Abstract
Southernmost South America provides significant wintering habitats for migrant shorebirds, most of which breed in the High Arctic tundra. Helminth species parasitizing these migratory birds have been well studied in North America; however, in South America they are poorly known. As part of an ongoing research on the helminth fauna from Patagonian birds in Argentina, we report Notocotylus chionis and Notocotylus sp. (Trematoda: Notocotylidae) parasitizing three shorebird species, the Nearctic migrants Calidris fuscicollis (WRSA) and Calidris bairdii (BASA) (Scolopacidae), and the Neotropical Charadrius falklandicus (TBPL) (Charadriidae). We provide a new morphological description of N. chionis considering that the previous one reported from the snowy sheathbill on Subantarctic islands are incomplete and based on few specimens. We also provided a morphometric characterization of Notocotylus sp. We obtained molecular data which confirmed the identification of specimens recovered from WRSA and TBPL as N. chionis. Phylogenetic analysis based on 28S ribosomal DNA sequences was performed. The results placed N. chionis close to other Patagonian species native to South America (i.e. N. primulus). Notocotylus chionis was found previously in the snowy sheathbill which inhabits in coasts of southern South America, Antarctic Peninsula and surrounding islands. Present finding in resident birds (TBPL) allows us to hypothesize that N. chionis is a Neotropical species whose life cycle is being completed in southern South America and Subantarctic islands and represents a valuable contribution to the knowledge of parasite diversity in the austral subpolar region of the western hemisphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Notocotylidae Lühe 1909 is a large, globally distributed group of digeneans parasitic in birds and mammals (Barton and Blair 2005). Its type genus Notocotylus Diesing, 1839 has a cosmopolitan distribution and includes from 48 to 63 valid species according to different authors (Kinsella and Tkach 2005; Boyce et al. 2012; Izrailskaia et al. 2019). Members of the genus are characterized by a high level of overall morphological uniformity with only a few differentiating characters traditionally used to distinguish between species. In addition, at least some species of Notocotylus are characterized by substantial, but insufficiently studied intraspecific variability of morphometric characteristics. Apparently, Notocotylus spp. demonstrate a greater specificity to the snail intermediate hosts than to avian definitive hosts (Stunkard 1959; Gonchar et al. 2019). All of this makes identification of Notocotylus spp. difficult.
The earliest record of Notocotylus in southern South America was the description of Notocotylus chionis Baylis 1928 from the snowy sheathbill Chionis alba Gmelin 1789 from Patagonia, Argentina by Baylis (1928) who did not indicate the exact geographic locality, or the number of specimens examined. Then, Jones and Williams (1968) reported this species from the same host in the South Orkney Islands. Other records of the genus in Argentina include Notocotylus tachyeretis Duthoit 1931 from the steamer duck Tachyeres patachonicus (King 1831), Notocotylus attenuatus (Rudolphi 1809) parasitizing the silver teal Spatula versicolor (Vieillot 1816) and the black-necked swan Cygnus melancoryphus (Molina 1782) in Buenos Aires Province, Notocotylus gibbus (Mehlis 1846) in the white-winged coot Fulica leucoptera Vieillot 1817 in Buenos Aires Province, and Notocotylus imbricatus (Looss 1893) in the Chiloe wigeon Mareca sibilatrix (Poeppig 1829) in Río Negro Province (Fernandes et al. 2015). Notocotylus biomphalariae Flores and Brugni 2005 was described from an experimental infection of chicks by metacercariae after the discovery of cercariae in Biomphalaria peregrina (Gastropoda) in the lakes Nahuel Huapi and Mascardi, Río Negro Province (Flores and Brugni 2005). The most recently described species was Notocotylus primulus Diaz, Gilardoni, Lorenti and Cremonte 2020 from the crested duck Lophonetta specularioides from the Patagonian coast (Diaz et al. 2020).
Our ongoing research on the trematode fauna from the Neotropical realm, both on the coast and inland, has revealed a significant digenean diversity, especially in shorebirds (Capasso et al. 2017, 2019; Capasso 2019; Diaz et al. 2020). Some of the Nearctic shorebirds that nest in Canada and the United States during the boreal summer, migrate south to spend their non-breeding period in coastal and inland wetlands of southern Patagonia. On the other hand, the Neotropical shorebirds are present all year in South America.
Until now, DNA sequences were available for only 3 out of 19 species of the Notocotylidae reported from vertebrate hosts in South America (Fernandes et al. 2015; Diaz et al. 2020), namely Hippocrepis hippocrepis (Diesing 1850) from Brazil (COI and 28S genes), Ogmogaster antarctica Johnston 1931 (18S and 28S genes) and N. primulus from Argentina (5.8S-ITS2-28S sequence).
The aim of this work is to provide morphological data for Notocotylus spp. parasitizing the Nearctic Baird’s sandpiper Calidris bairdii (Coues 1861), the white-rumped sandpiper Calidris fuscicollis (Vieillot 1819), and the Neotropical two-banded plover Charadrius falklandicus (Latham 1790) during their stay in Patagonia, Argentina. We also provide molecular data for Notocotylus chionis and perform a phylogenetic analysis to examine the phylogenetic affinities within the Pronocephaloidea.
Materials and methods
Specimen collecting and morphological study
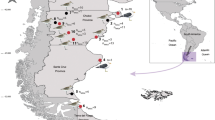
A total of 44 Baird’s sandpipers (BASA), 50 white-rumped sandpipers (WRSA) and five two-banded plovers (TBPL) from both marine and freshwater environments in Patagonia (Fig. 1) were examined for parasitic worms. Birds were either found dead or died accidentally during different research projects (see “Acknowledgements”). Birds were collected during January between 1999 and 2009, dissected in the field and the viscera were preserved in 10% formalin or 96% ethanol. In the laboratory, the viscera were examined under a stereomicroscope and the notocotylids were recovered from the intestinal caeca. For morphological studies the trematodes were post-fixed in 70% ethanol, stained with hydrochloric carmine or Gomori trichrome, dehydrated, cleared using eugenol, mounted in Canada balsam and studied using an Olympus BX51® (Olympus Corp., Tokyo, Japan) compound microscope equipped with a digital imaging system. Drawings were made with the aid of a drawing tube (Olympus BX51). All measurements are provided in micrometers.
One N. chionis specimen was dehydrated, critical point dried, mounted on an aluminum stub using conductive double-sided tape, coated with gold and examined under Jeol/SET 100® scanning electron microscope (SEM) (Tokyo, Japan) at an accelerating voltage of 15 kV.
Prevalence and mean intensity were calculated following Bush et al. (1997).
Voucher specimens were deposited in the Helminthological Collection of the Museo de La Plata, Buenos Aires, Argentina and in the Parasitological Collection of the Instituto de Biología de Organismos Marinos (IBIOMAR) (CCT CONICET-CENPAT), Puerto Madryn, Argentina.
DNA extraction, amplification, and sequencing
Two N. chionis specimens fixed in 96% ethanol, one from WRSA and one from TBPL, collected in Bahia San Sebastian, were used for molecular analysis. Genomic DNA for molecular analysis was isolated following the protocol of Tkach and Pawlowski (1999). An approximately 2700 base pairs long fragment of nuclear ribosomal DNA spanning the 3′ end of 18S nuclear rDNA gene, internal transcribed spacer region (ITS1 + 5.8S + ITS2) and 5′ end of the 28S gene were amplified by PCR on a T100™ thermal cycler (Bio-Rad) thermal cycler using forward primer ITSf (5′-CGC CCG TCG CTA CTA CCG ATT G-3′) and reverse primer 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) published by Tkach and Snyder (2008). PCRs were performed in a total volume of 25 μl using New England Biolabs One Taq quick load PCR mix according to the manufacturer’s protocol and using annealing temperature of 53 °C. PCR primers and several internal primers were used in sequencing reactions; internal forward primers: d58f (5′-GCG GTG GAT CAC TCG GCT CGT G-3′) and digl2 (5′-AAG CAT ATC ACT AAG CGG-3′); internal reverse primes: d58r1 (5′-GTC GAT GTT CAA AGC AGT ATG C-3′), digl2r (5′-CCG CTT AGT GAT ATG CTT-3′), ECD2 (5′-CTT GGT CCG TGT TTC AAG ACG GG-3′). PCR products were purified using ExoSap PCR clean-up enzymatic kit from Affymetrix following the manufacturer’s protocol, cycle-sequenced directly using BrightDye® Terminator Cycle Sequencing Kit (MCLAB, California, USA), alcohol precipitated, and run on an ABI 3130 automated capillary sequencer (Thermo Fisher Scientific, Waltham, MA, USA).
Contiguous sequences were assembled and edited using Sequencher software (GeneCodes Corp., ver. 4.1.4; Ann Arbor, Michigan), and submitted to GenBank under accession numbers MN877911 and MN877912.
Phylogenetic analysis
Phylogenetic analysis was conducted using newly obtained 28S sequences of N. chionis and 24 matching sequences of other representatives of the Pronocephaloidea available in the GenBank (Table 1). Diplodiscus subclavatus (Pallas 1760) Diesing 1836 (AY222212) was included as an outgroup based on the previously published phylogenies (Olson et al. 2003). Newly obtained and previously published sequences were aligned using ClustalW implemented in Mega7 (Kumar et al. 2016). The alignment was trimmed at both ends to the length of the shortest sequence and positions that could not be aligned unambiguously were excluded from the analysis.
Phylogenetic analysis was conducted using the Maximum Likelihood algorithm using MEGA 7 (Kumar et al. 2016) and the Bayesian inference (BI) as implemented in MrBayes Ver. 3.2.6 software (Ronquist and Huelsenbeck 2003). The 28S trimmed alignment presented 858 bp. The best substitution model (GTR +G) was estimated using jModelTest 2.1.10 (Darriba et al. 2012). Nodal support of ML analysis was estimated by performing 1000 bootstrap pseudoreplicates. The BI analysis was performed using MrBayes as follows: Markov chain Monte Carlo (MCMC) chains were run for 1000000 generations with sample frequency set at 100. Log-likelihood scores were plotted and only the final 75% of trees were used to produce the consensus trees by setting the “burn-in” parameter at 2500. This number of generations was considered sufficient because the SD dropped below 0.01.
Genetic distances (pairwise uncorrected p-distance) between ITS1-5.8S sequences were estimated from the sequences using the p-distance model in MEGA7.
Results
A total of 70 Notocotylus specimens (57 from WRSA, 8 from TBPL, and 5 from BASA) were recovered from the intestinal caeca of the examined birds. Specimens from WRSA and TBPL were identified as Notocotylus chionis Baylis 1928, whereas those from BASA as Notocotylus sp.
Morphological descriptions
Notocotylidae Lühe, 1909
Notocotylus chionis Baylis, 1928 (Table 2; Figs. 2 and 3).
Description (based on 23 stained specimens from WRSA and TBPL; measurements are provided in Table 2). Body with margins scalloped from level of beginning of uterus to end of body. Tegument unspined. Three rows of ventral papillae, variable in number, on average 25 papillae in each row; middle row with up to 27 papillae, lateral rows with up to 28 papillae. Papillae can be readily observed from the posterior end to seminal vesicle area. Anterior to this level papillae are inconspicuous and more difficult to observe. Middle row of papillae begins slightly anterior to lateral rows (Figs. 2a, 3a). Oral sucker rounded, terminal to subterminal. Esophagus very short, bifurcating into two ceca immediately posterior to oral sucker. Ceca long, curving between ovary and testes, nearly reaching posterior end of body. Testes at posterior end of body, longitudinally elongated, extracecal, opposite, lobed. External seminal vesicle extends posteriorly, dorsal to uterine loops. Cirrus sac median, straight, very elongated, broad posteriorly and sharply narrowed anteriorly, containing saccular internal seminal vesicle, pars prostatica and unarmed cirrus. Genital pore median, immediately ventral to cecal bifurcation. Ovary slightly elongated longitudinally, situated between anterior portions of testes. Vitellarium consisting of two elongated, narrow lateral groups of irregularly shaped follicles extending from anterior margin of testes to approximately level of half-length of uterine zone. Uterus long, sinuous, extending anteriorly from ovary and consisting of 24 to 31 transverse coils, some uterine coils slightly extending into extracecal space. Metraterm muscular, sinistral to cirrus pouch (Fig. 2b, c), opening via common genital pore (Fig. 3a). Eggs operculated, with long, single filament at each pole (Fig. 3b, c). Excretory pore opening dorsally at about level of posterior end of testes; excretory vesicle saccular.
Type host: Chionis alba (Charadriiformes, Chionidae).
Type locality: Patagonia, Argentina: exact locality was not indicated (Baylis 1928).
Other localities: Signy Island, (60°43′ S, 45° 36′ W), South Orkney Islands.
New hosts: Calidris fuscicollis, Charadrius falklandicus.
New localities: San Sebastian Bay, Tierra del Fuego Province (53°4′41.31″S, 68°14′10.32″W), Bahía Bustamante (45°5′18.80″ S; 66°25″ 44.77″ W), Chubut Province, Argentina.
Infection site: intestinal caeca.
Prevalence and mean intensity: 10% and 10.8 in WRSA, 20% and 1.6 in TBPL.
Voucher specimens: MLP-He-7638, CNP-Par 194.
Comments: some of the hosts (WRSA) harbored immature specimens.
Notocotylus sp. (Table 2).
Description (based on 5 stained specimens from BASA, measurements are provided in Table 2). Body with smooth margins. Tegument unspined. Three rows of ventral papillae, 21 papillae in the middle row, while the number varies between 17 and 19 in the lateral rows. Anteriormost papillae smaller in size than remaining papillae; in general, papillae appear at cirrus sac level. No ventral papillae are seen anterior to level of seminal vesicle. Oral sucker rounded, subterminal. Esophagus very short, bifurcating into two ceca immediately posterior to oral sucker. Testes at posterior end of body, longitudinally elongated, extracecal, opposite, lobed. Cirrus sac elongated, containing saccular internal seminal vesicle, pars prostatica and unarmed cirrus. Genital pore just posterior to the oral sucker, at level of cecal bifurcation. Ovary lobed, wider anteriorly, situated between anterior portions of testes. Vitellarium elongated, extending from anterior margin of testes to approximately level of posterior half-length of uterine zone. Uterus long, sinuous, consisting of 20 to 29 transverse coils. Metraterm muscular, sinistral to cirrus pouch. Eggs operculated; mature eggs in distal portion of uterus bearing single filament at each pole. Excretory pore opening dorsally at about level of posterior end of testes; excretory vesicle saccular.
Host: Calidris bairdii
Localities: Estancia María Cristina, Chubut Province (44°34′55.37″ S; 69°35′14.18″ W), Sarmiento, Chubut Province, (45°21′1.41″ S; 69°2′29.00″ W), Argentina.
Infection site: intestinal caeca.
Prevalence and mean intensity: 4.5% and 2.5.
Molecular results
Almost 2700 base pair long sequences spanning the ITS1 + 5.8 + ITS2 + partial 28S fragment of the ribosomal DNA operon obtained from specimens coming from WRSA and TBPL, were identical thus confirming the conspecificity of these specimens.
The BLAST search of the GenBank did not reveal identical matches for our sequences. ITS1-5.8S region obtained for N. chionis was 955 bp long and did not contain repetitive regions, unlike ITS1 sequences of N. atlanticus (Acc. Nos. MH818012-15), which contained two repeats at the 5′-end. Pairwise DNA analyses were performed based on 820 bp ITS1-5.8S rDNA of N. chionis and other Notocotylus species sequences available at GenBank, N. atlanticus and N. malhamensis (Acc. Nos. MH818012-15 and JQ766940). The genetic distance between N. chionis and N. atlanticus was 13.2% and between N. chionis and N. malhamensis was 9.2%.
The 28S tree topologies resulting from the ML and BI analyses were identical with BI producing higher branch support (Fig. 4). The isolates obtained in this study appeared on the tree closely related to the Argentinian Notocotylus primulus from L. specularioides. Notocotylus species did not form a monophyletic group. However, N. magniovatus, N. intestinalis, N. atlanticus, and N. attenuatus grouped together, and except by N. attenuatus, they are transmitted by caenogastropods. Similarly, Notocotylus sp. isolates (KY513158, AY222219 and EU712725) and N. malhamensis formed a well-supported clade, and except by N. malhamensis whose intermediate host remain unknown, they are transmitted by heterobranch mollusks. In general, all members of the Notocotylidae included in our analyses formed a monophyletic group without a clear geographical or host-related pattern. However, some clades shared similar intermediate hosts, either caenogastropod or heterobranch mollusks (Fig. 4).
Phylogenetic relationships between Notocotylus chionis (in bold) and other taxa of the superfamily Pronocephaloidea, as inferred from sequences of 28S rDNA (858 bp) analyzed by Maximum Likelihood (ML) and Bayesian Inference (BI) methods. Nodal support is indicated above internodes as BI/ML; values < 0.70 (BI) and < 50 (ML) are indicated by a dash. Sequence of Diplodiscus subclavatus (GenBank Acc. No: AY222212) was included as an outgroup. C Caenogastropoda, H Heterobranchia
Discussion
While Notocotylus spp. are commonly reported from waterfowl (mostly ducks) in the Nearctic region, there are only few records from shorebirds. Notocotylus attenuatus was found in Tringa flavipes and Steganopus tricolor from Texas (Yanez and Canaris, 1988; Enz and Canaris 2008), and in Arenaria melanocephala from Alaska (Bondarenko and Kontrimavichus, 1999), while Notocotylus sp. was reported parasitizing Calidris alpina in Alaska, Tringa semipalmata in Florida (USA), Alberta and Manitoba (Canada) (Dronen et al. 2002; Canaris and Kinsella 2007). Notably, these records were from scolopacid shorebirds while there are no reports of Notocotylus species in Charadriidae from this region.
Despite minor variation, the general morphology (the body shape and organ topology, the position of the genital pore at the level of cecal bifurcation, the arrangement and number of ventral papillae, the length of the cirrus sac) and morphometric characteristics of our specimens found in the WRSA and the TBPL fit the description of N. chionis. Although the species was reported several times from anseriform birds in Russia (Erkina 1954), Odening (1964) concluded that specimens identified as N. chionis in Russia actually belonged to Notocotylus parviovatus Yamaguti, 1934; recent studies propose this synonymization (Serbina 2016). Other species of the genus known from Patagonia are N. tachyeretis, N. imbricatus, N. biomphalariae and N. primulus. Our N. chionis specimens can be readily distinguished from all of them by the higher number of ventral papillae in both lateral and middle row (25–27 vs. 15–16, 14–16, 4–11 and 13–15, respectively, in the aforementioned species) (Duthoit 1931; Lunaschi and Sutton 1987; Flores and Brugni 2005; Diaz et al. 2020). Similar to the original description of N. chionis, the ventral papillae in our specimens commence immediately posterior to the oral sucker, whereas in N. tachyeretis, N. primulus, N. biomphalariae and N. imbricatus they commence at the level of the cirrus sac. Additionally, the genital pore in our specimens is located immediately ventral to the cecal bifurcation (similar to N. tachyeretis), whereas in N. primulus, N. biomphalariae and N. imbricatus it is postbifurcal.
Specimens obtained from BASA also resemble N. chionis in number and arrangement of ventral papillae, position of genital pore and general morphology. However, considering the small number of individuals found and the lack of sequence data from BASA specimens, we prefer to leave the identification as Notocotylus sp. until fresh specimens are available.
Gonchar and Galaktionov (2020) have recently stated that intraspecific variability in parasites with heteroxenous life cycles is driven by the host vagility and dispersal. This would explain the intraspecific morphological variability observed among conspecific notocotylid specimens found in the migratory shorebirds examined in the present study (see Table 2). Based on their morphological similarity, we cannot rule out the synonymy of N. tachyeretis and N. chionis. The host of N. tachyeretis, the steamer duck, is distributed in the Patagonian region of Argentina and Chile, thus overlapping the seasonal ranges of the shorebirds studied in this work. Notocotylus tachyeretis was described from a few specimens only and has not been found again since its original description (Duthoit 1931). Unfortunately, the type material deposited in the Natural History Museum of London was not accessible to the authors.
Molecular phylogenetic results support the close phylogenetic affinities between N. chionis and N. primulus, found in Patagonia from L. specularioides, a duck species native to South America (Diaz et al. 2020). This topology combined with the fact that N. chionis was found by us in resident birds (TBPL) and previously in the snowy sheathbill which inhabits in coasts of southern South America, coastal regions of the Antarctic Peninsula and surrounding islands, allows us to hypothesize that N. chionis is a neotropical species with its life cycle probably being completed in South America. We have found immature specimens in the WRSA from Patagonia in January (birds arrive to Patagonia in mid-November) which further corroborates the hypothesis that Nearctic shorebirds become infected upon arrival to Patagonia. Notocotylus chionis has not been found in Northern Hemisphere, and this is one reason to think that mollusk intermediate hosts are to be found only in South America and is absent on the North Hemisphere.
Our results also pose a question regarding the mollusk intermediate host that supports the circulation of N. chionis in Patagonia. It is known that many digenean taxa show a great specificity to their first intermediate host (Yamaguti 1975; Galaktionov and Dobrovolskij 2003). Dubois (1951) distinguished two biological groups within Notocotylus: one associated with pulmonate gastropods (Heterobranchia) and the other using prosobranch gastropods (Caenogastropoda) as intermediate hosts. Considering the results of our phylogenetical analysis (Fig. 4), we hypothesize that N. primulus and N. chionis most likely utilize caenogastropod mollusks as intermediate hosts.
The use of molecular tools allows for rapid progress in the studies of digenean life cycles including those of notocotylids. The availability of DNA sequences from properly identified adult stages is critical for identification of larval stages and elucidation of life cycles. Sequences of N. chionis reported in the present study represent a valuable addition to the growing database of sequence available for the family Notocotylidae from South America and provide a necessary basis for future studies of this group and life cycles of its members.
References
Assis JC, Lopez-Hernandez D, Pulido-Murillo EA, Melo AL, Pinto HA (2019) A morphological, molecular and life cycle study of the capybara parasite Hippocrepis hippocrepis (Trematoda: Notocotylidae). PLoS ONE 14:e0221662. https://doi.org/10.1371/journal.pone.0221662
Barton DP, Blair D (2005) Family Notocotylidae Lühe, 1909. In: Jones A, Bray RA, Gibson DI (eds) Keys to the trematoda. CABI, Wallingford, Oxfordshire, UK, pp 383–396
Baylis HA (1928) A new species of Notocotylus (Trematoda) with some remarks on the genus. Ann Mag Nat Hist Ser 10:582–585. https://doi.org/10.1080/00222932808672921
Besprozvannykh V, Ngo H, Ha N, Hung N, Rozhkovan K, Ermolenko A (2013) Descriptions of digenean parasites from three snail species, Bithynia fuchsiana (Morelet), Parafossarulus striatulus Benson and Melanoides tuberculata Müller, North Vietnam. Helminthologia 50:190–204
Bondarenko SK, Kontrimavichus VI (1999) The helminth fauna of Charadriiformes in Alaska: zoogeographical features and origin. Zool Zhurnal Moskva 78:643–653 (In Russian)
Boyce K, Hide G, Craig PS, Harris PD, Reynolds C, Pickles A, Rogan MT (2012) Identification of a new species of digenean Notocotylus malhamensis n. sp. (Digenea: Notocotylidae) from the bank vole (Myodes glareolus) and the field vole (Microtus agrestis). Parasitol 139:1630–1639. https://doi.org/10.1017/S0031182012000911
Canaris AG, Kinsella JM (2007) Helminth communities of three sympatric species of shorebirds (Charadrii) from four summer seasons at Bristol Bay, Alaska. J Parasitol 93:485–490. https://doi.org/10.1645/GE-3550.1
Capasso S (2019) Las comunidades de helmintos de aves playeras migratorias neárticas en humedales costeros e interiores de la Patagonia argentina. Doctoral Thesis, Universidad Nacional de La Plata
Capasso S, D’Amico VL, Diaz JI (2017) Odhneria odhneri Travassos, 1921 (Trematoda: Microphallidae) in Migrant Shorebirds from Patagonia, Argentina. Rev Arg Parasitol 6:15–20
Capasso S, D’Amico VL, Diaz JI (2019) A new species of Maritrema (Trematoda: Microphallidae) parasitizing the Baird’s sandpiper Calidris bairdii, and comments about diversity of Microphallidae in two Nearctic shorebirds at Patagonian sites in Argentina. Acta Trop 189:10–14. https://doi.org/10.1016/j.actatropica.2018.09.018
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Diaz JI, Gilardoni C, Lorenti E, Cremonte F (2020) Notocotylus primulus n. sp. (Trematoda: Notocotylidae) from the crested duck Lophonetta specularioides (Aves, Anatidae) from Patagonian coast, southwestern Atlantic Ocean. Parasitol Int 74:101976. https://doi.org/10.1016/j.parint.2019.101976
Dronen NO, Wardle WJ, Bhuthimethee M (2002) Helminthic Parasites from Willets, Catoptrophorus semipalmatus (Charadriiformes: Scolopacidae), from Texas, USA, with Descriptions of Kowalewskiella catoptrophori sp. n. and Kowalewskiella macrospina sp. n. (Cestoda: Dilepididae). Comp Parasitol 69:43–50
Dubois G (1951) Étude des trématodes nord-americains de la collection EL Schiller et revision du genre Notocotylus Diesing, 1839. Bull Soc Neuchl Sci Nat 74:42–76
Duthoit CM (1931) A new species of the Trematode genus Notocotylus. Ann Mag Nat Hist 7:290–293
Enz JJ, Canaris AG (2008) Metazoan parasites of lesser yellowlegs, Tringa flavipes (Charadriiformes) from southwestern United States and Alaska with a checklist of parasites reported from this host. J Parasitol 94:548–552. https://doi.org/10.1645/GE-1327.1
Erkina NG (1954) The life-cycle of the trematode Notocotylus chionis, parasite of aquatic birds. Doklady Akad SSSR 97:559–560
Fernandes BMM, Justo MCN, Cárdenas MQ, Cohen SC (2015) South American trematodes parasites of birds and mammals. Biblioteca de Ciências Biomédicas, ICICT, Rio de Janeiro
Flores V, Brugni N (2005) Notocotylus biomphalariae n. sp. (Digenea: Notocotylidae) from Biomphalaria peregrina (Gastropoda: Pulmonata) in Patagonia, Argentina. Syst Parasitol 61:207–214. https://doi.org/10.1007/s11230-005-3166-2
Fraija-Fernández N, Olson PD, Crespo EA, Raga JA, Aznar FJ, Fernández M (2015) Independent host switching events by digenean parasites of cetaceans inferred from ribosomal DNA. Int J Parasitol 45:167–173
Galaktionov KV, Dobrovolskij AA (2003) The main trends in Trematode evolution. In: Galaktionov D (ed) The biology and evolution of Trematodes. Springer, Dordrecht, pp 407–479
Gonchar A, Galaktionov KV (2020) Short communication: New data support phylogeographic patterns in a marine parasite Tristriata anatis (Digenea: Notocotylidae). J Helminthol 94:E79. https://doi.org/10.1017/s0022149x19000786
Gonchar A, Jouet D, Skírnisson K, Krupenko D, Galaktionov KV (2019) Transatlantic discovery of Notocotylus atlanticus (Digenea: Notocotylidae) based on life cycle data. Parasitol Res 118:1445–1456
Hanelt B (2009) Hyperparasitism by Paragordius varius (Nematomorpha: Gordiida) larva of monostome redia (Trematoda: Digenea). J Parasitol 95:242–244
Izrailskaia AV, Besprozvannykh VV, Tatonova YV, Nguyen HM, Ngo HD (2019) Developmental stages of Notocotylus magniovatus Yamaguti, 1934, Catatropis vietnamensis n. sp., Pseudocatatropis dvoryadkini n. sp., and phylogenetic relationships of Notocotylidae Lühe, 1909. Parasitol Res 118:469–481. https://doi.org/10.1007/s00436-018-6182-2
Jones NV, Williams IC (1968) The trematode parasites of the Sheathbill, Chionis alba (Gmelin), from Signy Island, South Orkney Islands. J Helminthol 42:65–80. https://doi.org/10.1017/S0022149X00027243
Kinsella JM, Tkach VV (2005) Notocotylus fosteri sp. nov. (Trematoda, Notocotylidae) from the rice rat, Oryzomys palustris in Florida. Acta Parasitol 50:194–198
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lunaschi LI, Sutton CA (1987) Fauna helmintológica de las aves del lago Pellegrini II. Limnobios 2:707–711
Odening K (1964) Zur trematodenfauna von Nettapus c. coromandelianus in Indien. Angew Parasitol 4:228–241
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol 33:733–755. https://doi.org/10.1016/s0020-7519(03)00049-3
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Serbina EA (2016) The role of bithyniid snails (Gastropoda: Bithyniidae) as hosts of trematodes of the family Notocotylidae in ecosystems of different climatic zones of the West Siberian Plain. Inland Water Biol 9:182–188. https://doi.org/10.1134/S1995082916020152
Soldánová M, Georgieva S, Roháčová J, Knudsen R, Kuhn JA, Henriksen EH, Siwertsson A, Shaw JC, Kuris AM, Amundsen PA, Scholz T, Lafferty KD, Kostandinova A (2017) Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. Int J Parasitol 47:327–345
Stunkard HW (1959) Studies on the morphology and life-history of Notocotylus minutus n. sp. a digenetic trematode from ducks. J Parasitol 46:803–809
Tkach V, Pawlowski JW (1999) A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitol 44:147–148
Tkach VV, Snyder SD (2008) Aptorchis glandularis n. sp. (Digenea: Plagiorchioidea) from the northwestern red-faced turtle, Emydura australis, (Pleurodira: Chelidae) in the Kimberley, Western Australia. J Parasitol 94:918–925. https://doi.org/10.1645/GE-1439.1
Tkach VV, Pawlowski J, Mariaux J, Swiderski Z, Littlewood DTJ, Bray RA (2001) Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor & Francis, London
Velez J, Hirzmann J, Lange MK, Chaparro-Gutierrez JJ, Taubert A, Hermosilla C (2018) Occurrence of endoparasites in wild Antillean manatees (Trichechus manatus manatus) in Colombia. Int J Parasitol 7:54–57
Yamaguti S (1975) Synoptical review of life histories of digenetic trematodes of vertebrates with special reference to the morphology of their larval forms. Keigaku Publishing Co., Tokyo
Yanez DM, Canaris AG (1988) Metazoan parasite community composition and structure of migrating Wilson’s Phalarope, Steganopus tricolor Viellot, 1819 (Aves), from El Paso County, Texas. J Parasitol 74:754–762
Acknowledgements
We would like to thank Mónica Abril, Graciela Escudero, Florencia Cremonte and Marcelo Bertellotti for providing birds and Guillermo Panisse for his help with processing them. We also thank Patricia Sarmiento from Servicio de Microscopía Electrónica de Barrido from Museo de La Plata and María Cristina Estivariz for the assistance with the line drawing and Nicolás Viera for his help with editing the Fig. 4. Fieldwork was conducted using permits from the Secretaría de Turismo y Áreas Protegidas, Chubut (Permit No. 19/04, 02/05, 92/05, 06/10, 02/08, 48/08 DF and FS) and from the Santa Cruz provincial government (Permit No. 406/05 DFS). Authors would like to thank Rodney Bray, Jong-Yil Chai, and one anonymous reviewer as well as the Editor, for the helpful comments on the manuscript.
Funding
This study was funded by Grants from ANPCyT (PICT 525 to JID), CONICET (PIP 698 to JID), and UNLP (N758, N859 to JID) and the U.S. National Science Foundation Grant #DEB-1120734 to VVT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capasso, S., Servián, A., Tkach, V.V. et al. Notocotylus chionis (Trematoda: Notocotylidae) and Notocotylus sp. from shorebirds in southern Patagonian wetlands of Argentina: morphological and molecular studies. Polar Biol 43, 1957–1966 (2020). https://doi.org/10.1007/s00300-020-02753-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02753-9