Abstract
To investigate whether impacts of reported climate change in the Antarctic marine environment have affected mesozooplankton populations, we compared the summertime abundances of four species of large calanoid copepods from samples taken during the Discovery Investigations (1926–1938) and contemporary times (1996–2013). Discovery samples were obtained using an N70V closing net fished vertically through three depth horizons encompassing the top 250 m of the water column, whereas contemporary samples were obtained using a Bongo net fished vertically through 200–0 m. Data from a previous study comparing catch efficiencies of the two nets were used to generate calibration factors which were applied to the N70V abundances. Following further corrections for net depth differences and seasonal biases in sampling frequency, three of the four species, Calanoides acutus, Rhincalanus gigas and Calanus simillimus, were found to be between ~ 20–55% more abundant in contemporary times than they were 70 years ago. Calanus propinquus was marginally more abundant in the Discovery era. These results were robust to sensitivity analyses for the net calibration factor, seasonal bias and net depth corrections. Although near-surface ocean temperatures within the Scotia Sea have increased by up to 1.5 °C during the last 70 years, we conclude that the most likely causes of increased copepod abundances are linked to changes in the food-web. In particular, we discuss the reported decrease in krill abundance in the South Atlantic that has potentially increased the amount of food available to copepods while at the same time decreasing predator pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impacts of climate change are being felt worldwide in the marine environment. Species and communities are responding to complex interactions of environmental forcing factors such as increasing temperature, ocean acidification and ocean-atmospheric coupling, which exert their effects over a range of spatial and temporal scales (Richardson 2008; Hátún et al. 2009; Burrows et al. 2011; Richardson et al. 2012; Poloczanska et al. 2013).
In the Southern Ocean, warming has been taking place for at least the last 50–70 years (Gille 2002; Meredith and King 2005; Whitehouse et al. 2008) and has been attributed to near-surface ocean–atmosphere-ice interactions (Turner et al. 2013). Consequences of warming have included regional changes in sea-ice extent and duration (Stammerjohn et al. 2008) which has subsequently been suggested as a major factor in the recent decline of Antarctic krill and increases in salp abundance (Loeb et al. 1997; Atkinson et al. 2004; Flores et al. 2012).
Impacts of environmental change on other planktonic groups are however less well understood (Constable et al. 2014). Copepoda are the dominant mesozooplankton group in the Southern Ocean but the factors affecting their distribution and abundance have been harder to establish, in part because of a lack of extensive time-series measurements. Changing patterns of atmospheric variability such as the Southern Annular Mode (SAM) which has an important influence on zonal winds (Sen Gupta and McNeil 2012) and the Southern Oscillation Index (SOI) have been linked to changes in plankton abundance. For example, near Elephant Island, Loeb et al. (2009, 2010) found significant correlations between the abundance and concentration of phytoplankton, zooplankton and krill with the SOI which exhibited 3–5 year frequencies characteristic of El Niño-Southern Oscillation (ENSO) variability. They found that abundances of Calanoides acutus, and Rhincalanus gigas, characteristic of the Antarctic Circumpolar Current (ACC), were positively correlated with chlorophyll a (Chl a) and the SOI. These changes appeared related to the influence of the SOI on water mass movements, with high copepod abundances associated with a southwards movement of ACC waters into the coastal regions off the northern-Antarctic Peninsula. Conversely, during periods when the sign of the SOI was negative, salps tended to become dominant. However, at South Georgia, abundances of krill and copepods were found to be negatively related across a range of scales suggesting direct interactions either as competitor or predator (Atkinson et al. 2001), rather than being solely mediated by ocean–atmosphere coupling. Thus, the balance of zooplankton composition represents a complex of oceanic-atmospheric—sea-ice and competitive interactions which are only just beginning to be teased apart.
Over a longer timescale, Tarling et al. (2018) compared copepod distributions in the Scotia Sea from Discovery Investigations (1920s–1930s) and contemporary times (1996–2013) and showed that, over intervening years, populations have essentially remained in the same geographical location despite ocean warming. Had they occupied the same thermal envelope which they inhabited in the 1930s, current distributions would be up to 500 km further south (see also Mackey et al. 2012). Reasons for maintenance of their historical distributions were attributed to food availability and the properties of the underlying water masses where a number of the species over-winter. It was also found that there had been a negligible difference in the rank order of abundance of dominant copepod species sampled over 70 years apart. However, ranked abundance can mask numerical changes, particularly if some species/taxa are extremely abundant and others less so. In this paper, we explore this further and have focussed on the commonly occurring biomass-dominant large calanoid copepods (C. acutus, R. gigas, Calanus simillimus and Calanus propinquus). We wished to establish whether abundances were the same between eras and, if not, to seek to understand what factors may lie behind any changes.
Methods
Copepod net sampling and abundance
Net sample stations
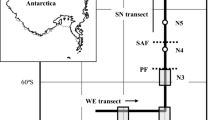
We analysed net samples from stations south of the Polar Front in the southwest Atlantic sector of the Southern Ocean, collected as part of the Discovery Investigations (1926–1938) and during contemporary cruises (1996–2013). Our analysis was confined to samples taken in the austral summer months of December-February, between the latitudes of 52–66°S.
The species under consideration have broad and overlapping distributions within the ACC although repeated sampling has shown that C. simillimus and R. gigas have more northerly distributions compared to C. acutus and C. propinquus which tend to inhabit colder waters to the south (Atkinson 1998; Schnack-Schiel 2001). The timing of their lifecycles and the presence of populations in near-surface waters vary according to latitudinal progression of the seasons (earlier in the north) with recruitment occurring up to 3 months earlier in some years in the northern parts of the ACC compared to the south (Ward et al. 2006, 2012a). In comparing between the two eras, we have assumed that any changes in the timing of the annual pattern of occurrence of species stages has been captured within the 3 summer months (December, January and February) on which the analysis focussed.
Stations were determined to be south of the Polar Front from the vertical temperature profile recorded at each station (Gordon et al. 1977; Tarling et al. 2018). The Discovery sample set accordingly comprised 53 N70V vertical closing net stations supplemented with an additional 10 N70V stations sampled during December 1926 and January 1927, for which catch data were extracted from Discovery Report 11 (appendix of Hardy and Gunther 1935). The contemporary dataset comprised catches made with a paired Bongo net at 147 stations (Fig. 1).
Net sample analysis
During sample analysis, the copepodite stages and adults of large calanoid copepod species were either enumerated from complete samples, or the whole sample placed in a Folsom plankton splitter and fractionated into replicate aliquots until countable numbers (~ 200 individuals) were estimated to be present. Abundances of taxa were standardised to numbers per net sample and the amount of water each net filtered was estimated based on mouth area and distance towed, to derive individual species concentrations (ind. m−3). Of the large calanoid species, four were consistently present across the majority of samples and became the focus of subsequent numerical analyses, those species being R. gigas, C. acutus, C. simillimus and C. propinquus.
Data preparation
Accounting for different integrated depths
At each Discovery station samples were collected from three depth horizons (50–0, 100–50 and 250–100 m) and abundances integrated from 250 to 0 m. Contemporary samples were collected between 200 and 0 m. Copepod abundances were determined in terms of concentrations as individuals per cubic metre (ind m−3) for both sets of samples. However, the majority of copepods reside in the top 200 m at this time of year (Atkinson 1991; Atkinson and Sinclair 2000) which potentially reduces concentrations in the Discovery samples relative to the contemporary samples because of the extra 50 m depth contributing to overall sample volume. Therefore, we multiplied Discovery abundances by 1.25 to account for this potential bias. Both sets of samples were subsequently multiplied by 200 m to derive a depth integrated abundance value for the 0–200 m surface layer (ind. m−2).
Accounting for net type bias
Different net types were used in the two eras of sampling. The Discovery Investigations collected N70V samples from nets deployed vertically between the 3 horizons (see above) within the surface 250 m (Kemp et al. 1929). Mesh sizes in this net decrease in stages from 5 mm in the upper part, to 440 µm and then 195 µm in the mid and lower parts respectively (measurements are metric equivalents of the original imperial units; see Kemp et al. 1929). In contrast, the contemporary samples were collected from Bongo net deployments (net dia. 0.61 m, 200 µm mesh net) fished vertically from 200 to 0 m.
To enable a comparison between the two net types, an N70V net was reconstructed using nylon mesh of the nearest metric equivalent to the imperial measurements of the bolting silks originally specified in Kemp et al. (1929). The nets were fished alongside each other at a series of stations in Marguerite Bay on the Antarctic Peninsula and across the Scotia Sea to determine a broad spectrum calibration factor (Ward et al. 2012b). For the present study, we re-analysed these data to establish species-specific inter-calibration factors for the four principal calanoid species under consideration.
One particular issue was to take into account the different developmental stages (CI to adult) of the sampled copepods, since the changes of size may alter respective catchabilities and retentions by the two different nets. We therefore compared the abundances of each individual developmental stage of each of the 4 species in every calibration station of Ward et al. (2012b) to determine the average residual difference (Res), as follows:
where N is abundance (ind. m−2) of either the calibration Bongo net, B, or the calibration N70V net, N70, s is species and d is developmental stage from CI to adult (male and female).
It was also necessary to take into account the relative contribution of each of these developmental stages to total species abundance. In certain Discovery samples, some individuals had previously been removed and although numbers taken were specified on sample labels, stage distributions were not. Data taken from Hardy and Gunther (1935) were also simply reported as species numbers rather than by stage. Therefore, it was necessary to infer typical proportional stage distributions from the contemporary samples. Hence, Ress,d was multiplied by the relative proportion of stages within species in the contemporary samples (Prs‚d), so that a standardised residual difference, SRess,d, could be determined as follows:
SRess,d was divided by the average abundance of the N70V calibration hauls to produce a normalised developmental stage specific calibration factor (Cals,d) which was then summed across all stages to produce a species-specific calibration factor (Cals):
where d1 is development stage 1 (CI), dt is final adult stage (male and female).
Discovery sample abundances (NDI,s) were then multiplied by 1 + Cals to determine a calibrated abundance (NDIcal,s) with which to compare against contemporary abundances (NC,s):
Accounting for seasonal bias
Further data analyses revealed a seasonal trend in datasets whereby there was a substantial increase in abundances in January compared to December and February in both the Discovery Investigations and contemporary sample sets. However, sampling effort varied between the two eras, with there being a proportionally greater sampling effort in January in the contemporary dataset compared to the Discovery dataset. To account for this potential bias, we resampled the two datasets so that there was even selection of datapoints across the 3 months. For both the Discovery and contemporary datasets (NDIcal,s and NC,s), 10 datapoints were selected at random (with replacement) from each of the 3 months and combined to make a new resampled dataset of 30 datapoints, for which an average was determined. The process was repeated 100 times for each species, to which statistical tests were then applied (see below).
Sensitivity analyses
To determine the sensitivity of the inter-era comparison of species abundance to the various stages in data preparation, a series of sensitivity analyses were run. The two main treatments to the original datasets were the inter-calibration of abundances caught by the two different nets and the resampling to account for seasonal sampling bias, so the objective of the sensitivity analysis was to determine whether species-specific abundances remained significantly different between eras when treatments were altered. For the calibration factor sensitivity tests, the factors were increased by 25, 50 and 100% or removed completely. Multiplying the calibration factor by percentages below 0 was not considered given that this would always act to increase the level of difference between the two eras. For the seasonality sensitivity tests, runs were carried out to determine the effect of removing the resampling process. The 1.25 multiplication factor to the Discovery nets was also removed in a further test to determine its implications.
Statistical tests
Comparisons of abundances between eras were tested either using an unpaired t test or a Mann–Whitney Rank Sum test (U test), the latter being used in instances where the datasets failed a priori tests for normality (Shapiro–Wilk test) or equal variance. A Kruskall-Wallis 1-way ANOVA on ranks test was applied to differences between months. Tests producing significant differences were further tested using a Dunn’s Method all Pair-wise Multiple Comparison Procedure.
Results
Calibration
To determine the calibration factor, it was necessary to consider the relative proportion of stages within species in the contemporary samples (Fig. 2). Although there was inter-specific variation in the relative abundance of developmental stages, the CIII and CIV stages were generally among the most frequent (Fig. 2). For instance, CIII was the most frequent stage in R. gigas, with stages CII and CI also being relatively abundant. A similar pattern was apparent in C. propinquus although stage CI was comparatively low in abundance while CIV had a similarly high frequency to stage CII. In C. acutus and C. simillimus, the later developmental stages (CIV and CV) had higher frequencies than the earlier developmental stages. Adult females were more abundant than males in all species, although both were relatively infrequent compared to the earlier developmental stages.
The calibration factor also required the residual difference in species and stage specific abundances to be determined in matched Bongo and N70 hauls. In these hauls, it was found that more individual copepods were captured by Bongo nets than N70V nets, with the majority of residual differences (i.e. Bongo minus N70V, Ress,d) being positive (Fig. 3). The residual differences were much greater in C. acutus and C. simillimus than they were in R. gigas and C. propinquus. In C. acutus, some of the greatest differences were observed in the early developmental stages, although CIV also exhibited a high value for Ress,d. Only CI and CII showed notably high values for Ress,d in C. simillimus with a further minor peak in the females. R. gigas exhibited a similar peak in Ress,d, but there was little pattern in the low values of Ress,d in C. propinquus.
The calibration factor (1 + Cals) is a function of both the residual difference between calibration hauls and relative proportion of stages within species (Table 1). The highest calibration factors were observed in C. acutus and C. simillimus, for which the highest values for Ress,d were observed. However, the corresponding stage distribution downweighs the calibration factor in C. acutus in relation to C. simillimus. The calibration factors for R. gigas and C. propinquus were low since both have comparatively low species abundances and low residual differences.
Comparison of abundances between eras
There were substantial differences in species abundances between the three summer months included in the analysis, with abundance levels in January being almost double those of December and February in both the Discovery Investigations and contemporary samples (Fig. 4). The difference between months was significant in both eras (Discovery Investigations, Kruskall-Wallis test, H = 7.328, 2 df, p = 0.026; Contemporary, H = 7.475, 2 df, p = 0.024).
There was a difference in sampling effort between the respective months, with January containing the highest sampling effort in the contemporary dataset and the lowest in the Discovery Investigations dataset (Fig. 4). This necessitated data resampling in order to dampen any temporal bias in the comparison of abundances between the two datasets (see "Methods").
The calibrated abundances of R.gigas, C. acutus and C. simillimus in the Discovery samples were considerably and significantly lower than those in the contemporary samples (Fig. 5) (Discovery vs Contemporary; R gigas: Mann–Whitney U Test, T = 6590.000 n(small) = 100 n(big) = 100, p < 0.001, C. acutus: T = 7590, n(small) = 100 n(big) = 100, p < 0.001; C. simillimus: T = 7053.000 n(small) = 100 n(big) = 100, p < 0.001). In the case of C. acutus, calibrated abundances were around 80% of the values observed in contemporary times (mean ± SE of 3553 ± 101 and 4374 ± 116 ind m−2 respectively) while, in R. gigas and C. simillimus, Discovery samples were between 65 and 70% of contemporary values (respective mean ± SE of 1020 ± 22 and 1525 ± 47 ind m−2 for R. gigas and 2377 ± 70 and 3711 ± 139 ind m−2 for C. simillimus). However, in C. propinquus, the opposite trend was observed, with values being significantly higher in the Discovery era (mean ± SE of 903 ± 21 ind m−2 vs 812 ± 41 ind m−2 for contemporary era), although the absolute or proportional differences (91 ind m−2 and 90% respectively) were not as substantial as for the other species.
Discovery Investigations versus Contemporary abundance Box plot of estimated abundance (ind m−2) of Rhincalanus gigas, Calanoides acutus, Calanoides simillimus and Calanoides propinquus during the Discovery Investigations and contemporary times. Horizontal line represents the median, limits of boxes, 25th and 75th percentiles, limits of whiskers, 10th and 90th percentiles, dots, 5th and 95th percentiles. ˄ indicates abundances during contemporary era were significantly larger than those during the Discovery Investigation era, ˅, that Discovery Investigation era abundances were significantly larger than those in the contemporary era (p < 0.001)
Levels of significance in these results were relatively insensitive to the calibration factor (Table 2). When removing the calibration factor altogether or increasing its value by 25 or 50%, values in contemporary times were still significantly larger in R. gigas, C. acutus and C. simillimus. Only when the calibration factor was increased by 100% was there any change to this result, with C. acutus no longer significantly more abundant in the contemporary era. Greater sensitivity was exhibited in relation to seasonality in abundance levels, with the removal of the resampling procedure to dampen the effect of different levels of sampling effort between months increasing the level of difference between eras, with even C. propinquus now exhibiting significantly greater abundances in contemporary times. Removal of the 1.25 multiplication factor to accommodate the different integrated depth intervals between the Discovery and contemporary nets had a similar effect, with abundances being significantly greater in contemporary times in all species.
Discussion
In this study we have demonstrated that three of the four species of large calanoids studied have increased in abundance within the Scotia Sea over the past 70 years. Over the same period the Southern Ocean has changed profoundly. There have been significant increases in temperature and, in some regions, reductions in sea-ice, alongside a decline in krill biomass (Atkinson et al. 2004). The commercial extinction of the great whales during the 20th century is also conjectured to have had significant impacts on the functioning of food-webs (Laws 1977, 1985; Willis 2007, 2014; Smetacek 2008; Nicol et al. 2010).
We can rule out methodological differences as the cause of the changes in abundance even though estimates of abundance from the different periods were derived from different nets. Our inter-net calibration determined size-related differences in catch efficiency and appropriate correction factors were applied to N70V catches.
A study carried out in the Weddell Sea comparing historical Discovery N70V (1929–1939) and contemporary WP-2 (1989–1993) net samples concluded that there had been marginally significant long-term changes among large calanoids but overall, no consistent trend was apparent (Vuorinen et al. 1997). However, and importantly, no inter-calibration of net performance was carried out.
Calibration factor
The survey data from which we generated the calibration factors were originally reported in Ward et al. (2012b). When considered across the entire catch, that study estimated that the Bongo net caught ~ 3 times as many individuals as the N70 net. This increased to ~ 4 times greater when limited only to copepod developmental stages or individuals that were < 0.5 mm body length. However, between body lengths of 1 and 7 mm, the Bongo net caught between 1.5 and 2 times as many individuals as the N70 net. Given the large dependence of Bongo:N70 abundance ratio on body size, we considered it necessary to develop a specific calibration factor for each of our four chosen calanoid copepod species that took developmental stages into account. We could only examine developmental stage composition in the contemporary samples, since specimens had been previously extracted from Discovery Investigation samples without any record of their respective developmental stages. The contemporary samples showed that mid developmental stages (CII–CIV) dominated the summertime populations of three of the four calanoid species, the exception being C. acutus, where the dominant stage was late development stage, CV. Calanoides acutus is the only one of the four calanoid species known to enter true diapause for a large part of the year (Drits et al. 1994). Tarling et al. (2004) showed that the population in the Scotia Sea consists of a mixture of 1- or 2-year life-cycle types, with CV being the dominant over-wintering stage. CV therefore dominate the summertime population of C. acutus since their abundance comprises both 1 and 2 year old individuals. The other calanoid species appear to have summertime populations that are dominated by newly recruiting individuals from that same season. Although we cannot be certain that summertime populations had the same structure during the Discovery Investigations era as during contemporary times, we deliberately designed our analysis to encompass all of the summer months so as to average over any minor variations in life-cycle phenology between the two eras.
Through combining developmental stage composition with the residual differences in abundance between Bongo and N70 samples for each developmental stage, we derived calibration factors between 1.2 and 1.7. This reflects the fact that even though relatively large residual differences were observed in the early developmental stages, these stages were not that common in the population during the summer. These calibration factors are somewhat lower than those originally proposed by Ward et al. (2012b). That study considered the entire copepod community, which was numerically dominated by smaller species such as Oithona similis and Ctenocalanus citer. The calanoid species we analyse here are comparatively larger in body size even during the earlier developmental stages and the residual differences between the Bongo and N70 nets were correspondingly smaller. Nevertheless, the sensitivity analyses showed that even increasing the value of the calibration factors by 50%, which would act to inflate abundances during the Discovery era, did not change the overall pattern of significantly greater abundances in the contemporary era in three out of the four calanoid species.
Climate variability
Recent investigations carried out around the western Antarctic Peninsula and Elephant Island are unanimous in finding links between decadal changes in abundance of plankton and the dominant modes of climate variability such as SAM and ENSO, which importantly influence sea-ice extent (Stammerjohn et al. 2008). It has been suggested that sea-ice extent in the first part of the 20th century may have been greater than in recent times (de la Mare 1997; Cotté and Guinet 2007). However data derived from satellite measurements from 1979 to 2006 show a positive trend of around 1% per decade reaching a new record maximum for the satellite era in 2012 (Turner et al. 2009, 2014). If ice extent was greater over the Scotia Sea in the early part of the last century we might have expected changes in cycles of productivity and hence in the timing of appearance in surface waters of some species, particularly large calanoids that overwinter at depth and appear in the surface waters in spring. Such a phenological change is not borne out by the data (Fig. 4) which show similar trends in relative abundance by month.
Movements of frontal zones have also been recorded in response to atmospheric forcing. During El Niño events, north-west winds in the vicinity of Drake Passage decrease, allowing colder water from the Weddell Sea to flow north and penetrate into the Bransfield Strait. Increased winds and a southwards movement of the SACCF allows warmer water to mix with cold coastal waters during the La Niña phase (Loeb et al. 2009, 2010). This increased oceanic influence results in more Chl a, more copepods and better krill recruitment in the coastal area whereas, under the El Niño regime, salps dominate, Chl a is low and krill recruitment is poor. Aside from sea-ice reduction, climate variability also induces physical changes in the marine environment such as water column stability which influences primary productivity and links to species abundance in space and time (Saba et al. 2014; Steinberg et al. 2015). We have only incomplete data on prevailing atmospheric conditions and their impacts during the time of the Discovery Investigations. Our data are also insufficient to allow us to test for changes in regional abundance of species across the Scotia Sea which we acknowledge may be a possibility. However, averaged over the entire region, and across two decadal periods, differences in abundances are proportionately large and strongly suggest wider changes within the ecosystem, rather than local displacements of water masses and changes in nutrient supply.
Temperature and food availability
It is hard to see how the observed increases in temperature between eras would impact on population demography and account for the differences observed. The increases of ~ 1.5 °C are apparent only within the near-surface ocean, although lesser warming has been observed at depth (Gille 2002). We might in any case have expected species to respond differently to changing temperature since we considered species with both warm (R. gigas and C. simillimus) and cold (C. acutus and C. propinquus) water preferences and yet, with the exception of C. propinquus, which showed a marginal decrease in contemporary times, all have increased in overall abundance. In terms of food availability, there have been a number of studies suggesting both recent decreases and increases in primary production in the Southern Ocean during the satellite era. Gregg et al. (2003) found a 10% decline in productivity when comparing satellite mounted Coastal Zone Colour Scanner (CZCS) data for the period 1979–1986 compared to more recent SeaWiFS (Sea-viewing Wide Field-of-view Sensor) measurements (1997–2002). In contrast, Smith and Comiso (2008) found that productivity in the entire Southern Ocean showed a substantial and significant increase during their 9-year observation period (1997–2006), with much of this increase due to changes during the austral summer months. However, we have no direct way of knowing how present levels of phytoplankton compare to those found 70 years ago during the Discovery Investigations.
It is also important to consider how changes elsewhere in the ecosystem may have brought about increased abundances by virtue of trophic cascade effects. An increase in abundance could have arisen due to an increase in available food, a relaxation of predation pressure, or both. Antarctic krill (Euphausia superba) might provide a key to understanding some of the ecosystem interactions as it has been argued that krill occupy a position in the Southern Ocean food-web whereby they influence trophic levels above and below themselves, in a so called ‘wasp-waist’ ecosystem (Flores et al. 2012; Atkinson et al. 2014). For example, it has been demonstrated that intense krill grazing can alter phytoplankton species composition by preferentially grazing diatoms leading to a dominance of flagellates < 20 µm (Jacques and Panouse 1991; Kopczynska 1992; Granéli et al. 1993). Equally, through fluctuations in biomass, their availability to higher predators varies and can impact breeding success and population size (Trathan et al. 2007). It has been suggested that, historically, both whales and krill were able to act as ‘ecosystem engineers’ in the sense that by virtue of their great abundance they were, and are, important recyclers of nutrients essential for phytoplankton growth (Tovar-Sanchez et al. 2007; Willis 2007, 2014; Smetacek 2008; Nicol et al. 2010; Schmidt et al. 2011). In this way, increased phytoplankton production would have supported a greater krill population ultimately benefiting whales and perhaps placing greater pressure on copepods, both as competitors and as potential prey.
The degree of competition for food resources is likely to be highly variable in space and time reflecting plankton densities and distributions as well as conditions conducive to primary production. However, food limitation is commonly observed in the world ocean, particularly among large copepods (Saiz and Calbet 2011). In the Southern Ocean, egg production rates of C. acutus and R. gigas reach an asymptote at around 3 mg m−3 Chl a (Shreeve et al. 2002) which is a relatively high concentration for much of the predominantly high nutrient low chlorophyll Southern Ocean. Longhurst (1998) notes that, within the southern part of the ACC, only 5% of underway-sampled chl-a concentration data exceeds 1 mg m−3 and most are a quarter of this (Tréguer and Jacques 1992). Not only copepod abundance but carbon mass and condition have also been found to be related closely to proxies of past production levels such as silicate levels and nutrient deficits (Shreeve et al. 2002; Ward et al. 2007), showing that bottom up control is important. Microphytoplankton (> 20 µm) has also been found to account for a large part of the variance in copepod abundance and carbon mass around South Georgia and elsewhere (Berggreen et al. 1988; Paffenhöfer 1988; Shreeve et al. 2002). Krill grazing may selectively remove microphytoplankton, thus disadvantaging large calanoid copepods which require blooms of large diatoms to optimise recruitment (Ward et al. 2005). However, of the 4 species, only C. propinquus showed a marginal but significant decrease in contemporary abundance, suggesting other factors may be paramount in this case. All species have broad and overlapping distributions within the ACC but life history traits are variable. For example, C. acutus is the most herbivorous and has a clear period of diapause in winter (Atkinson 1998), whereas C. propinquus has a closer association with ice-covered waters to the south of the Scotia Sea and, along with the northerly distributed C. simillimus, has extended periods of reproduction, with at least part of each population remaining active during winter (Bathmann et al. 1993; Atkinson 1998; Pasternak and Schnack-Schiel 2001). In contrast to C. acutus and R. gigas, in which wax esters are the main storage lipid, triacylglycerides dominate in both species of Calanus, suggesting more or less continuous feeding throughout the year (Hagen et al. 1993; Ward et al. 1996) and it has been found that microzooplankton can form a considerable part of the diet of both Calanus species (Hopkins et al. 1993; Atkinson 1995, 1996). The extent to which the diet of C. propinquus includes sea-ice algae is currently debateable. It has generally been found to be more abundant in open water than in the ice and marginal ice zones and was shown to have a higher proportion of empty guts when found under sea-ice (Burghart et al. 1999). However, recent data from the Scotia Sea show areas of recruitment for C. propinquus and, to an extent, C. acutus, which match surface concentrations of an isoprenoid ice-algae biomarker in the wake of the retreating ice edge (Schmidt et al. 2018). It is possible that a reduction in sea-ice means that under-ice productivity available to C. propinquus has declined or that any historical increase in chlorophyll available to copepods did not occur in the more southern parts of the Scotia Sea.
Krill may also directly prey on copepods (Atkinson and Snÿder 1997; Atkinson et al. 1999; Cripps et al. 1999; Hernández-León et al. 2001), particularly at times of low phytoplankton production and biomass. Through either preying directly upon or outcompeting copepods for food, krill may therefore, to a greater or lesser extent, control copepod population numbers. An overall increase in the number of large calanoids therefore suggests that control on this group has relaxed since the time of the Discovery Investigations. The reported decline of krill in the Atlantic sector of the Southern Ocean since the 1970s (Atkinson et al. 2004) could therefore be a mechanism by which competition and or predation has reduced, allowing copepod numbers to increase.
Ecosystem impacts
There is little doubt that a decreased abundance of krill will have had a significant impact on the amount of carbon passing through direct diatom- krill -higher predator food-chains. Copepod and krill food-webs have different characteristics in terms of carbon demand and fate depending on which is the dominant organism. Krill grazing can decrease phytoplankton standing stocks, particularly when swarms are present, although copepods rarely do, unless standing stocks are low (Atkinson 1996; Dubischar and Bathmann 1997). Within the Scotia Sea, krill and copepods are the dominant crustaceans, with krill tending to be more abundant in the southern part and copepods towards the north (Ward et al. 2012a). In a modelling study, Priddle et al. (2003) found that the biogeochemical consequences of grazing by krill and copepods were also different in terms of nutrient regeneration and resupply to primary producers. In a low krill-high copepod scenario, higher phytoplankton biomass and production, lower mixed layer ammonium, nitrate and silicate concentrations and higher detrital carbon were predicted than for a high krill low copepod scenario. Phytoplankton chlorophyll biomass was negatively related to krill biomass, and mixed layer nutrients were positively correlated with krill biomass in these data. Both observations and model results suggest that variation in biogeochemical carbon and nitrogen cycles in the South Georgia pelagic ecosystem is determined largely by changes in zooplankton community composition and its impact on phytoplankton dynamics. Contemporary estimates of krill and copepod biomass suggest that copepod standing stocks are at least equal to those of krill or indeed exceed them (Voronina 1998). Estimates of copepod vs krill production around South Georgia (where the biomass of both groups is generally high) suggest that the copepod community as a whole may be four times as productive as krill (Shreeve et al. 2005). Over the wider scale, Voronina (1998) estimates that 92% of annual zooplankton production in the Southern Ocean can be attributed to copepods whereas Conover and Huntley (1991) estimate productivity to be three times higher than krill-based estimates of ingestion and assimilation. Given that the biomass of baleen whales was so much higher in the past, it is axiomatic that krill biomass must also have been higher than contemporary estimates to support this biomass (Willis 2007, 2014; Smetacek 2008). The balance of production would also have changed but, even with large calanoids being less abundant in the past, as shown by our study, copepods would still have contributed significantly to secondary production.
Our previous analysis has shown that over the last 70 years, despite warming, the geographical distribution of the plankton community of the Scotia Sea has not changed (Tarling et al. 2018). This study has shown that, despite the rank order of species abundance staying broadly the same, there have been changes in absolute abundance of biomass-dominant copepod species. The factors we consider responsible are linked through to changes occurring within the food chain brought about by decreasing krill abundance both as a result of warming induced habitat loss and also the commercial exploitation of whales.
References
Atkinson A (1991) Lifecycles of Calanoides acutus, Calanus simillimus and Rhincalanus gigas (Copepoda: Calanoida) within the Scotia Sea. Mar Biol 109:79–91. https://doi.org/10.1007/BF01320234
Atkinson A (1995) Omnivory and feeding selectivity in five copepod species during spring in the Bellingshausen Sea, Antarctica. ICES J Mar Sci 52:385–396. https://doi.org/10.1016/1054-3139(95)80054-9
Atkinson A (1996) Subantarctic copepods in an oceanic, low chlorophyll environment: ciliate predation, food selectivity and impact on prey populations. Mar Ecol Prog Ser 130:85–96. https://doi.org/10.3354/meps130085
Atkinson A (1998) Life cycle strategies of epipelagic copepods in the Southern Ocean. J Mar Syst 15:289–311. https://doi.org/10.1016/S0924-7963(97)00081-X
Atkinson A, Sinclair JD (2000) Zonal distribution and seasonal vertical migration of copepod assemblages in the Scotia Sea. Polar Biol 23:46–58. https://doi.org/10.1007/s003000050007
Atkinson A, Snÿder R (1997) Krill-copepod interactions at South Georgia, Antarctica, I. Omnivory by Euphausia superba. Mar Ecol Prog Ser 160:63–76. https://doi.org/10.3354/meps160063
Atkinson A, Ward P, Hill A, Brierley AS, Cripps GC (1999) Krill-copepod interactions at South Georgia, Antarctica, II. Euphausia superba as a major control on copepod abundance. Mar Ecol Prog Ser 176:63–79. https://doi.org/10.3354/meps176063
Atkinson A, Whitehouse MJ, Priddle J, Cripps GC, Ward P, Brandon MA (2001) South Georgia, Antarctica: a productive, cold water, pelagic ecosystem. Mar Ecol Prog Ser 216:279–308. https://doi.org/10.3354/meps216279
Atkinson A, Siegel V, Pakhomov E, Rothery P (2004) Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103. https://doi.org/10.1038/nature02996
Atkinson A, Hill SL, Barange M, Pakhomov EA, Raubenheimer D, Schmidt K, Simpson SJ, Reiss C (2014) Sardine cycles, krill declines, and locust plagues: revisiting ‘wasp-waist’ food webs. Trends Ecol Evol 29:309–316. https://doi.org/10.1016/j.tree.2014.03.011
Bathmann UV, Makarov RR, Spiridonov VA, Rohardt G (1993) Winter distribution and overwintering strategies of the Antarctic copepod species Calanoides acutus, Rhincalanus gigas and Calanus propinquus (Crustacea, Calanoida) in the Weddell Sea. Polar Biol 13:333–346. https://doi.org/10.1007/BF00238360
Berggreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol 99:341–352. https://doi.org/10.1007/BF02112126
Burghart SE, Hopkins TL, Vargo GA, Torres JJ (1999) Effects of a rapidly receding ice edge on the abundance, age structure and feeding of three dominant calanoid copepods in the Weddell Sea, Antarctica. Polar Biol 22:279–288. https://doi.org/10.1007/s003000050421
Burrows MT, Schoeman DS, Buckley LB et al (2011) The pace of shifting climate in marine and terrestrial ecosystems. Science 334:652–655. https://doi.org/10.1126/science.1210288
Conover RJ, Huntley M (1991) Copepods in ice-covered seas—distribution, adaptations to seasonally limited food, metabolism, growth patterns and life cycle strategies in polar seas. J Mar Syst 2:1–41. https://doi.org/10.1016/0924-7963(91)90011-I
Constable AJ et al (2014) Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob Change Biol 20:3004–3025. https://doi.org/10.1111/gcb.12623
Cotté C, Guinet C (2007) Historical whaling records reveal major regional retreat of Antarctic sea ice. Deep-Sea Res I 54:243–252. https://doi.org/10.1016/j.dsr.2006.11.001
Cripps GC, Watkins JL, Hill HJ, Atkinson A (1999) Fatty acid content of Antarctic krill Euphausia superba at South Georgia related to regional populations and variations in diet. Mar Ecol Prog Ser 181:177–188. https://doi.org/10.3354/meps181177
de la Mare WK (1997) Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature 389:57–60. https://doi.org/10.1038/37956
Drits AV, Pasternak AF, Kosobokova KN (1994) Physiological characteristics of the Antarctic copepod Calanoides acutus during late summer in the Weddell Sea. Hydrobiologia 293:201–207. https://doi.org/10.1007/BF00229942
Dubischar CD, Bathmann UV (1997) Grazing impact of copepods and salps on phytoplankton in the Atlantic sector of the Southern ocean. Deep Sea Res II 44:415–433. https://doi.org/10.1016/S0967-0645(96)00064-1
Flores H, Atkinson A, Kawaguchi S, Krafft BA, Milinevsky G, Nicol S, Reiss C, Tarling GA, Werner R, Rebolledo EB, Cirelli V (2012) Impact of climate change on Antarctic krill. Mar Ecol Prog Ser 458:1–9. https://doi.org/10.3354/meps09831
Gille ST (2002) Warming of the Southern Ocean since the 1950s. Science 295:1275–1277. https://doi.org/10.1126/science.1065863
Gordon AL, Georgi DT, Taylor HW (1977) Antarctic polar front zone in the western Scotia Sea—Summer 1975. J Phys Oceanogr 7:309–328.https://doi.org/10.1175/1520-0485(1977)007%3c0309:APFZIT%3e2.0.CO;2
Granéli E, Granéli W, Rabbani MM, Daugbjerg N, Fransz G, Cuzin-Roudy J, Alder VA (1993) The influence of copepod and krill grazing on the species composition of phytoplankton communities from the Scotia Weddell Sea. Polar Biol 13:201–213. https://doi.org/10.1007/BF00238930
Gregg WW, Conkright ME, Ginoux P, O’Reilly JE, Casey NW (2003) Ocean primary production and climate: global decadal changes. Geophys Res Lett 30:1809. https://doi.org/10.1029/2003GL016889
Hagen W, Kattner G, Graeve M (1993) Calanoides acutus and Calanus propinquus, Antarctic copepods with different lipid storage modes via wax esters or triacylglycerols. Mar Ecol Prog Ser 97:135–142
Hardy AC, Gunther ER (1935) The plankton of the South Georgia whaling grounds and adjacent waters, 1926–1927. Discov Rep 11:1–456
Hátún H, Payne MR, Beaugrand G, Reid PC, Sandø AB, Drange H, Hansen B, Jacobsen JA, Bloch D (2009) Large bio-geographical shifts in the north-eastern Atlantic Ocean: from the subpolar gyre, via plankton, to blue whiting and pilot whales. Prog Oceanogr 80:149–162. https://doi.org/10.1016/j.pocean.2009.03.001
Hernández-León S, Portillo-Hahnefeld A, Almeida C, Bécognée P, Moreno I (2001) Diel feeding behaviour of krill in the Gerlache Strait, Antarctica. Mar Ecol Prog Ser 223:235–242. https://doi.org/10.3354/meps223235
Hopkins TL, Lancraft TM, Torres JJ, Donnelly J (1993) Community structure and trphic ecology of zooplankton in the Scotia Sea marginal ice zone in winter (1988). Deep Sea Res I 40:81–105. https://doi.org/10.1016/0967-0637(93)90054-7
Jacques G, Panouse M (1991) Biomass and composition of size fractionated phytoplankton in the Weddell-Scotia Confluence area. Polar Biol 11:315–328. https://doi.org/10.1007/BF0023902
Kemp S, Hardy AC, Mackintosh NA (1929) The Discovery Investigations, objects, equipment and methods. Discov Rep 1:143–251
Kopczynska EE (1992) Dominance of microflagellates over diatoms in the Antarctic areas of deep vertical mixing and krill concentrations. J Plankton Res 14:1031–1054. https://doi.org/10.1093/plankt/14.8.1031
Laws RM (1977) Seals and whales of the Southern Ocean. Phil Trans R Soc B 279:81–96. https://doi.org/10.1098/rstb.1977.0073
Laws RM (1985) The ecology of the Southern Ocean: the Antarctic ecosystem, based on krill, appears to be moving toward a new balance of species in its recovery from the inroads of whaling. Am Sci 73:26–40
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900. https://doi.org/10.1038/43174
Loeb VJ, Hofmann EE, Klinck JM, Holm-Hansen O, White WB (2009) ENSO and variability of the Antarctic Peninsula pelagic marine ecosystem. Antarct Sci 21:135–148. https://doi.org/10.1017/S0954102008001636
Loeb V, Hofmann EE, Klinck JM, Holm-Hansen O (2010) Hydrographic control of the marine ecosystem in the South Shetland-Elephant Island and Bransfield Strait region. Deep Sea Res II 57:519–542. https://doi.org/10.1016/j.dsr2.2009.10.004
Longhurst AR (1998) Ecological geography of the sea. Academic Press, Cambridge
Mackey AP, Atkinson A, Hill SL, Ward P, Cunningham NJ, Johnston NM, Murphy EJ (2012) Antarctic macrozooplankton of the southwest Atlantic sector and Bellingshausen Sea: Baseline historical distributions (Discovery Investigations, 1928–1935) related to temperature and food, with projections for subsequent ocean warming. Deep Sea Res II 59:130–146. https://doi.org/10.1016/j.dsr2.2011.08.011
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32:L19604. https://doi.org/10.1029/2005GL024042
Nicol S, Bowie A, Jarman S, Lannuzel D, Meiners KM, Van Der Merwe P (2010) Southern Ocean iron fertilization by baleen whales and Antarctic krill. Fish Fisheries 11:203–209. https://doi.org/10.1111/j.1467-2979.2010.00356.x
Paffenhöfer GA (1988) Feeding rates and behavior of zooplankton. Bull Mar Sci 43:430–445
Pasternak AF, Schnack-Schiel SB (2001) Seasonal feeding patterns of the dominant Antarctic copepods Calanus propinquus and Calanoides acutus in the Weddell Sea. Polar Biol 24:771–784. https://doi.org/10.1007/s003000100283
Poloczanska ES, Brown CJ, Sydeman WJ et al (2013) Global imprint of climate change on marine life. Nat Clim Change 3:919–925. https://doi.org/10.1038/nclimate1958
Priddle J, Whitehouse M, Ward P, Shreeve RS, Brierley AS, Atkinson A, Watkins JL, Brandon MA, Cripps GC (2003) Biogeochemistry of a Southern Ocean plankton ecosystem: using natural variability in community composition to study the role of metazooplankton in carbon and nitrogen cycles. J Geophys Res 108:8082. https://doi.org/10.1029/2000JC000425
Richardson AJ (2008) In hot water: zooplankton and climate change. ICES J Mar Sci 65:279–295. https://doi.org/10.1093/icesjms/fsn028
Richardson AJ, Brown CJ, Brander K, Bruno JF, Buckley L, Burrows MT, Duarte CM, Halpern BS, Hoegh-Guldberg O, Holding J, Kappel CV (2012) Climate change and marine life. Biol Lett 23:907–909. https://doi.org/10.1098/rsbl.2012.0530
Saba GK, Fraser WR, Saba VS, Iannuzzi RA, Coleman KE, Doney SC, Ducklow HW, Martinson DG, Miles TN, Patterson-Fraser DL, Stammerjohn SE (2014) Winter and spring controls on the summer food web of the coastal West Antarctic Peninsula. Nat Commun 5:4318. https://doi.org/10.1038/ncomms5318
Saiz E, Calbet A (2011) Copepod feeding in the ocean: scaling patterns, composition of their diet and the bias of estimates due to microzooplankton grazing during incubations. Hydrobiologia 666:181–196. https://doi.org/10.1007/s10750-010-0421-6
Schmidt K, Atkinson A, Steigenberger S, Fielding S, Lindsay M, Pond DW, Tarling GA, Klevjer TA, Allen CS, Nicol S, Achterberg EP (2011) Seabed foraging by Antarctic krill: implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol Oceanogr 56:1411–1428. https://doi.org/10.4319/lo.2011.56.4.1411
Schmidt K, Brown TA, Belt ST, Ireland LC, Taylor KWR, Thorpe SE, Ward P, Atkinson A (2018) Do pelagic grazers benefit from sea ice? Insights from the Antarctic sea ice proxy IPSO25. Biogeosciences 15:1987–2006. https://doi.org/10.5194/bg-15-1987-2018
Schnack-Schiel SB (2001) Aspects of the study of the life cycles of Antarctic copepods. Hydrobiologia 453:9. https://doi.org/10.1023/A:1013195329066
Sen Gupta A, McNeil B (2012) Variability and change in the ocean. In: Henderson-Sellers A, McGuffie K (eds) The future of the world’s climate. Elsevier, Amsterdam, pp 141–165
Shreeve RS, Ward P, Whitehouse MJ (2002) Copepod growth and development around South Georgia: relationships with temperature, food and krill. Mar Ecol Prog Ser 233:169–183. https://doi.org/10.3354/meps233169
Shreeve RS, Tarling GA, Atkinson A, Ward P, Goss C, Watkins J (2005) Relative production of Calanoides acutus (Copepoda: Calanoida) and Euphausia superba (Antarctic krill) at South Georgia, and its implications at wider scales. Mar Ecol Prog Ser 298:229–239. https://doi.org/10.3354/meps298229
Smetacek V (2008) Are declining krill stocks a result of global warming or of the decimation of the whales? In: Duarte CM (ed) Impacts of global warming on polar ecosystems. Bilbao, Fundación BBVA, pp 47–83
Smith WO, Comiso JC (2008) Influence of sea ice on primary production in the Southern Ocean: a satellite perspective. J Geophys Res 113:C05S93. https://doi.org/10.1029/2007JC004251
Stammerjohn SE, Martinson DG, Smith RC, Yuan X, Rind D (2008) Trends in Antarctic annual sea ice retreat and advance and their relation to El Niño–Southern Oscillation and Southern Annular Mode variability. J Geophys Res 113:C03S90. https://doi.org/10.1029/2007JC004269
Steinberg DK, Ruck KE, Gleiber MR, Garzio LM, Cope JS, Bernard KS, Stammerjohn SE, Schofield OM, Quetin LB, Ross RM (2015) Long-term (1993–2013) changes in macrozooplankton off the Western Antarctic Peninsula. Deep Sea Res I 101:54–70. https://doi.org/10.1016/j.dsr.2015.02.009
Tarling GA, Shreeve RS, Ward P, Atkinson A, Hirst AG (2004) Life-cycle phenotypic composition and mortality of Calanoides acutus (Copepoda: Calanoida) in the Scotia Sea: a modelling approach. Mar Ecol Prog Ser 272:165–181. https://doi.org/10.3354/meps272165
Tarling GA, Ward P, Thorpe SE (2018) Spatial distributions of Southern Ocean mesozooplankton communities have been resilient to long-term surface warming. Glob Change Biol 24:132–142. https://doi.org/10.1111/gcb.13834
Tovar-Sanchez A, Duarte CM, Hernández-León S, Sañudo-Wilhelmy SA (2007) Krill as a central node for iron cycling in the Southern Ocean. Geophys Res Lett 34:L11601. https://doi.org/10.1029/2006GL029096
Trathan PN, Forcada J, Murphy EJ (2007) Environmental forcing and Southern Ocean marine predator populations. In: Rogers AD, Johnston NM, Murphy EJ, Clarke A (eds) Antarctic ecosystems: an extreme environment in a changing world. Blackwell, Hoboken. https://doi.org/10.1002/9781444347241.ch11
Tréguer P, Jacques G (1992) Review Dynamics of nutrients and phytoplankton, and fluxes of carbon, nitrogen and silicon in the Antarctic Ocean. In: Hempel G (ed) Weddell Sea Ecology. Springer, Berlin, pp 149–162https://doi.org/10.1007/978-3-642-77595-6_17
Turner J, Comiso JC, Marshall GJ, Lachlan-Cope TA, Bracegirdle T, Maksym T, Meredith MP, Wang Z, Orr A (2009) Non-annular atmospheric circulation change induced by stratospheric ozone depletion and its role in the recent increase of Antarctic sea ice extent. Geophys Res Lett 36:L08502. https://doi.org/10.1029/2009GL037524
Turner J, Bracegirdle TJ, Phillips T, Marshall GJ, Hosking JS (2013) An initial assessment of Antarctic sea ice extent in the CMIP5 models. J Clim 26:1473–1484. https://doi.org/10.1175/JCLI-D-12-00068.1
Turner J, Barrand NE, Bracegirdle TJ et al (2014) Antarctic climate change and the environment: an update. Polar Rec 50:237–259. https://doi.org/10.1017/S0032247413000296
Voronina NM (1998) Comparative abundance and distribution of major filter-feeders in the Antarctic pelagic zone. J Mar Syst 17:375–390. https://doi.org/10.1016/S0924-7963(98)00050-5
Vuorinen I, Hänninen J, Bonsdorff E, Boormann B, Angel MV (1997) Temporal and spatial variation of dominant pelagic Copepoda (Crustacea) in the Weddell Sea (Southern Ocean) 1929 to 1993. Polar Biol 18:280–291. https://doi.org/10.1007/s003000050189
Ward P, Shreeve RS, Cripps GC (1996) Rhincalanus gigas and Calanus simillimus: lipid storage patterns of two species of copepod in the seasonally ice-free zone of the Southern Ocean. J Plankton Res 18:1439–1454. https://doi.org/10.1093/plankt/18.8.1439
Ward P, Shreeve R, Whitehouse M, Korb B, Atkinson A, Meredith M, Pond D, Watkins J, Goss C, Cunningham N (2005) Phyto- and zooplankton community structure and production around South Georgia (Southern Ocean) during Summer 2001/02. Deep Sea Res I 52:421–441. https://doi.org/10.1016/j.dsr.2004.10.003
Ward P, Shreeve R, Korb B, Whitehouse M, Thorpe S, Pond D, Cunningham N (2006) Plankton community structure and variability in the Scotia Sea: austral summer 2003. Mar Ecol Prog Ser 309:75–91. https://doi.org/10.3354/meps309075
Ward P, Whitehouse M, Shreeve R, Thorpe S, Atkinson A, Korb R, Pond D, Young E (2007) Plankton community structure south and west of South Georgia (Southern Ocean): links with production and physical forcing. Deep Sea Res I 54:1871–1889. https://doi.org/10.1016/j.dsr.2007.08.008
Ward P, Atkinson A, Tarling G (2012a) Mesozooplankton community structure and variability in the Scotia Sea: a seasonal comparison. Deep Sea Res II 59:78–92. https://doi.org/10.1016/j.dsr2.2011.07.004
Ward P, Tarling GA, Coombs SH, Enderlein P (2012b) Comparing Bongo net and N70V mesozooplankton catches: using a reconstruction of an original net to quantify historical plankton catch data. Polar Biol 35:1179–1186. https://doi.org/10.1007/s00300-012-1163-x
Whitehouse MJ, Meredith MP, Rothery P, Atkinson A, Ward P, Korb RE (2008) Rapid warming of the ocean around South Georgia, Southern Ocean, during the 20th century: forcings, characteristics and implications for lower trophic levels. Deep Sea Res I 55:1218–1228. https://doi.org/10.1016/j.dsr.2008.06.002
Willis J (2007) Could whales have maintained a high abundance of krill? Evol Ecol Res 9:651–662
Willis J (2014) Whales maintained a high abundance of krill; both are ecosystem engineers in the Southern Ocean. Mar Ecol Prog Ser 513:51–69. https://doi.org/10.3354/meps10922
Acknowledgements
We are grateful to the close attention paid by the three reviewers to improving this manuscript. We thank the officers and crew of RRS James Clark Ross for enabling our various contemporary field sampling programmes and above all the personnel of the Discovery Investigations who laid the foundations of our understanding of the Southern Ocean. We also thank the Natural History Museum (London) for conserving many of the extant Discovery samples and Miranda Lowe for organising their loan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors were supported by the Ocean Ecosystems programme at British Antarctic Survey (NERC). The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ward, P., Tarling, G.A. & Thorpe, S.E. Temporal changes in abundances of large calanoid copepods in the Scotia Sea: comparing the 1930s with contemporary times. Polar Biol 41, 2297–2310 (2018). https://doi.org/10.1007/s00300-018-2369-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2369-3