25752
Manno C, Giglio F, Stowasser G, Fielding S, Enderlein P, Tarling GA (2018) Threatened species drive the strength of the carbonate pump in the northern Scotia Sea. Nat Commun 9:4592. https://doi.org/10.1038/s41467-018-07088-y
Mekkes L, Sepúlveda-Rodríguez G, Bielkinaitë G, Wall-Palmer D, Brumme G-JA, Dämmer LK, Huisman J, van Loon E, Renema W, Peijnenburg KTCA (2021) Effects of ocean acidification on calcification of the Sub-Antarctic Pteropod Limacina retroversa. Front Mar Sci 8:581432. https://doi.org/10.3389/fmars.2021.581432
Nishizawa Y, Sasaki H, Takahashi KT (2016) Interannual variability in shelled pteropods (Limacina spp.) in the Indian sector of the Southern Ocean during austral summer. Antarct Rec 60:35–48. https://doi.org/10.15094/00012672
Ojima M, Takahashi KT, Iida T, Odate T, Fukuchi M (2013) Distribution patterns of micro- and meso-zooplankton communities in sea ice regions of Lützow-Hom Bay, East Antarctica. Polar Biol 36:1293–1304. https://doi.org/10.1007/s00300-013-1348-y
Ojima M, Takahashi KT, Tanimura A, Odate T, Fukuchi M (2015) Spatial distribution of micro- and meso-zooplankton in the seasonal ice zone of east Antarctica during 1983–1995. Polar Sci 9:319–326. https://doi.org/10.1016/j.polar.2015.05.002
Orsi AH, Whitworth T III, Nowlin WD Jr (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res 42:641–673. https://doi.org/10.1016/0967-0637(95)00021-W
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner G-K, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig M-F, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686. https://doi.org/10.1038/nature04095
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press Inc., New York
Pinkerton MH, Décima M, Kitchener JA, Takahashi KT, Robinson KV, Stewart R, Hosie GW (2020) Zooplankton in the Southern Ocean from the continuous plankton recorder: Distributions and long-term change. Deep-Sea Res I 162:103303. https://doi.org/10.1016/j.dsr.2020.103303
Seibel BA, Dierssen HM (2003) Cascading trophic impacts of reduced biomass in the Ross Sea Antarctica: just the tip of the iceberg? Biol Bull 205:93–97. https://doi.org/10.2307/1543229
Sokolov S, Rintoul SR (2002) Structure of Southern Ocean fronts at 140°E. J Mar Syst 37:151–184. https://doi.org/10.1016/S0924-7963(02)00200-2
Steinberg DK, Ruck KE, Gleiber MR, Garzio LM, Cope JS, Bernard KS (2015) Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res I 101:54–70. https://doi.org/10.1016/j.dsr.2015.02.009
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J Oceanogra 46:190–194. https://doi.org/10.1007/BF02125580
Takahashi KT, Hosie GW (2021)The status and trends of Southern Ocean zooplankton based on the SCAR Southern Ocean Continuous Plankton Recorder (SO-CPR) survey. SCAR Bulletin, No. 206, p 97
Takahashi KT, Hosie GW, Kitchener JA, McLeod DJ, Odate T, Fukuchi M (2010) Comparison of zooplankton distribution patterns between four seasons in the Indian Ocean sector of the Southern Ocean. Polar Sci 4:317–331. https://doi.org/10.1016/j.polar.2010.05.002
Takahashi KT, Hosie GW, Kitchener JA, McLeod DJ, Stevens C, Robinson K, Jonas T, Fukuchi M (2011) Report on “Southern Ocean CPR Standards Workshop -SCAR Expert Group on CPR Research-.” Nankyoku Shiryô (antarctic Record) 55:277–284
Takahashi KT, Ojima M, Tanimura A, Odate T, Fukuchi M (2017) The vertical distribution and abundance of copepod nauplii and other micro- and mesozooplankton in the seasonal ice zone of Lützow−Holom Bay during austral summer 2009. Polar Biol 40:79–93. https://doi.org/10.1007/s00300-016-1925-y
Thibodeau PS, Steinberg DK, Stammerjohn SE, Hauri C (2019) Environmental controls on pteropod biogeography along the Western Antarctic Peninsula. Limnol Oceanogr 64:S240–S256. https://doi.org/10.1002/lno.11041
Thibodeau PS, Steinberg DK, McBride CE, Conroy JA, Keul N, Ducklow HW (2020) Long-term observations of pteropod phynology along the Western Antarctic Peninsula. Deep-Sea Res I 166:103363. https://doi.org/10.1016/j.dsr.2020.103363
Thibodeau PS, Song B, Moreno CM, Steinberg DK (2022) Feeding ecology and microbiome of the pteropod Limacina helicina antarctica. Aquat Microb Ecol 88:19–24. https://doi.org/10.3354/ame01981
Wang K, Hunt BPV, Liang C, Pauly D, Pakhomov EA (2017) Reassessment of the life cycle of the pteropod Limacina helicina from a high resolution interannual time series in the temperate North Pacific. ICES J Mar Sci 74:1906–1920. https://doi.org/10.1093/icesjms/fsx014
Weldrick CK, Trebilco R, Davies DM, Swadling KM (2019) Trophodynamics of Southern Ocean pteropods on the southern Kerguelen Plateau. Ecol Evol 9:8119–8132. https://doi.org/10.1002/ece3.5380
Weldrick CK, Makabe R, Mizobata K, Moteki M, Odate T, Takao S, Trebilco R, Swadling KM (2021) The use of swimmers from sediment traps to measure summer community structure of Southern Ocean pteropods. Polar Biol 44:457–472. https://doi.org/10.1007/s00300-021-02809-4
Acknowledgements
We express our heartfelt appreciation to all members of the 38–47th Japanese Antarctic Research Expeditions, who collected the samples and oceanographic data used in this study. We would like to thank Patricia Thibodeau and one anonymous reviewer for their constructive comments on an earlier version of this manuscript. We are also grateful to the officers and crew of the icebreaker Shirase for their assistance during the cruise. We thank Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Funding
This research was supported in part by the National Institute of Polar Research (NIPR) through Project Research no. KP-308, and a Grant-in-Aid for Science (No. 16H02970) to K.T. Takahashi from the Japan Society for the Promotion of Science. The study is also part of the Science Program of JARE AMB-0902.
Author information
Authors and Affiliations
Contributions
KTT directed the JARE monitoring program and wrote the manuscript. HU carried out field sampling aboard the icebreaker Shirase and processed the samples.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, K.T., Umeda, H. The variability in abundance and shell size of the thecosome pteropods Limacina spp. in the seasonal ice zone of the Southern Ocean in March. Polar Biol 46, 523–537 (2023). https://doi.org/10.1007/s00300-023-03141-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03141-9