Abstract
Testate protists in the genus Gromia (‘gromiids’, supergroup Rhizaria) are common and diverse in marine settings. However, their ecological significance is not well understood, partly because they remain largely undescribed with most records being of the type species, G. oviformis. To enhance our knowledge of gromiid biodiversity, we use morphological and genetic data to describe four new species with tests of different shapes from sublittoral depths (21–136 m) in South Georgia fjords: G. pashukae (ovate), G. landrethi (spherical), G. amygdaliformis (almond-shaped), and G. saoirsei (elongate), maximum lengths 3.4, 2.5, 1.4 and 4.5 mm, respectively. We also describe two smaller ( ~1 mm) gromiid species from intertidal sites on the Falkland Islands: G. cedhageni (ovate test with finely granular, orange-coloured contents), and Gromia psammophila (subrectangular test with numerous small mineral grains embedded in pale, finely granular material). Gromia landrethi is also represented by DNA sequences from specimens collected in Ushuaia (Argentina) and G. psammophila by sequenced specimens from southern Chilean fjords, but the other species are known only from localities in South Georgia or the Falkland Islands. Analysis of partial SSU rRNA genes confirms the presence of these six new species, as well as an undescribed species represented by a clade of three sequences branching separately. Our results increase the number of described gromiid species from 10 to 16, and underline the importance of these poorly known relatives of the foraminifera in higher-latitude intertidal and fjord settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Gromia includes testate marine rhizarian protists that have often been confused with foraminifera, although they are clearly distinct from foraminifera in terms of their pseudopodial morphology (Rhumbler 1904; Hedley 1958), wall structure (Hedley and Bertaud 1962; Arnold 1982), and phylogenetic position (e.g., Longet et al. 2004; Sierra et al. 2013). Gromiids, together with various predatory and parasitic amoeboid protists, are currently assigned to the Endomyxa, a group that is either sister to or includes the Retaria (foraminifera plus acantharean and polycystine radiolarians) (Adl et al. 2019). They are often relatively large (> 1 mm), making them easily recognisable in intertidal and other coastal settings. This led to the early discovery and description of the first, and for many years the only, gromiid species, Gromia oviformis, described by Dujardin (1835) based on samples from the Gulf of Lyon (Mediterranean) and the French coast of the English Channel. With the exception of G. schultzei Norman 1892 (= Gromia sp. of Schultze 1875), all other species assigned to this genus by nineteenth century and early twentieth century authors belong to other protistan groups, mainly freshwater foraminifera (Holzmann et al. 2021).
More recent studies have shown that species of Gromia are in fact quite diverse and occur in various marine habitats, ranging from hadal to coastal settings across temperate and tropical latitudes around the world (Gooday et al. 2000; Gooday and Bowser 2005; Aranda da Silva et al. 2006; Matz et al. 2008; Aranda da Silva and Gooday 2009; Rothe et al. 2009, 2011; Goldstein et al. 2011; Cedhagen et al. 2013; LeDuc and Rowden 2017; Sergeeva et al. 2017; Henderson 2021; Pavel et al. 2021). Gromiids are also common in fjords and other higher latitude coastal environments (Schulze 1875; Heron-Allen and Earland 1922; Bowser et al. 1996; Gooday et al. 1996, 2005). Recently, we described three species that were collected in fjords on the west coast of Greenland (Gooday et al. 2021). Here, we extend these studies by describing new gromiid species from contrasting higher latitude settings in the southern hemisphere, four from fjords located on the northeast coast of the sub-Antarctic island of South Georgia and two from intertidal environments on the more temperate Falkland Islands.
Study areas
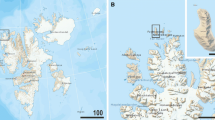
The island of South Georgia is about 170 km long, 40 km wide, mountainous, heavily glaciated, and flanked by a broad continental shelf (Hodgson et al. 2014). It is situated some 350 km to the south of the Antarctic Polar Front (Fig. 1), which forms part of the Antarctic Circumpolar Current (ACC), and deviates northwards to the west of the island (Orsi et al. 1995). It also lies on the Atlantic side of the Southern ACC Front, the southern boundary of the ACC that wraps around the South Georgia shelf to the east and north (Fig. 1).
Map of sampling localities in the Falkland Islands and on the north coast of South Georgia. Map data from google map (upper), https://freevectormaps.com (middle), as well as South Georgia and The Shackleton Crossing map 1: 200 000, published by British Antarctic Survey in 2017 and Nautical chart BA 3588 Approaches to Stromness and Cumberland Bays 1: 50 000, published by UK Hydrographic Office (lower). Position of the southern Antarctic Circumpolar Current front (SACCF), the Polar Front (PF), and the Subantarctic Front (SAF) are after Orsi et al. (1995). The SACCF and SAF mark the southern and northern limits, respectively, of the Antarctic Circumpolar Current (ACC)
Although South Georgia lies in the path of the ACC, the shelf waters surrounding it are different from those of the open ocean, being highly productive with strong phytoplankton blooms as well as exhibiting considerable complexity and variability (Atkinson et al. 2001; Gilpin et al. 2002; Korb and Whitehouse 2004; Meredith et al. 2005). As a result of the high productivity, the area supports a rich ecosystem and high biomass. Our study is based on samples from three fjords, Cumberland Bay East and West (maximum depth 265 m) and the shallower Fortuna and Stromness Bays (maximum depth 160 m). These and other fjords on the north coast of South Georgia have received less attention than the adjacent shelf areas, with the Cumberland Bay system being the most extensively studied. Data on seafloor topography from recent multibeam swath bathymetry surveys (Hodgson et al. 2014) show that South Georgia fjords typically comprise an inner basin and a deeper outer basin, both delimited by terminal moraines. The shallow depth of the inner basins likely reflects the deposition of sediment derived from glaciers at the heads of the fjords, with a variety of sedimentary features suggesting a high sediment supply. Some fjords are linked to submarine troughs of glacial origin that extend outwards across the adjacent shelf, although of the three fjords that we sampled, only Cumberland Bay is linked to one of these features (Graham et al. 2008). Localised methane seepage has been detected in both branches of Cumberland Bay (Römer et al. 2014), possibly linked to peat-rich sediments buried at a depth of some ten of meters (Geprägs et al. 2016). In some nearshore areas, organic material originating from whaling stations is visible in the shallow sediment record (Platt 1979).
Some additional environmental data are available for Cumberland Bay (Römer et al. 2014; Geprägs et al. 2016). The summer water column is stratified, with a surface lens of low-salinity water near the fjord head. Bottom-water temperatures range from 2.5° C at 100 m depth to 1.6° C at 250 m, oxygen from 6.5 to 5.1 ml/l, while salinity does not rise above 34.2. Sediments are generally muds, sandy muds, black, dark green, or grey in colour. The organic carbon accounts for 0.65% wt. and the sediments show signs of bioturbation.
The Falkland Islands comprise two main islands (Falkland West and East), situated on the edge of the Patagonian shelf about 1450 km to the northwest of South Georgia and 460 km to the east of the tip of South America. Unlike South Georgia, the Falklands lie to the north of the Polar Front. It therefore has a milder, more temperate climate. Nearshore water temperatures can range from 2º C in winter to 14º C in summer (Otley et al. 2008). Our samples come from inter-tidal locations, strongly affected by these annual temperature changes, as well as by fresh water discharge.
Methods
Sampling, sample processing and morphological methods
Samples were collected between 26 November and 6 December 2019 in Fortuna Bay, Stromness Bay and Cumberland East and West Bays at depths between 21 and 250 m using a Van Veen grab deployed from the yacht Saoirse (Table 1; Fig. 1). Surface sediment was removed with a spoon and washed on deck through a series of sieves with mesh sizes of 500, 250, and 125 µm. The residues were transferred to plastic jars with ambient seawater and stored in a laboratory refrigerator in the British Antarctic Survey’s King Edward Point Research Station, Cumberland East Bay. Samples were collected opportunistically in the Falkland Islands at two intertidal sites on December 18th and 19th, 2019, placed in plastic jars and sieved later through the same series of meshes. As soon as possible after collection, the different residues were sorted in seawater in Petri dishes for gromiids and foraminifera, all the time being kept chilled using a freezer pack. Most gromiids were obtained from the 250–500-µm and particularly the > 500-µm fractions. They were always picked where present, but because of time constraints they were not picked exhaustively, except in the case of the > 500-µm fractions from Stations SG-08 and SG-12. Specimens for genetic analysis were preserved in RNAlater; samples for morphological analysis were preserved in 4% formalin buffered with borax.
Following the expedition, the gromiids for genetic analysis were returned to Geneva, where they were photographed using a Leica M205 C microscope fitted with a Leica DFC450 C camera. Specimens to be described morphologically were returned to Southampton where they were photographed using an Olympus SZX7 stereo-microscope and an Olympus BH2 compound microscope, in both cases equipped with a Canon 60D SRL digital camera.
DNA extraction, PCR amplification and sequencing
Sixty specimens of Gromia were extracted individually using DNeasy Plant Mini Kit (Qiagen). Isolate numbers are given in Supplementary Table S1. Semi-nested PCR amplification was carried out for the 3′ end fragment of the SSU using eukaryotic SSU forward primer s12.2 (GATYAGATACCGTCG) at the first amplification step and the gromiid-specific SSU forward primer s13.3 (CGTTGGATAGGACTC) for the reamplification. The eukaryotic SSU reverse primer 20R (5′GACGGGCGGTGTGTACAA) was used for both amplification steps.
The amplified PCR products were purified using the High Pure PCR Cleanup Micro Kit (Roche Diagnostics). Sequencing reactions were performed using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and analyzed on a 3130XL Genetic Analyzer (Applied Biosystems). The resulting sequences were deposited in the EMBL/GenBank database (accession numbers MT906533-34, MT906547-48, MZ468155-203, MZ701627-33; Online Resource 1).
Phylogenetic analysis
The obtained sequences were added to an existing database using the Muscle automatic alignment option as implemented in SeaView vs. 4.3.3. (Gouy, et al. 2010). The alignment contains 97 sequences with 883 sites used for analysis. Nucleotide frequencies are 0.24 (A), 0.21 (C), 0.26 (G) and 0.29 (T).
The phylogenetic tree was constructed using maximum likelihood phylogeny (PhyML 3.0) as implemented in ATGC: PhyML (Guindon et al. 2010). An automatic model selection by SMS (Lefort et al. 2017) based on Akaike Information Criterion (AIC) was used, resulting in a GTR substitution model being selected for the analysis. The initial tree is based on BioNJ. Bootstrap values (BV) are based on 100 replicates. Our tree is unrooted and represents the branching order.
Results: systematic descriptions
The type material is deposited in the Natural History Museum, London (Protist Collection). Specimens are stored in 10% formalin in 1.25 ml cryovials.
RHIZARIA Cavalier-Smith, 2002.
ENDOMYXA Cavalier-Smith, 2002.
Class GROMIIDEA Cavalier-Smith, 2003.
Order GROMIIDA Claparède and Lachmann, 1856.
Family GROMIIDAE Reuss, 1862.
Gromia Dujardin, 1835
Gromia pashukae Gooday & Holzmann sp. nov.
Gromia pashukae Gooday & Holzmann sp. nov.; Station SG-08, Cumberland West Bay. a Paratype 3, reg. no NHMUK 2021.11.2.4. b Holotype, reg. no. NHMUK 2021.11.2.1. c Paratype 1, reg. no. NHMUK 2021.11.2.2. d Paratype 2, reg. no. NHMUK 2021.11.2.3. e Paratype 4, reg. no. NHMUK 2021.11.2.5. f Smaller, unregistered specimens. Scales 1 mm (a–e), 0.5 mm (f)
Gromia pashukae Gooday & Holzmann sp. nov., oral capsules; Station SG-08, Cumberland West Bay. a Holotype, reg. no. NHMUK 2021.11.2.1. b Paratype 1, reg. no. NHMUK 2021.11.2.2. c Paratype 2, reg. no. NHMUK 2021.11.2.3. d Paratype 3, reg. no. NHMUK 2021.11.2.4. e Paratype 4, reg. no. NHMUK 2021.11.2.5. f Smaller, unregistered specimens. Scales 0.25 mm (a–e), 0.5 mm (f)
Diagnosis: Species of Gromia with ovoid test measuring up to 3.0 mm or more in length, although specimens < 1.1 mm long are sometimes common; length/width ratio ranging from 1.05 to 1.60. Apertural end may be somewhat flattened. Oral capsule relatively large (up to 490 µm diameter), circular, plano-convex in side view with distinct rim and flat outer surface, typically projecting only slightly from apertural end. Wall transparent, sometimes (particularly in larger specimens) with more or less obvious furrows radiating down from oral capsule but small-scale surface ornamentation absent.
ZooBank registration: urn:lsid:zoobank.org:act:CCB7177F-D9E6-4A41-8990-0E8AC1B7A6E2.
Type material: Station SG-08, Cumberland West Bay; 54° 12.038′ S, 36° 34.267′ W, water depth 23 m. The holotype (reg. no. NHMUK 2021.11.2.1) and four paratypes (reg. no. NHMUK 2021.11.2.2–5) are preserved in 10% formalin.
Other material: Station SG-08 (as above). 203 specimens for morphology; five specimens for genetics (isolates 21148, 21153–21156).
Station SG-09, Cumberland West Bay; 54° 12.044′ S, 36° 34.253′ W, water depth 60 m. 162 specimens for morphology, two specimens for genetics (isolates 21128–21129).
Station SG-12, Cumberland East Bay; 54° 16.980′ S, 36° 29.977′ W, water depth 21 m. Approximately, 360 specimens for morphology, seven specimens for genetics (isolates 21199, 21201–21206).
Etymology: Named in honour of Keri-Lee Pashuk, Co-Captain of the yacht Saoirse, who undertook all of the boat-related logistics.
Description: The test is ovoid and resembles a grape in overall shape (Fig. 2). The adapertural end is evenly rounded and the apertural end more gently rounded or flattened, occasionally almost straight. Specimens span a wide size range. Test lengths vary from 0.40 to 3.36 mm, widths from 0.32 to 2.63 mm, and length/width ratios from 1.04 to 1.61 (Table 2). Those from Station SG-09 in Cumberland West Bay are generally somewhat smaller than those from the nearby type locality (SG-08). A large collection (n = 303) from Station SG-12 in Cumberland East Bay are dominated by specimens less than 1 mm in length, but also include some that are unusually large (Online Resource 2).
The oral capsule projects only slightly beyond the test outline. In plan view, it appears as a circular structure, in the type and figured specimens measuring 270–490 µm in diameter, including a more or less distinct rim, 30–50 µm wide (Fig. 3). In side view (Online Resource 3), it appears as a plano-convex structure, with a flat outer surface, a curved inner surface, and obliquely truncated outer edges that form the rim visible in plan view. The interior gives the impression of being an empty space, bounded by a flat outer organic layer and a saucer-shaped inner layer (Fig. 3a–d). A central axial column of material, presumably largely cytoplasmic in nature, extends through the oral structure, from the interior to the exterior. In one specimen, several filaments, possibly cyanobacteria in the process of being ingested, emerge from the mouth of the oral capsule (Online Resource 3). Particularly in smaller specimens, the flat outer surface of the capsule is overlain by, and appears to be firmly attached to, a delicate ‘superstructure’ that projects as a distinct mound and is generally light in colour (Online Resource 3).
The test wall is rather thin and the surface smooth with no obvious ornamentation. However, the apertural end of the test often displays a pattern of furrows that radiate down from the oral capsule; these features appear to be folds in the test surface (Fig. 3e, f). The test contents are brownish grey and comprise small stercomata.
Molecular characteristics: Gromia pashukae (100% BV) branches as sister to G. cedhageni, the branching being strongly supported (98% BV). Gromia sp. 2, G. marmorea and G. botelliformis cluster as sister group to both species, but their branching is not well supported by the bootstrap value (Fig. 4). The partial SSU rDNA sequences of G. pashukae contain 633 nucleotides and the GC content is 46%. Three large and three small specimens from Cumberland East Bay (SG-12) yielded identical sequences.
PhyMl phylogenetic tree based on the 3′end fragment of the SSU rRNA gene, showing evolutionary relationships of 97 gromiid taxa. Sequences obtained for this study are marked in bold. The tree is unrooted and represents the branching order but does not show ancestory relationships. Numbers at nodes indicate bootstrap values (BV’s) > 70%
Remarks: Gromia pashukae is by far the most abundant species in our South Georgia collections, being represented by hundreds of specimens at each of three sites in both branches of Cumberland Bay. At stations SG-08 in the western branch, and SG-12 in the eastern branch, we picked all specimens from sieve fractions > 500 µm (Online Resource 2). The sample from SG-12 yielded 302 specimens spanning a size range from 0.41 to 3.36 mm. However, there was a strong bias towards smaller specimens, with three quarters (75.2%) having a maximum dimension of less than 1 mm and a peak at 0.70–0.79 mm. The possibility that this distribution reflects a recent reproductive event is strengthened by the long tail of larger specimens with a much smaller and more diffuse size class peaking at 2.30–2.39 mm. In contrast, the sample from SG-08 yielded 199 specimens with lengths ranging from 0.80 to 2.74 mm and only five (2.51%) being less than 1 mm long (Online Resource 2). In this case there was no indication of distinct size classes.
Gromiids with an ovoid test, resembling that of G. pashukae, are recorded from localities in different parts of the world. These include G. oviformis of Jepps (1926, Pl. 37, Figs. 9, 10, 11 therein; from SW England) and Burki et al. (2002, Fig. 4a therein; from McMurdo Sound, Antarctica), Gromia sp. 5 (Fig. 2h–k in Aranda da Silva et al. 2006; from the bathyal Arabian Sea), some specimens of G. marmora (Fig. 2c in Rothe et al. 2009; from the bathyal Weddell Sea), Gromia sp. 5A (Fig. 4a, c in Rothe et al. 2011; from the bathyal Weddell Sea), Gromia sp. (Fig. 1 in Goldstein et al. 2011; from Florida), and Gromia sp. (Fig. 4a–c in Pavel et al. 2021; from the Black Sea). In three cases (Burki et al. 2002; Aranda da Silva et al. 2006; Rothe et al. 2009), genetic data show that these ovoid morphotypes are not closely related to G. pashukae.
The oral capsule of G. pashukae is unusual, forming a flat feature surrounded by a circular collar that projects only slightly from the surface of the test. These characteristics are particularly evident in larger specimens, in which the capsule most closely resembles that of G. marmora (Fig. 4a in Rothe et al. 2009). In many smaller specimens, this flat structure is covered by a mound of whitish material, forming a more prominent feature (Online Resource 3). It is not clear whether this mound is part of the capsule itself or composed of a coherent mass of detrital material that is attached to it.
Distribution: Currently known only from Cumberland West Bay and East Bay, South Georgia.
Gromia landrethi Gooday & Holzmann sp. nov.
Gromia landrethi Gooday & Holzmann sp. nov. a–d Station SG-18, Stromness Bay. a Holotype, reg. no. NHMUK 2021.11.2.6. b Paratype 1, reg. no. NHMUK 2021.11.2.7. c Paratype 2, reg. no. NHMUK 2021.11.2.8. d Paratype 3, reg. no. NHMUK 2021.11.2.9. (e–f) Station SG-04; unregistered specimens. Scales 1 mm
Diagnosis: Species of Gromia with almost spherical test, typically ~ 1.5 to 2.5 mm diameter; length/width ratio ranging from 1.01 to 1.07. Oral capsule low and flat, around 300 µm in diameter, sometimes with overlying structure of material resembling detritus. Wall transparent with no obvious surface ornamentation.
ZooBank registration: urn:lsid:zoobank.org:pub:775F4FDE-7B6A-487C-BFF1-0A57EE9640DA.
Type material: Station SG-18, Stromness Bay; 54° 10.783′ S, 36° 39.979′ W, water depth 102 m. The holotype (reg. no. NHMUK 2021.11.2.6) and three paratypes (reg. no. NHMUK 2021.11.2.7–9) are preserved in 10% formalin.
Other material: Station SG-18 (as above). Two specimens for genetics (isolates 21141–21142).
Station SG-04, Fortuna Bay; 54° 07.658′ S, 36° 47.598′ W, water depth 135 m. Two specimens for morphology; four specimens for genetics (isolates 21149–21152).
Station SG-28, Stromness Bay near Grass Island; 54° 09.861′ S, 36° 40.175′ W, water depth 37 m. Five specimens for genetics (isolates 21161–21164, 21168).
SG-09, Cumberland West Bay; 54° 12.044′ S, 36° 34.253′ W, water depth 60 m. One specimen for genetics (isolate 21130).
Ushuaia: USH/ARC2005, St. 5, 12 m depth; five specimens for genetics (isolates 7711–7715).
Etymology: In honour of Greg Landreth, Co-Captain of the yacht Saoirse.
Description: The test is almost spherical, except for a slight flattening of the oral end compared to the more rounded adapertural end (Fig. 5). Lengths range from 0.95 to 2.54 mm (including the oral capsule), widths from 0.90 to 2.44 mm, and the length/width ratio from 1.01 to 1.07. Paratype 3 (Fig. 5d) is by far the smallest specimen (Table 3).
The oral capsule is sometimes partly obscured by a mass of detrital material (Fig. 5b, d), particularly noticeable in a specimen from Fortuna Bay (Fig. 5e). The capsule, where visible, projects only slightly beyond the test surface and bordered by a collar-like feature, measuring approximately 330 µm in the holotype and 280 µm in paratype 1. In specimens from the type locality (SG-18), the collar extends into a delicate structure that resembles an accumulation of detrital material but appears firmly attached to the collar (Fig. 6). Whether this is part of the oral capsule is not clear.
The test wall is transparent. In most of the specimens preserved in formalin, it appears slightly crumpled, although not strongly deformed. There is no sign of any surface ornamentation. The test contents are greyish and consist of small (10–20 µm) stercomata that collapse to varying extents in preserved specimens.
Molecular characteristics: Gromia landrethi (100% BV, 17 specimens sequenced) branches as sister to G. amygdaliformis, with G. brevis and G. saoirsei clustering at the base of both species (Fig. 4). The branching order of these four species is not supported by a high bootstrap value. The partial SSU rDNA sequences of G. landrethi contain 655 nucleotides and the GC content is 46%. Pairwise distances range from 0 to 0.003.
Remarks: Several more or less spherical gromiid species with some resemblance to Gromia landrethi have been described, or at least illustrated. Gromia sphaerica from the Arabian Sea is clearly distinguished from the new species by its much larger test (4.7–38 mm diameter) and the presence of multiple oral capsules (Gooday et al. 2000). Gromia sp. 4, another spherical Arabian Sea species, is closer in size to G. landrethi and has a single oral capsule, although this structure is much more prominent than in the new species. The oral capsule of G. landrethi, where clearly visible, appears to resemble that of G. pashukae in having a flat outer surface surrounded by a circular collar and surmounted by a delicate mound of detritus-like material. As noted above, the oral capsule of G. marmora is rather similar, and this Weddell Sea species also includes specimens that are more or less spherical, as well as similar in size (length 1.0–3.4 mm) to G. landrethi (Rothe et al. 2009). Genetic data, however, clearly distinguishes G. landrethi from all of the above-mentioned species.
Distribution: Currently known from Fortuna Bay, Stromness Bay and Cumberland West Bay (South Georgia) and coastal waters near Ushaia, Tierra del Fuega, Argentina.
Gromia saoirsei Gooday & Holzmann sp. nov.
Gromia saoirsei Gooday & Holzmann sp. nov.; Station SG-27, Stromness Bay. a Holotype, reg. no. NHMUK 2021.11.2.10. b Holotype; detail of apertural end showing sediment mass around the oral capsule and clear cytoplasm immediately inside the oral capsule. c Paratype 1, reg. no. NHMUK 2021.11.2.11. d Detail of Paratype 1 showing indeterminate globular structure. e Paratype 2, reg. no. NHMUK 2021.11.2.12. f Paratype 3, reg. no. NHMUK 2021.11.2.13. Scales 1 mm (a, c), 0.50 mm (b, d–f)
Gromia saoirsei Gooday & Holzmann sp. nov., unregistered specimens; Station SG-27, Stromness Bay. a–b Specimen 1 and corresponding oral capsule. c–d Specimen 2 and oral capsule. e–f Specimen 3 and oral capsule. g–j Specimen 4. g Entire test. h Test wall with finely reticulated ornamentation. i–j Detail of reticulated ornamentation, viewed using Nomarski interference optics. Scales 1 mm (a, c, e, g, h), 100 µm (b, d, f, i, j)
Diagnosis: Species of Gromia with fairly elongate, sausage-shaped test, 1.6–4.5 mm (usually 2.1–3.7 mm) long; length/width ratio typically ranging from 2.2 to 3.0. Oral capsule relatively small (diameter 108–186 µm) and low with flat outer surface. Wall transparent; delicate reticulated surface ornamentation sometimes visible.
ZooBank registration: urn:lsid:zoobank.org:act:FE52D1FB-F8FB-4867-83DF-5B1A6DFBABF1.
Etymology: Named for the yacht Saoirse, from which the samples for this study were collected. The species name is pronounced ‘Surshai’.
Type material: Station SG-27, Stromness Bay; 54° 09.372′ S, 38° 38.426′ W, water depth 136 m. The holotype (reg. no. NHMUK 2021.11.2.10) and three paratypes (reg. no. NHMUK 2021.11.2.11–13) are preserved in 10% formalin.
Other material: Station SG-27 (as above). 14 specimens for morphology, five specimens for genetics (isolates 21143–21147).
Description: The test is elongate and approximately cylindrical (Fig. 7a, c). The adapertural end is rounded and the apertural end more gently rounded and often somewhat truncated. The sides are usually more or less parallel, but the width sometimes varies. In some cases, the adapertural part of the test is somewhat inflated compared to the apertural part (Figs. 7e, f, 8a, g), although one specimen is wider at the apertural end (Fig. 8e). The length ranges from 1.58 to 4.48 mm (typically 2.17–3.65 mm), mean 2.91 ± 0.81 mm, the width from 0.55 to 1.89 mm (typically 0.83–1.48 mm), mean 1.15 ± 0.37 mm, and the length/width ratio from 2.19 to 3.72 (typically 2.25–3.02), mean 2.60 ± 0.37 (n = 18 in all cases).
The oral capsule is often obscured by a more or less globular mass of rather featureless material (Fig. 7a–c). This may be detritus and sediment collected by the organism, but in at least one case, it appears to include cytoplasm extruded from the test interior. Where visible, the capsule forms a rather small, low, flat structure (Fig. 8b, d, f). In three larger specimens, it is 160–186 µm wide and 28–35 µm high. In three smaller specimens, it is 108–110 µm wide and 26–28 µm high. The central canal is funnel-shaped, widening rapidly at the top.
The test wall is transparent, very thin and slightly crinkled in preserved specimens. The surface of one partly empty test bears a distinct system of narrow ridges forming an irregular, polygonal mesh with most individual polygons in the size range 25–55 µm, although some are smaller (Fig. 8h–j). In a few places, the mesh breaks down into a much finer and irregular pattern. In most other specimens the test contents make the wall difficult to see, but a similar surface pattern is sometimes evident, albeit fainter and less clearly developed than shown in Fig. 8i, j.
The test contents are greyish and mainly comprise small stercomata (usually 15–30 µm in diameter). Larger inclusions, notably several globular structures (Fig. 7d) and an elongate black object (Fig. 7f), are occasionally present. A distinct orange stain is often developed on the underside of the test, the result of denser iron-rich particles (xanthosomes) settling under gravity.
Molecular characteristics: Gromia saoirsei (100% BV) branches at the base of a clade containing G. brevis, G. amygdaliformis and G. landrethi (Fig. 4). However, its branching is not supported by a high bootstrap value. The partial SSU rDNA sequences of G. saoirsei contain 662 nucleotides and the GC content is 48%. Identical sequences were obtained from five specimens.
Remarks: Gromia saoirsei is more elongate than the other new species described here, and has a rather distinctive, low oral capsule that is much smaller than those of G. pashukae and G. landrethi. It is more similar to the Arctic species G. botelliformis (Gooday et al. 2021), but has a rather larger and relatively wider test (mean length 2.90 mm and length/width ratio 2.60 compared to 1.50 mm and 3.50, respectively) and is genetically distinct. Gromia schultzei (= Gromia sp. of Schultze 1875) from Norwegian fjords, is also similar in shape, but larger and more elongate (length 8–9 mm, length/width ratio − 5.7) than G. saoirsei. Allogromia marina from the Gullmar fjord (Sweden) (Nyholm and Gertz 1973), described as a foraminifera but with all the characteristics of a gromiid (Gooday et al. 2021), is similar. Smaller specimens are rounded or egg-shaped but larger ones are more elongate, as in G. saoirsei. There are no genetic data for either of these Scandinavian species and so their relationship to our new gromiid is unknown.
The inflated adapertural part of the test (most obvious in Fig. 8g) is a feature also seen in an undescribed species from the bathyal Weddell Sea (Gromia sp. 76; Fig. 3c in Rothe et al. 2011) and some specimens of Gromia pyriformis from the Arabian Sea (Gooday and Bowser 2005). However, the Weddell Sea and Arabian Sea forms are clearly more elongate (length/width ratio 4.1–4.7) and less elongate (1.40–1.90), respectively, than the new species (2.19–3.72).
An interesting feature of this species is the finely reticulated ornamentation of the test surface, clearly visible in one of our specimens using a compound microscope (Fig. 8h–j). Rather similar but less obvious patterns are present in G. botelliformis (Fig. 9e in Gooday et al. 2021) and in the deep Weddell Sea species G. melinus (Fig. 5e in Rothe et al. 2009). Aranda da Silva et al. (2006) and Aranda da Silva and Gooday (2009) mention a ‘honeycomb-like structure’ in Gromia sp. 2 from the Arabian Sea, although this reticulated pattern has a larger mesh size (about 100–160 µm) than that seen in G. saoirsei (25–55 µm). It also appears to be a more complicated structure in the Arabian Sea species, comprising at least two layers of polygons, of which only the outer is exposed on the test surface (Aranda da Silva 2005).
Gromia amygdaliformis Gooday & Holzmann sp. nov. a–b Unregistered specimens from Station SG-04, Fortuna Bay. c–f Type specimens, Station SG-18, Stromness Bay. c Holotype, reg. no. NHMUK 2021.11.2.14. d Paratype 1, reg. no. NHMUK 2021.11.2.15. e Paratype 2, reg. no. NHMUK 2021.11.2.16. f Paratype 3, reg. no. NHMUK 2021.11.2.17. Scales 1 mm (a), 0.50 mm (b–f)
Distribution: Currently known only from Stromness Bay in South Georgia.
Gromia amygdaliformis Gooday & Holzmann sp. nov.
Gromia amygdaliformis Gooday & Holzmann sp. nov.; Station SG-18, Stromness Bay. a–d Unregistered specimens and corresponding oral capsules. e–h Type specimens, oral capsules. e Holotype, reg. no. NHMUK 2021.11.2.14. f Paratype 1, reg. no. NHMUK 2021.11.2.15. g Paratype 2, reg. no. NHMUK 2021.11.2.16. h Paratype 3, reg. no. NHMUK 2021.11.2.17. Scales 500 µm (a, c), 100 µm (b, d–h)
Diagnosis: Species of Gromia with more or less elongate, streamlined, almond-shaped test; widest behind mid-point with fairly broadly rounded adapertural end and tapering toward small oral capsule at the more narrowly rounded apertural end. Length typically 1.0–1.4 mm; length:width ratio typically 1.5–1.8. Oral capsule small (70–130 µm diameter), more or less subconical, merging with test outline. Wall transparent and devoid of obvious features.
ZooBank registration: urn:lsid:zoobank.org:act:A257CDA5-B2A4-482E-A148-BCC3C310AE67.
Type material: Station SG-18, Stromness Bay; 54° 10.783′ S, 36° 39.979′ W, water depth 102 m. The holotype (reg. no. NHMUK 2021.11.2.14) and three paratypes (reg. no. NHMUK 2021.11.2.15–17) are preserved in 10% formalin.
Other material: Station SG-18 (as above); 12 specimens for morphology, two specimens for genetics (isolates 21139, 21140).
Station SG-04, Fortuna Bay; 54° 07.658′ S, 36° 47.598′, 135 m water depth; 18 specimens for morphology.
Station SG-28, near Grass Island, Stromness Bay; 54° 09.861′ S, 36° 40.175′ W, 37 m water depth; three specimens for genetics (isolates 21165–21167).
Etymology: Latin—amygdalus, almond, referring to shape of the test.
Description: The test is streamlined, more or less almond-shaped, widest behind the mid-point with a broadly rounded adapertural end and tapering towards the narrowly rounded (sometimes almost pointed) apertural end (Fig. 9). Specimens (n = 24) from the type locality have the following dimensions: length 0.85–1.51 mm (typically 1.11–1.47 mm), mean 1.20 ± 0.19 mm; width 0.52–0.84 mm, mean 0.70 ± 0.19. The length/width ratio ranges from 1.37 to 2.18 (usually 1.67–1.97), mean 1.72 ± 0.14. Specimens (n = 18) from Station SG-04 in Fortuna Bay tend to be somewhat smaller: length 0.83 to 1.40 mm (typically 0.91–1.23 mm), mean 1.10 ± 0.12 mm; width 0.54–0.77 mm (typically 0.62–0.72 mm), mean 0.66 ± 0.06; length/width ratio 1.42–1.80 (usually 1.54–1.78), mean 1.65 ± 0.10.
The oral capsule generally forms a low mound or truncated cone that merges with the test outline (Fig. 10). The top is often rather flattened, and in the holotype the cone has a stepped appearance (Fig. 10e). The capsule measures 130 µm wide and 37 µm high in the holotype, while in the paratypes it is 98–115 µm wide and 27–35 µm high. Two smaller specimens from the type locality (length = 0.91 µm) have correspondingly smaller oral capsules, 74 and 71 µm wide and 20 and 47 µm high, respectively (Fig. 10a–d). A central canal, widening towards the upper end, is sometimes visible.
The thin, transparent test wall has a reflective surface and no obvious ornamentation. It tends to crumple somewhat in preserved specimens. The test contents are brownish or greyish and consist largely of small round stercomata. An orange, ferruginous stain is often present.
Molecular characteristics: Gromia amygdaliformis (100% BV, five specimens sequenced) branches between G. landrethi and G.brevis (Fig. 4), but this branching is not supported by a high bootstrap value. The partial SSU rDNA sequences of G. amygdaliformis contain 655–670 nucleotides and the GC content is 48%. Pairwise distances range from 0 to 0.557.
Remarks: This species has a distinctively-shaped test, resembling a somewhat inflated almond, tapering at its apertural end towards a small oral capsule. Gromia brevis from the Nuuk fjord system of western Greenland (Gooday et al. 2021) is rather similar, but the test is less streamlined. It is also smaller than that of G. amygdalifomis (0.30–0.67 mm compared to 0.85–1.50 mm, respectively), but has a relatively larger oral capsule. A number of undescribed grape-shaped species from the Arabian Sea (Aranda da Silva et al. 2006; Aranda da Silva and Gooday 2009) and the Black Sea (Pavel et al. 2021) have tests that are more nearly oval and again less streamlined than that of the new species.
Distribution: Currently known only from Stromness Bay, South Georgia.
Gromia cedhageni Gooday et Holzmann sp. nov.
Figure 11
Gromia cedhageni Gooday & Holzmann sp. nov., type specimens; Falklands Station FK-08. a Holotype, reg. no. NHMUK 2021.11.2.24. b Holotype, oral capsule. c Paratype 1, reg. no. NHMUK 2021.11.2.25. d Paratype 1, oral capsule. e Paratype 2, reg. no. NHMUK 2021.11.2.26. f Paratype 3, reg. no. NHMUK 2021.11.2.27. Scales 0.25 mm (a, c, e, f), 50 µm (b, d)
Diagnosis: Species of Gromia with small, more or less oval test, 0.85–0.95 mm long; length/width ratio of 1.20–1.40. Oral capsule forming small nipple-shaped structure, 100–140 µm wide and ~ 40 µm high. Wall transparent with no obvious ornamentation. Test contents predominately orange and very finely granular.
ZooBank registration: urn:lsid:zoobank.org:act:ED931ABF-5A40-43F6-8D35-F57D33288D80.
Type material: Station FK-08; 51° 48.020′ S, 58° 57.892′ W, intertidal. The holotype (reg. no. NHMUK 2021.11.2.24) and three paratypes (reg. no. NHMUK 2021.11.2.25–27) are preserved in 10% formalin.
Other material: Station FK-08 (as above). Six specimens for genetics (isolates 21182–21184, 21186–21188).
Etymology: In honour of Dr. Tomas Cedhagen, who has made important contributions to our knowledge of monothalamid foraminifera and gromiids.
Description: The test is broadly ovoid (egg-shaped), fairly symmetrical in the holotype and paratype 1 (Fig. 11a, c), slightly wider in front of the mid-point in paratype 2 (Fig. 11e), and inflated in front of the mid-point in paratype 3 (Fig. 11f). The four specimens range in length from 0.85 to 0.90 mm, and width from 0.63 to 0.71 mm, with a length/width ratio of 1.20–1.40 (Table 4). The oral capsule is at least partially visible in three specimens, measuring 100–140 µm wide and 38–40 µm high. It forms a fairly low, mound-like structure in the holotype (Fig. 11b), whereas in paratypes 2 and 3 it is relatively higher and narrower (Fig. 11d). The contents of the central canal of the holotype and paratype 1 merge into a coherent mass of detrital material that extends well beyond the capsule itself. In paratype 3, a larger accumulation of similar material completely obscures the oral capsule (Fig. 11f).
The wall is thin and transparent without any surface ornamentation. The test contents are mainly orange, particularly at the apertural end, but with a greyish tinge towards the adapertural end. They are finely granular on a scale of a few microns (< 5 µm) and appear to consist of very small stercomata. A few larger, brownish particles are also usually present.
Molecular characteristics: Gromia cedhageni (100% BV) branches as sister to G. pashukae, the branching is highly supported (98%BV) (Fig. 4). The partial SSU rDNA sequences of G. cedhageni contain 643 nucleotides and the GC content is 46%. The obtained sequences of six specimens are identical.
Remarks: This ovoid gromiid is quite similar in shape to a specimen identified as G. oviformis from McMurdo Sound (Antarctica) (Burki et al. 2002), and Gromia sp. of Goldstein et al. (2011) from Florida Keys (USA). However, it is smaller than the former (0.8–0.9 mm long compared with − 2.5 mm), larger than the latter (− 0.35 mm), and not related genetically to any of the gromiids identified as G. oviformis (including that of Burki et al. 2002) for which genetic data are available (Fig. 4).
A distinctive feature of G. cedhageni is that the test contents are very fine-grained, unlike the larger stercomata that fill the interiors of most gromiids, and predominantly orange in colour. This colour is not to be confused with the ferruginous stain often seen within the tests of larger gromiids. However, it may be a consequence of the food being consumed by the gromiids at this particular locality (Jepps 1926; Henderson, 2021), rather than being a morphological feature.
Distribution: Currently known only from Darwin Cove, East Falkland.
Gromia psammophila Gooday and Holzmann sp. nov.
Gromia psammophila Gooday & Holzmann sp. nov., type specimens; Station FK-07. a Holotype, reg. no. NHMUK 2021.11.2.18. b Paratype 1, reg. no. NHMUK 2021.11.2.19. c Paratype 2, reg. no. NHMUK 2021.11.2.20. d Paratype 3, reg. no. NHMUK 2021.11.2.21. e Paratype 4, reg. no. NHMUK 2021.11.2.22. f Paratype 5, reg. no. NHMUK 2021.11.2.23. Scales 0.25 mm
Gromia psammophila Gooday & Holzmann sp. nov., Station FK-07. a–g Oral capsules. a Holotype, reg. no. NHMUK 2021.11.2.18. b Paratype 1, reg. no. NHMUK 2021.11.2.19. c Paratype 2, reg. no. NHMUK 2021.11.2.20. d Paratype 3, reg. no. NHMUK 2021.11.2.21. e Paratype 4, reg. no. NHMUK 2021.11.2.22. f Paratype 5, reg. no. NHMUK 2021.11.2.23. g Unregistered specimen. h Test contents with reflective mineral particles. Scales 50 µm (a–g), 100 µm (h)
Diagnosis: Species of Gromia with subcylindrical test, 0.78–1.09 mm long; length/width ratio 1.65–1.94. Oral capsule small (< 100 µm wide), forming stepped and truncated cone. Wall transparent with no obvious ornamentation. Test contents pale, finely granular, but including large numbers of mineral grains creating speckled appearance.
ZooBank registration: urn:lsid:zoobank.org:pub:0E6E567C-1DCC-4F01-9C3C-C040AC6C81FA.
Type material: Station FK-08, Teal Inlet; 51° 33.800′ S, 58° 25.432′ W, intertidal. The holotype (reg. no. NHMUK 2021.11.2.18) and three paratypes reg. no. (NHMUK 2021.11.2.19–23) are preserved in 10% formalin.
Other material: Station FK-07 (as above). Ten specimens for morphology, six specimens for genetics (isolates 21175, 21178, 21180–21181).
Chile, Rio Amarillo; 53° 27.683′ S; 70° 58.417′ W. Four specimens for genetics (isolates 5794, 5796, 5893, 5894).
Etymology: Greek, derived from psámmos (‘sand’) and phílos (‘friend’).
Description: The test is approximately subrectangular to ovoid with a broadly rounded adapertural end and a more or less truncated apertural end (Fig. 12). In the holotype (the longest specimen) the adapertural end is slightly inflated (Fig. 12a), whereas Paratype 3 is ovoid and widest towards the apertural end (Fig. 12d). The length of the test varies from 0.78 to 1.09 mm, the width from 0.40 to 0.60 mm, and the length/width ratio from 1.65 to 1.94.
The oral capsule forms a small, truncated, roughly conical structure, usually with an uneven, stepped profile (Fig. 13a–g). It measures 27–37 µm high, 60–95 µm wide at the base, and 36–50 µm wide at the top (n = 7). Where clearly visible, the central canal tapers upwards from a relatively wide base, before widening again shortly before reaching the top of the capsule.
The test wall is transparent and devoid of obvious ornamentation. The contents are pale yellowish-brown, a darker shade in some specimens than in others, and speckled with numerous mineral grains (Fig. 12). These are often fairly evenly distributed throughout the interior, but also tend to accumulate on the underside of specimens after they have been lying in a dish for some time, presumably as a result of gravitational settling. Many of the grains are whitish or transparent (possibly quartz) but some are darker, either brownish or black (Fig. 13h). Most are 18–28 µm in size, but some of the darker grains are elongate and have maximum dimensions > 30 µm. They are embedded within a mass of very small (-10 µm), pale stercomata-like granules.
Molecular characteristics: Gromia psammophila specimens from Falklands and Chile form a highly supported (98% BV) group that branches as sister to a clade containing undescribed Gromia specimens from South Georgia and Greenland. However, this branching is not supported by a high BV. The partial SSU rDNA sequences of G. psammophila contain 608 nucleotides and the GC content is 47%. The obtained sequences of ten specimens are identical.
Remarks: This relatively small intertidal gromiid has several distinctive features. The subrectangular (subcylindrical in three dimensions) shape of the test is unusual. The only similar species is Gromia sp. 6 of Aranda da Silva et al. (2006) and Aranda da Silva and Gooday (2009), but this tapers towards the apertural end and is much larger (several millimetres long) than G. psammophila. The stepped profile of the oral capsule is also notable, although the capsule has a similar shape in at least one specimen of G. amygdaliformis (see also Fig. 6a in Henderson 2021). However, the most characteristic feature of this new species is the presence of numerous small mineral particles that impart a speckled appearance to the pale test interior. Larger gromiids often accumulate considerable amounts of foreign material, including mineral particles, within their test (e.g., Jepps 1926; pp. 18–19 in Arnold, 1972), but these are usually obscured by stercomata. Henderson (2021) recently described gromiids from Scotland that had ingested numerous dark magnetite particles, rendering them magnetic. In the case of G. psammophila, the mineral particles are mainly light in colour and not obviously magnetic. However, like those of Henderson (2021), they tend to settle to the undersides of tests, indicating that they are denser than the finely granular matrix in which they are embedded.
Distribution: Currently known only from Teal Inlet, East Falkland and Rio Amorillo (Chile).
Gromia sp.
Material: SG-07, Cumberland East Bay; 54° 16.052′ S; 36° 26.227′ W, 250 m depth. Three specimens used for genetics (isolates 21126, 21171, 21172).
Description: (based on a photograph of the freshly collected specimen before preservation). The test is elongate and slender, length 2.29 mm, width decreasing from 0.21 mm near the apertural end, but tapering to 0.13 near the adapertural end.
Remarks: The single specimen that was photographed in a fresh state (isolate 21126) was more elongate than any of the others in our South Georgia collection (Online Resource 4).
Distribution: South Georgia, Cumberland East Bay.
Molecular characterization
The newly described species (Fig. 4) are sustained by strong bootstrap values (96–100%). Two species (G. pashukae and G. cedhageni) build a strongly supported sister clade. Gromia species from South Georgia and the Falklands branch with deep-sea species (Gromia sp. 1, Gromia sp. 6, Gromia sp. 8, G. winnetoui, G. marmorea, G. melinus, G. sphaerica) as well as with shallow- water species from Arctic regions (G. brevis, G. cucumiformis, G. botelliformis, Gromia spp.), the Antarctic shelf (Gromia sp. 7) and the Mediterranean (G. oviformis).
Relationships between these groups are not sustained by bootstrap values except for three clades. One of these clades consists of Gromia sp. 1, G. winnetoui and Gromia sp. (MT906519, MT906521, from Svalbard and Greenland, respectively) and is moderately supported (73%BV), another includes G. botelliformis, G. marmorea and Gromia sp. 2 (86%BV), and the third comprises the undescribed elongate Gromia sp. from South Georgia (MZ468155-157) and Gromia sp. from Greenland (MT906549-551) (100%BV). Complete SSU rDNA sequences would be necessary to establish more robust phylogenetic relationships.
See Online Resource 1 for references linked to species mentioned above that were not described in this paper.
Discussion
Our new gromiid species can be recognised fairly easily based on their morphological characteristics. Like those found in other restricted geographical areas (e.g., Gooday et al. 2005, 2021), they are distinguished mainly by the overall shape of the test. Other characters, such as the thickness and ornamentation (if any) of the test wall, the shape and size of the oral capsule (but see Fig. 6 in Henderson 2021 for an example of capsule shape variation within a species), the number of oral capsules and the nature of the test contents, may also be important. In addition to test shape, distinctive features of the gromiids described here include reticulated surface ornamentation (G. saoirsei), a wide oral capsule with a flat outer surface and a distinct rim (G. pashukae and G. landrethi), and fine-grained test contents (G. cedhageni and G. psammophila, speckled with mineral grains in the latter). When species are compared more widely, however, most conform to a relatively small set of repetitive morphotypes (e.g., spherical, grape-shaped, pear-shaped, sausage-shaped). As a result, species from distant localities often look similar. This is a common issue among many marine and non-marine protistan taxa, including freshwater monothalamid foraminifera (Siemensma et al. 2021), the rotaliid foraminiferal genus Ammonia (Hayward et al. 2021), testate amoebae (Kosakyan et al. 2015; Singer et al. 2018), naked amoebae (De Jonckheere 2006) and flagellates (Grossmann et al. 2016). Given the relatively limited taxonomic value of gromiid test morphology, it is particularly important for species descriptions to include genetic as well as morphological data.
Evidence gathered over recent decades has shown that species of the genus Gromia are considerably more diverse than previously realised (Gooday et al. 2000, 2021; Aranda da Silva et al. 2006; Aranda da Silva and Gooday 2009; Rothe et al. 2009, 2011; Goldstein et al. 2011; Pavel et al. 2021). Our material from South Georgia and the Falkland Islands reinforces this conclusion. Gromiids are known from shallow and deep Antarctic waters (Bowser et al. 1996; Pawlowski et al. 2005; Rothe et al. 2009, 2011), but have not been reported before from either of these Southern Hemisphere islands. The present study reveals them to be a common and visually conspicuous component of the benthic macrofauna in the sub-Antarctic fjords of South Georgia, as they are also in sub-Arctic and Arctic fjords of Greenland, Svalbard, the White Sea (Gooday et al. 2005, 2021). Further sampling around South Georgia, particularly along the southern coastline and in deeper offshore waters, would probably yield additional species. Although time available for sampling in the Falkland Islands was more limited. Two new species, both smaller and more delicate than those from South Georgia, were discovered at muddy intertidal localities, suggesting that gromiids may be quite diverse in this region as well.
There is little reliable information about the geographical ranges of gromiid species. Gromia oviformis is reported from localities on different continents but this is almost certainly a species complex rather than a single cosmopolitan species. Genetic data show that G. cucumiformis and several undescribed species have broad ranges (up to > 3600 km) in the Arctic (Gooday et al. 2021). In the Southern Hemisphere, Gromia melinus occurs at two sites 14 km apart but at different depths (3103 and, 4392 m) off Kap Norvegica in the eastern Weddell Sea, and two undescribed species are separated by distances of 707 km (2600, 4800 m depth) and 1707 km (2600, 3103 m) in the western Weddell Sea, although in the latter case ranges are not supported genetically (Rothe et al. 2011). Two of the species described in the present study, Gromia landrethi and G. psammophila, span ranges of 2034 km (Stromness Bay in South Georgia to Ushaia) and 876 km (Teal Inlet in East Falkland to southern Chile), respectively. All other gromiid species are currently known from single localities. Whether this is because they are endemic or simply a consequence of undersampling is unclear, although the latter interpretation seems more likely. Understanding the biogeography, as well as the biodiversity, of these important but understudied rhizarians clearly presents major challenges, particularly in the Southern Ocean, a region where complex tectonic and climatic factors have shaped the evolution and distribution of species over geological time scales (Majewski et al. 2021).
Data availability
All data generated and analysed during this study are included in this published article. Type specimens are deposited in the Natural History Museum, London, under registration numbers NHMUK 2021.11.2.1–27.
References
Adl SM, Bass D, Lane CE, Lukes J et al (2019) Revisions to the classification, nomenclature, and diversity of eukaryotes. J Eukaryot Microbiol 66:4–119. https://doi.org/10.1111/jeu.12691
Aranda da Silva AAS, Gooday AJ (2009) Large organic-walled Protista (Gromia) in the Arabian Sea: density, diversity, distribution and ecology. Deep-Sea Res II 56:422–433. https://doi.org/10.1016/j.dsr2.2008.12.027
Aranda da Silva AAS, Pawlowski J, Gooday AJ (2006) High diversity of deep-sea Gromia from the Arabian Sea revealed by small subunit rDNA sequence analysis. Mar Biol 148:769–777. https://doi.org/10.1007/s00227-005-0071-9
Aranda da Silva AAS (2005) Benthic protozoan community attributes in relation to environmental gradients in the Arabian Sea. PhD dissertation. University of Southampton
Arnold ZM (1972) Observations on the biology of the protozoan Gromia oviformis Dujardin. Univ Calif Publ Zool 100:1–144
Arnold ZM (1982) Shell-wall lamination in Gromia oviformis Dujardin. J Foram Res 12(4):298–316
Atkinson A, Whitehouse MJ, Priddle J, Cripps GC, Ward P, Brandon MA (2001) South Georgia, Antarctica: a productive, cold water, pelagic ecosystem. Mar Ecol Prog Ser 216:279–308. https://doi.org/10.3354/meps07498
Bowser SS, Marko M, Bernhard JM (1996) Occurrence of Gromia oviformis in McMurdo Sound. Antarct J US 31:122–124
Burki F, Berney C, Pawlowski J (2002) Phylogenetic position of Gromia oviformis Dujardin inferred from nuclear-encoded small subunit ribosomal DNA. Protist 153:251–260
Cedhagen T, Aungtonya C, Banchongmanee F, Sinniger FA, Pawlowski J (2013) Gromiids and monothalamous foraminiferans (Rhizaria) from the Andaman Sea, Thailand—taxonomic notes. Phuket Mar Biol Cent Res Bull 72:1–17
De Jonckheere JF (2006) Isolation and molecular identification of free-living amoebae of the genus Naegleria from Arctic and sub-Antarctic regions. Eur J Protist 42:115–123. https://doi.org/10.1016/j.ejop.2006.02.001
Dujardin F (1835) Recherches sur les organismes inférieurs. Ann Sci Nat, Zool Ser 2, 4: 343–377
Geprägs P, Torres ME, Mau S, Kasten RM, Bohrmann G (2016) Carbon cycling fed by methane seepage at the shallow Cumberland Bay, South Georgia, sub-Antarctica. Geochem, Geophys Geosyst 17:1401–1418. https://doi.org/10.1002/2016GC006276
Gilpin LC, Priddle J, Whitehouse MJ, Savidge G, Atkinson A (2002) Primary production and carbon uptake dynamics in the vicinity of South Georgia—balancing carbon fixation and removal. Mar Ecol Prog Ser 242:51–62
Goldstein ST, Bowser SS, Richardson EA (2011) Towards understanding the biodiversity of gromiids: the fine structure of Gromia sp. from Zane Grey Creek Florida Keys. Microsc Microanal 17(Suppl. 2):348–349. https://doi.org/10.1017/S1431927611002613
Gooday AJ, Bowser SS (2005) The second species of Gromia (Protista) from the deep sea: its natural history and association with the Pakistan margin oxygen minimum zone. Protist 156:113–126. https://doi.org/10.1016/j.protis.2004.11.002
Gooday AJ, Bowser SS, Bernhard JM (1996) Benthic foraminiferal assemblages in Explorer’s Cove, Antarctica: a shallow water site with deep-sea characteristics. Prog Oceanogr 37:219–267
Gooday AJ, Bowser SS, Bett BJ, Smith CR (2000) A large testate protist, Gromia sphaerica sp. nov. (Order Filosea), from the bathyal Arabian Sea. Deep-Sea Res II 47:55–73. https://doi.org/10.1016/S0967-0645(99)00100-9
Gooday AJ, Bowser SS, Cedhagen T, Cornelius N, Hald M, Korsun S, Pawlowski J (2005) Monothalamous foraminiferans and gromiids (Protista) from western Svalbard: a preliminary survey. Mar Biol Res 1:290–310. https://doi.org/10.1080/1745100051009150
Gooday AJ, Holzmann M, Goetz E, Cedhagen T, Korsun S, Pawlowski J (2021) Three new species of Gromia (Protista, Rhizaria) from western Greenland fjords. Polar Biol 44:1037–1053. https://doi.org/10.1007/s00300-021-02858-9
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. https://doi.org/10.1093/molbev/msp259
Graham AGC, Fretwell PT, Larter RD, Hodgson DA, Wilson CK, Tate AJ, Morris P (2008) New bathymetric compilation highlights extensive paleo-ice sheet drainage on the continental shelf, South Georgia, sub-Antarctica. Geochem Geophys Geosyst 9:701. https://doi.org/10.1029/2008GC001993
Grossmann L, Bock C, Schweikert M, Boenigk J (2016) Small but manifold – hidden diversity in ‘Spumella-like flagellates.’ J Eukaryot Microbiol 63:419–439. https://doi.org/10.1111/jeu.12287
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Hayward BW, Holzmann M, Pawlowski J, Parker JH, Kaushik T, Toyofuku MS, Tsuchiya M (2021) Molecular and morphological taxonomy of living Ammonia and related taxa (Foraminifera) and their biogeography. Micropaleontology 67:109–313. https://doi.org/10.47894/mpal.67.2-3.01
Hedley RH (1958) Confusion between Gromia oviformis and Allogromia ovoidea. Nature 182:1391–1392
Hedley RH, Bertaud WS (1962) Electron-microscopic observations of Gromia oviformis (Sarcodina). J Protozool 9:79–87
Henderson Z (2021) Magnetite-bearing Gromia protists of the Lorn area, Scotland. Quekett J Microsc 44:109–120
Heron-Allen EA, Earland A (1922) Protozoa, Part 2. Foraminifera. Natural History Rep Br Antarctic (“Terra Nova”) Expedition 6:25–268
Hodgson DA, Graham AGC, Griffiths HJ, Roberts SJ, Cofaigh CÓ, Bentley MJ, Evans DJA (2014) Glacial history of sub-Antarctic South Georgia based on submarine geomorphology of its fjords. Quat Sci Rev 89:129–147. https://doi.org/10.1016/j.quascirev.2013.12.005
Holzmann M, Gooday AJ, Siemensma F, Pawlowski J (2021) Review: freshwater and soil foraminifera a story of long forgotten relatives. J. Foram. Res. 51(4):318–331. https://doi.org/10.2113/gsjfr.51.4.31801-10-2021
Jepps MW (1926) Contribution to the study of Gromia oviformis Dujardin. Q J Microsc Sci 70:701–719
Korb RE, Whitehouse M (2004) Contrasting primary productivity regimes around South Georgia, Southern Ocean: large blooms versus high nutrient, low chlorophyll waters. Deep-Sea Res II 51:721–738. https://doi.org/10.1016/j.dsr.2004.02.006
Kosakyan A, Gomaa F, Lara E, Lahr DJG (2015) Current and future perspectives on the systematics, taxonomy and nomenclature of testate amoebae. Europ J Protistol 55:105–117. https://doi.org/10.1016/j.ejop.2016.02.001
LeDuc D, Rowden AA (2017) Not to be sneezed at: does pollen from forests of exotic pine affect deep ocean trench ecosystems? Ecosystems 21:237–247. https://doi.org/10.1007/s10021-017-0146-8
Lefort V, Longueville JE, Gascuel O (2017) SMS: smart model selection in PhyML. Mol Biol Evol 34:2422–2424. https://doi.org/10.1093/molbev/msx149
Longet D, Burki F, Flakowski J, Berney C, Polet S, Fahrni J, Pawlowski J (2004) Multigene evidence for close evolutionary relations between Gromia and Foraminifera. Acta Protozool 43:303–311
Majewski W, Holzmann M, Gooday AJ, Majda A, Mamos T, Pawlwoski J (2021) Cenozoic climatic changes drive evolution and dispersal of coastal benthic foraminifera in the Southern Ocean. Sci Rep 11:19869. https://doi.org/10.1038/s41598-021-99155-6
Matz MV, Frank TM, Marshall NJ, Widder EA, Johnson S (2008) Giant deep-sea protist produces bilaterian-like traces. Curr Biol 18:1849–1854. https://doi.org/10.1016/j.cub.2008.10.028
Meredith MP, Brandon MA, Murphy EJ, Trathan PN et al (2005) Variability in hydrographic conditions to the east and northwest of South Georgia, 1996–2001. J Mar Syst 53:143–167. https://doi.org/10.1016/j.jmarsys.2004.05.005
Norman AM (1892) Museum Normanianum, or a catalogue of the Invertebrata of Europe, and the Arctic and North Atlantic Oceans, which are contained in the collection of the Rev. Canon A.M. Norman. VII. Spongozoa. VIII. Rhizopoda, Thomas Caldcleugh, Durham, p 21
Nyholm K-G, Gertz I (1973) To the biology of the monothalamous foraminifer Allogromia marina n. sp. Zoon 1:89–93
Orsi AH, Whitworth TW, Nowlin WD (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res I 42:641–673
Otley H, Munro G, Clausen A, Ingham B (2008) Falkland Islands State of the Environment Report 2008. Falkland Islands Government and Falklands Conservation, Stanley
Pavel AB, Menabit S, Pop C (2021) New records of soft-shelled monothalamous Foraminifera and gromiids on the Romanian Black Sea shelf. Biologia 76:2241–2251
Pawlowski J, Fahrni J, Guiard J, Conlan K, Hardecker J, Habura A, Bowser SS (2005) Allogromiid foraminifera and gromiids from under the Ross Ice Shelf: morphological and molecular diversity. Polar Biol 28:514–522. https://doi.org/10.1007/s00300-005-0717-6
Platt HM (1979) Sedimentation and the distribution of organic matter in a sub-Antarctic marine bay. Estuar Coast Shelf Sci 9:51–63. https://doi.org/10.1016/0302-3524(79)90006-9
Rhumbler L (1904) Systematische Zusammenstellung der recenten Reticulosa. Arch Protistenk 3:181–294
Römer M, Torres M, Kasten S, Kuhn G, Graham AGC, Mau S, Little CTS et al (2014) First evidence of widespread active methane seepage in the Southern Ocean, off the sub-Antarctic island of South Georgia. Earth Planet Sci Lett 403:166–177. https://doi.org/10.1016/jepsl.2014.06.036
Rothe N, Gooday AJ, Cedhagen T, Fahrni J, Hughes JA, Page A, Pearce RA, Pawlowski J (2009) Three new species of deep-sea Gromia (Protista, Rhizaria) from the bathyal and abyssal Weddell Sea, Antarctica. Zool J Linn Soc 157:451–469
Rothe N, Gooday AJ, Cedhagen T, Hughes JA (2011) Biodiversity and distribution of the genus Gromia (Protista, Rhizaria) in the deep Weddell Sea (Southern Ocean). Pol Biol 34:69–81. https://doi.org/10.1007/s00300-010-0859-z
Schulze FE (1875) Zoologischen Ergebnisse der Nord-seefahr vom 21 Juli bis 9 September, 1872. I. Rhizopoden. Jahresbericht Der Kommission Zur Wissenschaftlichen Untersuchung Der Deutschen Meere in Kiel 1872–1873:97–114
Sergeeva N, Ṻrkmez D, Dovgal IV, Sezgin M (2017) Protists (Ciliophora, Gromiida, Foraminifera) in the Black Sea meiobenthic communities. J Black Sea/medit Environ 23:121–155
Siemensma F, Holzmann M, Apothéloz-Perret-Gentil L, Clauß S, Voelcker E, Bettighofer S, Roshan SK, Walden S, Dumack K, Pawlowski J (2021) Broad sampling of monothalamids (Rhizaria, Foraminifera) gives further insight into diversity of non-marine Foraminifera. Eur J Protistol 77:125744. https://doi.org/10.1016/j.ejop.2020.125744
Sierra R, Matz MK, Aglyamova G, Pillet L, Decelle J, Not F, de Vargas C, Pawlowski J (2013) Deep relationships of Rhizaria revealed by phylogenomics: a farewell to Haeckel’s Radiolaria. Mol Phylogenet Evol 67:53–59. https://doi.org/10.1016/j.ympev.2012.12.011
Singer D, Kosakyan A, Seppey CVW, Pillonel A, Fernández LD, Fontaneto D, Mitchell EAD, Lara E (2018) Environmental filtering and phylogenetic clustering correlate with the distribution patterns of cryptic protist species. Ecology 99:904–914. https://doi.org/10.1016/j.ejop.2014.11.004
Acknowledgements
Sampling in South Georgia would not have been possible without the help and dedication of Keri Pashuk and Greg Landreth with their SRV Saoirse. We also thank Paul Brickle (SAERI) for help in organizing fieldwork in the Falklands and South Georgia, Martin Collins and other British Antarctic Survey staff at the King Edward Point Laboratory for making available laboratory facilities and equipment on South Georgia, and Witold Szczuciński and Piotr Rozwalak for assisting with sampling in South Georgia. We are grateful to Sergei Korsun and an anonymous reviewer for comments and suggestions that helped to improve the manuscript.
Funding
Fieldwork for this study was supported by a grant from the Polish National Science Centre, NCN-2018/31/B/ST10/02886, to Wojciech Majewski and Swiss National Science Foundation grant 31003A_179125, to Jan Pawlowski. The molecular work was supported by Schmidheiny Foundation grant (MH).
Author information
Authors and Affiliations
Contributions
WM was responsible for organising and leading the overall South Georgia/Falklands campaign, obtaining funding, planning the sampling strategy in South Georgia, and preparing Fig. 1. JP provided additional funding. JP, MH and AJG conceived the study and picked gromiids from the samples. AJG undertook the descriptive work, photography, drafted the manuscript, and compiled most of the the figures. MH was responsible for DNA extraction, amplification and sequencing, carrying out the phylogenetic analysis, preparing Fig. 4, and writing the genetic parts of the text. All authors participated in field work and provided edits to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial or personal interests that could have influenced this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gooday, A.J., Holzmann, M., Majewski, W. et al. New species of Gromia (Protista, Rhizaria) from South Georgia and the Falkland Islands. Polar Biol 45, 647–666 (2022). https://doi.org/10.1007/s00300-022-03017-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03017-4