Abstract
Oxidative stress is considered the main physiological mechanism involved in short- and long-term life histories, operating through the progressive deterioration of tissues, with consequences in the reproductive performance, growth patterns, aging, and survival. Besides, the Antarctic Peninsula environmental change, registered in the last decades, makes essential the antioxidant status knowledge of fauna and flora to optimize current management plans to protect Antarctic wildlife. Therefore, the present study aimed to investigate the differences between the oxidant-antioxidant status in adults and chicks´ blood of the skuas Stercorarius antarcticus and Stercorarius maccormicki. Through spectrophotometric techniques, the enzyme activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST) as well as the level of total glutathione (TG), total protein (TP), lipid peroxidation (LPO), and protein oxidation (PO) were measured. The adults of both species showed higher TG levels than the chicks. S. antarcticus adults were characterized by showing high GST activity and the highest total protein (TP) levels. Instead, S. maccormicki adults showed high levels of oxidative damage (PO and LPO) and high CAT activity. On the other hand, the chicks of both species were typified by the augmented SOD activity compared to those registered in adults. In turn, S. maccormicki chicks had increased GPx activity. Considering the multiple factors that affect the antioxidant status of both skua species, the information reported, herein, is a first promising dataset that would allow giving reference values for them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of the Brown Skua (Stercorarius antarcticus) and the South Polar Skua (Stercorarius maccormicki) is clearly different. After the breeding season, South Polar Skuas migrate to the Northern Atlantic, Northern Pacific, and some birds wintered in the Southern Hemisphere (Kopp et al. 2011). On the other hand, Brown Skuas reach parts of the Patagonian Shelf, Argentine Basin, and South Brazil Shelf (Phillips et al. 2007; Krietsch et al. 2017). Besides, these two skua species are considered Antarctic top predators which nest sympatrically in a few areas, mostly along the Antarctic Peninsula between 61° and 65° S (Ritz et al. 2006). At these sites, feeding habits of S. maccormicki are determined by a variety of complex interacting factors, such as the abundance of resources and competitive exclusion between species (Montalti et al. 2009; Graña Grilli et al. 2011). It was described that S. maccormicki foraged almost exclusively at sea (mainly fish and krill) (Malzof and Quintana 2008; Montalti et al. 2009), whereas S. antarcticus monopolized the terrestrial food source of penguin eggs and chicks (Reinhardt et al. 2000; Graña Grilli and Montalti 2015). Besides, it is commonly accepted that seabirds that feed at sea take longer trips than those feeding on terrestrial resources near their nests (Schreiber and Burger 2001).
Like most Antarctic organisms, seabirds are exposed to severe environmental conditions such as extremely low temperatures and high levels of UV radiation, which can increase the generation of reactive oxygen species (ROS) altering the cellular redox homeostasis. The imbalance between ROS production/antioxidant defenses will lead to oxidative stress (OS) (Sies 1985). The result of the increased oxidant level may also lead to reversible or irreversible cell damage (mainly lipid peroxidation, protein oxidation, and DNA damage) and eventually to cell death (Halliwell and Gutteridge 2015).
The OS levels are not constant in organisms and vary for the action of several known factors such as reproduction, developmental stage, immune responses, physical activity, environmental conditions, and food availability (Costantini 2008; Monaghan et al. 2009; Metcalfe and Alonso-Alvarez 2010; Beaulieu and Costantini 2014). Particularly, oxidative damage can also be positively related to the growth rate and reproduction. Both processes involve a high metabolic demand, which diverts resources away from self-maintenance (Smith et al. 2016). The cells have evolved their protective processes to counterbalance the oxidative damage neutralizing the ROS effect. It is well known that different enzymes and non-enzymatic compounds participate in the antioxidant chain in biological systems. The enzymes, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST), are important for inside cell protection, since they are manufactured within cells like other proteins, and their levels do not depend on the ingested diet (Cohen et al. 2009). Otherwise, the intake of low molecular weight antioxidants like reduced glutathione (GSH), ascorbate, and uric acid are largely dependent on the diet (Cohen et al. 2009), and their presence enhances or inhibits OS (Metcalfe and Alonso-Alvarez 2010).
Oxidative stress may play a central role in the trade-off between growth or reproduction and self-maintenance (Costantini 2014). Growth is a period where levels of OS are augmented due to a high metabolic rate required for development, while the OS is increased through all steps of the cycles implied in reproduction (Kim et al. 2010; Hall et al. 2010). Besides, in adulthood, OS would not only arise from endogenous metabolism, but also from oxidative challenges induced by environmental conditions such as seasonal variability of diet composition, oxygen availability, and temperature (Metcalfe and Alonso-Alvarez 2010).
According to Beaulieu and Costantini (2014), it is very important to know the antioxidant status in birds; the measured markers can be used not only to describe the physiological state, they may predict their chances of reproduction and survival in the wildlife also. In this way, the present study aims to determine and establish reference values of oxidative status in breeding adults and growing chicks of S. antarcticus and S. maccormicki.
Materials and methods
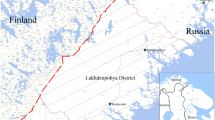
Stercorarius maccormicki and S. antarcticus specimens were captured using a rope with a snare trap. Blood samples were collected from adults and chicks of both species, which were nesting on Potter Peninsula (62° 15′ S, 58° 40′ W) (25 de Mayo/King George Island, South Shetland Islands, Antarctica) (Fig. 1). A total of 33 skuas: 6 adults and 12 chicks of S. maccormicki and 7 adults and 8 chicks of S. antarcticus were sampled independently of sex. Chicks were between 10 and 15 days old, weighting 200 g approximately. Instead, the adults were sampled during the early care of chicks in the breeding stage. Neither the adults nor the chicks were related to each other. Approximately 3 mL of blood was drawn from the brachial vein of the birds using a heparinized syringe with 23 gauge sterile needle. Whole-blood samples were centrifuged at 2000×g for 15 min at 4 °C, to separate erythrocytes from plasma. Plasma samples were stored in liquid N2 until their analysis. The erythrocytes were washed twice with cold saline solution, centrifuged to remove the leucocytes layer, and then hemolysis was carried out through the freeze/thaw procedure. After that, samples were centrifuged at 14,000×g for 10 min and the supernatants were stored in liquid N2 until they were processed in the laboratory.
Location of the study site at Poter Peninsula, 25 de Mayo (King George) Island, South Shetland Islands, Antarctic Peninsula. Image made with Quantarctica 3 (Quantum GIS [Since 2.0 just QGIS] + Antarctica = "Quantarctica") (Norwegian Polar Institute, 2018)
The enzyme activities were measured in the erythrocyte fraction using spectrophotometric techniques. For the determination of samples’ parameters, all the reagents and solvents used in the processing and measurement techniques were of analytical grade. Before the SOD activity measure, a chloroform: ethanol (3:5) extraction from the erythrocyte fraction was done followed by centrifugation at 5000×g (for 15 min) to get rid of hemoglobin (Hb+) interference. Then, the aqueous supernatant was saved for determining the SOD activity by the Misra and Fridovich (1972) protocol. One SOD unit was the amount of enzyme necessary to inhibit 50% of the rate of autocatalytic adrenochrome formation, measured at 480 nm (ε480 = 2.96 M−1 cm−1).
For the CAT determination, the erythrocyte’s samples were diluted (1:500 v/v), and the CAT activity was measured sensing the decrease in hydrogen peroxide concentration (H2O2 10 mM solution) at 240 nm (ε240 = 40 M−1 cm−1) (Aebi 1984). One CAT unit was the amount of enzyme necessary to degrade 1 µmole of H2O2.
The GST enzyme activity was determined according to Habig et al. (1974) protocol using 1-chloro-2.4-dinitrobenzene (CDNB) 100 mM as substrate and reduced glutathione (GSH) 100 mM. One GST unit represents the amount of enzyme required to conjugate 1 µmole of 1-chloro-2.4-dinitrobenzene to GSH in the erythrocyte’s fraction, at 340 nm (ε340 = 9.6 mM−1 cm−1).
Both SOD, CAT, and GST units were expressed as enzyme units per mg protein per minute of assay reaction (U enzyme mg protein−1 min−1).
The GPx activity was registered at 340 nm (ε340 = 6.22 mM−1 cm−1) through the glutathione/NADPH/glutathione reductase system, by the dismutation of tertbutyl hydroperoxide (Flohé and Günzler 1984). In this assay, KCN 50 mM was added to the reaction medium to avoid the methemoglobin interference present in the samples. One GPx unit was the amount of enzyme necessary to oxidize 1 nmole of NADPH per mg of erythrocyte’s protein per minute at 340 nm and was expressed as nmole of NADPH mg protein−1 min−1.
To determine the total glutathione (TG) content, red cells were deproteinized with perchloric acid 2 mM, ethylene diamine tetra-acetic solution (EDTA) (1:1) 4 mM, and the supernatant was neutralized with potassium hydroxide 2 M. The reaction medium contained phosphate buffer (100 mM, pH 7.2), nicotinamide dinucleotide phosphate acid (NADPH) 0.2 mM, glutathione reductase 0.2U mL−1, 5,5′-dithiobis (2-nitrobenzoic acid) 70 µM (ε412 = 13.5 mM−1 cm−1), and 30 µL of the previously described extract (Akerboom and Sies 1981). The results are expressed in µmole mL hemolysed erythrocytes−1.
Plasma lipid peroxidation (LPO) level was measured according to Buege and Aust (1978), by the formation of thiobarbituric acid reactive substances (TBARS). Fresh plasma samples were added to the reaction mixture (trichloroacetic acid 15% (w/v), 2-thiobarbituric acid 0.375% (w/v), and butylhydroxytoluene 0.147 mM) in a ratio of 1:5 (v/v). The mixture was vigorously shaken, maintained in boiling water for 60 min, and immediately cooled at 5 °C for 5 min (Ohkawa et al. 1979). Then it was centrifuged at 5000×g for 10 min, and the supernatant was measured spectrophotometrically at 535 nm (ε535 = 153 M−1 cm−1).
Plasma protein oxidation (PO) level was evaluated according to Reznick and Packer (1994) modified by Ansaldo et al. (2007), by detecting the formation of protein hydrazones as a result of the reaction of dinitrophenylhydrazine (DNPH) with protein carbonyls. The carbonyl content was calculated from the absorbance measurement at 375 nm, using an absorption coefficient ε375 = 22,000 M−1 cm−1.
Erythrocyte and plasma total protein (TP) concentration was measured by the method of Lowry et al. (1951) using bovine serum albumin as standard.
Statistical analysis
The InfoStat software version 2015 (Di Rienzo et al. 2015) was used for all the statistical analyses employed. Data were analyzed through an extended and mixed linear model (MLM) using two fixed factors. This type of analysis was chosen because it was statistically robust and allowed the evaluation of the studied parameters' variation between species (S. maccormicki and S. antarcticus) or age (adults and chicks). Furthermore, through this analysis, it was possible to estimate the interactions between both factors. Since in some cases the assumption of equality of variances was not fulfilled, the MLM analysis allowed us to model the variance function. Consequently, if the error variance structure was not homogeneous, the variance identity function (varIdent) was used to model the heteroscedasticity. The best model was obtained by Akaike (1992) and Schwarz (1978) IC comparison. For multiple comparisons, a post test called DGC was performed (Di Rienzo et al. 2002). All possible interactions were taken into account and included. Besides, the principal component analysis (PCA) was also performed (1) to obtain the smallest number of the studied biomarkers capable of expressing the variability in the data and (2) to determine which of them contribute more to the separation among the groups. To assess if there were significant differences among the groups concerning the principal component retained, an ANOVA was performed considering the two factors (species and age) and PC1 and PC2 as the variable responses. ANOVA post hoc comparisons were done using the DGC test (Di Rienzo et al. 2002) when results were significant. All the values of the studied parameters were expressed as means ± standard error, and the level of significance was considered p < 0.05.
Results
The variance´s function was only used to model the heteroscedasticity in GPx; meanwhile, for the other analyzed parameters, the best model was obtained without modeling the error variance structure. Table 1 shows the more relevant outcome parameters of the extended and mixed linear model using the factors: species (S. maccormicki and S. antarcticus) and age (adults and chicks).
SOD and CAT activities are shown in Fig. 2. No difference was observed for SOD activity comparing both species. However, a significantly lower value (MLM, F1,29 = 8.38, p = 0.0073) was registered in adults compared with chicks. This condition was more evident in S. antarcticus than in S. maccormicki (Fig. 2a). CAT antioxidant activity showed significant differences among factors (Fig. 2b). Moreover, S. antarcticus enzyme activity was lower (MLM, F1,29 = 19.41, p = 0.0001) than that observed in S. maccormicki. Besides, chick erythrocytes showed CAT activities significantly lower (MLM, F1,29 = 4.93, p = 0.0349) than those registered in their respective adults (S. maccormicki 18.5% and S. antarcticus 33%) (Fig. 2b).
GST activity did not show any difference between age and species (Table 1), but values were higher in adults than chicks of both species (Fig. 3a).
Enzyme activities of a GST (glutathione S-transferase) and b GPx (glutathione peroxidase) in the erythrocyte´s fraction and c TG (total glutathione) levels in the erythrocyte’s extract. White bar: Stercorarius antarcticus, gray bar: Stercorarius maccormicki. Values are expressed as means ± standard error. (N): sample size. Different letters mean significant differences (p < 0.05)
Among the considered factors, significant interaction (MLM, F1,28 = 10.44, p = 0.0032) was registered for GPx activities. The S. antarcticus adults and chicks showed similar GPx activity (Fig. 3b), their values being lower than those measured in S. maccormicki. Adults of S. maccormicki had a GPx activity 40% lower than their chicks (Fig. 3b).
When the TG content was analyzed (Fig. 3c), significantly lower levels (MLM, F1,31 = 26.1, p < 0.0001) were registered in chicks compared to adults (70% in S. maccormicki and 86% in S. antarcticus). No significant difference between species was found.
Plasma lipid peroxidation level (LPO) measured as TBARS did not show any significant difference when species and age were analyzed (Fig. 4a), although LPO showed high values in S. maccormicki adults compared to chicks.
Levels of a TBARS (lipid peroxidation), b PO (protein oxidation), and c TP (total protein) in plasma fraction. White bar: Stercorarius antarcticus, gray bar: Stercorarius maccormicki. Values are expressed as means ± standard error. (N): sample size. Different letters mean significant differences (p < 0.05)
S. maccormicki skuas exhibited protein oxidation levels (PO) significantly higher (MLM, F1,26 = 10.28, p = 0.0037) than those registered for S. antarcticus. Moreover, S. maccormicki adults showed the highest levels of PO (2.66 ± 0.39 nmole [carbonyl] mg protein−1, n = 6) (Fig. 4b). On the other hand, TP displayed significant interaction (MLM, F1,26 = 11.29, p = 0.0025) between species and age. Furthermore, S. antarcticus adults evidenced the highest plasma total protein levels (45.95 ± 2.14 mg mL plasma−1, n = 7) (Fig. 4c), and these values contrasted with those obtained from their chicks and S. maccormicki chicks and adults.
The results of PC analysis are shown in Fig. 5 and Table 2. The analysis showed the ordering of species-age groups along with the two retained components; 51% of the total variance was explained by both PCs (Fig. 5). The first component (PC1 = 28.8%) completely separated two groups corresponding to the species: S. antarcticus (on the left end of the axis) and S. maccormicki (on the right end of the axis). Furthermore, the second component (PC2 = 22.1%) ordered the chicks at the axis upper end and adults in the lower end. Therefore, the four groups (species-age) were distinguished with both PCs (Fig. 5).
Biplot Principal Component Analysis. The circles and triangles correspond to the PC1 and PC2 scores of each observation. See the Results for more explanation. Solid circle: Stercorarius maccormicki adults; open circle: Stercorarius antarcticus adults; solid triangles: S. maccormicki chicks; and open triangles: S. antarcticus chicks. The lines represent the correlation between the original variables with PC1 and PC2. Contours correspond to confidence ellipses of four groups of species*age
Positive and negative loadings indicate a positive or negative correlation between the biomarker and PC1 or PC2 (Table 2). In bold, the significant absolute values indicate those variables that most contributed to each component. The variables that most contributed to PC1 are CAT, PO, and GPx. Meanwhile, the principal contribution of SOD, TG, and GST are observed at PC2 (Table 1). The eigenvectors graphed for PC1 are -0.12 SOD + 0.56 CAT + 0.41 GPx + 0.02 TG − 0.28 GST + 0.28 LPO + 0.52 PO − 0.3 TP and those for PC2 are : + 0.62 SOD − 0.14 CAT + 0.17 GPx − 0.58 TG − 0.44 GST + 0.01 LPO − 0.14 PO − 0.14 TP (Fig. 4). The cophenetic correlation coefficient was 0.884. This value indicated the quality of the reduction achieved.
Finally, a two-way ANOVA (species and age) was performed for the PC1 and PC2 variables. In the case of PC1, significant differences (ANOVA, F1,20 = 31.17, p < 0.0001) were observed between species, while for PC2 , significant differences (ANOVA, F1,20 = 33.75, p < 0.0001) were observed between ages.
Discussion
The studied skua populations showed a clear pattern either between age or species for antioxidant enzyme activities and oxidative damage biomarkers. Thus, it was evident for the age, higher SOD activity registered in chicks than in adults (Fig. 2a), as well as higher GST and TG observed in adults compared to chicks (Fig. 3a,c). Besides, higher PO levels (Fig. 4b) were observed in S. maccormicki than in S. antarcticus when species were compared.
For a better understanding of how the whole blood antioxidant status was affected, the studied variables were analyzed simultaneously and it was possible to observe that the chosen biomarkers established a clear division into four groups: S. antarcticus adults, S. antarcticus chicks, S. maccormicki adults, and S. maccormicki chicks. Each group was characterized by a particular set of variables (Fig. 5; Table 2). The adults of both species showed higher TG levels than the chicks. S. antarcticus adults were characterized by showing high GST activity and the highest TP levels, while S. maccormicki adults showed high levels of oxidative damage (PO and LPO) and high CAT activity. Additionally, the chicks of both species were typified by the SOD activity augmented which was higher than that registered in adults. In turn, S. maccormicki chicks had higher GPx activity values.
The tripeptide glutathione protects the cell against the free radical attack and is considered the most abundant low molecular weight thiol in animal cells (Wu et al. 2004). In endotherm vertebrates, TG levels are composed of GSH (99%) plus the oxidized form GSSG (1%) (Halliwell and Gutteridge 2015). In particular, for birds, Özkan et al. (2007) and Alonso-Alvarez et al. (2010) have shown the influence of nutritional factors and age on glutathione metabolism. In a previous study, Ansaldo et al. (2005) registered non-enzymatic antioxidant levels as a whole, using the total antioxidant potential (TRAP) technique. They determined that the TRAP levels of both skua species were similar either for species or age. In agreement with that study, the present results did not register significant differences between species for TG levels. But differences appeared when adults were compared to chicks: fourfold higher in S. maccormicki and sixfold higher in S. antarcticus, respectively (Fig. 3c; Table 1). Romero Haro and Alonso-Alvarez (2015) registered lower GSH concentrations in Zebra Finch (Taeniopygia guttata) when adults were compared to chicks. Such differences may be explained because the rate of GSH synthesis depends on the availability of the amino acid cysteine, which can be obtained directly from food or by the metabolism of dietary methionine (Meister and Anderson 1983). Then, low glutathione levels registered during the growth period could indicate low amino acid availability in the environment, producing altered phenotypes in adulthood (Romero Haro and Alonso-Alvarez 2015). Moreover, Isaksson et al. (2005) registered high TG levels in adult plasma samples of the Great Tit (Parus major) which were different from those of chick. They argued that the latter was mostly immobile, warm, and safe in the nest along with their not fully developed defense system. In this context, the high TG levels could be part of the response to physical effort and other stressors (e.g., adverse climate, migration, or poor nutrition during the preceding winter) experienced by breeding adults. During the winter, regarding the preferred locations where adults conduct the non-breeding season, they may display a feeding behavior according to their personality and availability of resources. Moreover, depending on the age and experience of the individuals, they may optimize the resources consumed trying to improve their body condition. Altogether, these factors will be associated with and will determine the timing of the breeding season, as well as, the nutritional condition. Adults with a better body condition will possibly have more resources to be reallocated to avoid oxidative damage. It was previously described for breeding Brown Skuas during the intensive care of chicks that body mass and overall nutritional status decline because of the parental effort (Ibañez et al. 2018; Graña Grilli et al. 2018). During these breeding activities, a trade-off between oxidative stress damage and other physiological functions occur. High investment in breeding performance is expected to lower future fecundity or survival perspectives because of resources, such as antioxidants and nutrients, assigned to reproduction that are no longer available for self-maintenance. Briefly, the cyclic production of oocytes, the ovulation, the number of eggs, and the breeding effort, all of them as part of the reproductive cycle can induce OS (Costantini 2008; Metcalfe and Alonso-Alvarez 2010). The composition of the diet is determined, among other factors, by the availability of food and the ability of the animals to obtain it. At the time of sampling, the adults were in the parental care stage, so the time spent away from the nest as well as the distance traveled in search of food could be reduced. Since the present study is observational, the high TG values could be due to both the availability of food in the vicinity of the nest and the reproductive effort, so it was not possible to discriminate the effect of each separately.
Contrary to those observed by Alonso-Alvarez et al. (2010) and Romero Haro and Alonso-Alvarez (2015), the blood LPO and PO levels registered, herein, were associated with the skua species but not with age (Fig. 4a,b; Table 1). This could be explained as differences between feeding strategy which are positively related to the effort in foraging trips. Frequently, S. maccormicki exploit large terrestrial colonies of penguins and also feed extensively on fish. Seal carcasses, garbage, and marine invertebrates including krill Euphausia spp are occasionally used by this species. On the other hand, S. antarcticus feed on eggs and chicks of penguins, burrowing petrels, and seal carcasses or placentae (Reinhardt et al. 2000; Graña Grilli and Montalti 2012, 2015). In the studied area, S. maccormicki fed mainly on fish, as a consequence of the feeding territory overlap with S. antarcticus, which monopolized the land resources (Montalti et al. 2009). The flight effort (e.g., high metabolic activity) is normally associated with altered redox homeostasis and the augmented risk of OS (Costantini et al. 2008; Nikolaidis et al. 2012). In the Zebra Finch (Taeniopygia guttata), Costantini et al. (2013) tested if the exercise-induced OS could alter the integrity of the pro-oxidant/antioxidant system by manipulating the flight activity levels. They found that, when exposed to a short-term major increase in physical activity, individuals suffer more OS. Specifically, the birds had high levels of PO. Therefore, as was expected for the study area, S. maccormicki showed higher levels of oxidative damage than S. antarcticus, especially their adults who make the foraging trips on marine prey.
A study done over 92 bird species showed substantial differences in antioxidant capacity due to the effect of dietary constituents (Cohen et al. 2007). The incorporation of low molecular weight non-enzymatic antioxidants such as tocopherols, carotenoids, GSH, ascorbic acid, and uric acid was highly diet dependent, and their presence attenuates or inhibits oxidative stress. Therefore, TG levels measured, herein, might be directly related to the election of the diet. Besides, depending on the type of the chosen prey, the levels of oxidative damage (PO and LPO) could be modified indirectly. For example, it was observed in White-Throated Sparrows (Zonotrichia albicollis) that, when fed diets rich in polyunsaturated fatty acids (PUFA), they showed greater oxidative damage compared with those fed with low concentrations of the same PUFA (Alan and McWilliams 2013). Another study done on Adélie penguins (Pygoscelis adeliae) during two consecutive summers showed that the year they fed a higher percentage of fish, instead of krill, they had lower antioxidant capacity and higher oxidative damage than the following year (Beaulieu et al. 2010). Thus, the levels of TG, PO, and LPO registered in the present work might be affected by the diet.
The chick´s development is a stage of life in which OS levels can rise as a result of the high metabolic rate with an immature antioxidant system (López Arrabé et al. 2016 and references therein). For S. maccormicki, particularly, chicks were characterized by their high SOD and GPx activities more than by their blood oxidative damage (Fig. 5). This could indicate that, at the time of sampling, the chicks’ antioxidant system was already mature or the antioxidants from the diet provided by their parents were sufficient to keep the redox balance. The chicks of both species did not show high levels of oxidative damage, although it was noted that the S. maccormicki chicks have higher PO levels than the S. antarcticus ones. This observation was consistent with that detected in the embryos of piscivorous birds which were particularly susceptible to peroxidative damage due to the prevalence of long-chain high PUFA in the maternal diet and, consequently, in the yolk (Surai et al. 2001).
Circulating antioxidants concentration intra- and inter-species could, therefore, be viewed as a function of multiple factors, including but not limited to diet, antioxidant metabolism that appears to differ across species and ontogeny irrespective of diet (Cohen et al. 2009). In short, apparently, the adults would choose the resources to exploit because of the quality or availability of prey. The competition between both species was evident, each one took advantage of a different ecological niche while nesting in the same area at the same time (personal observation). On the other hand, during the parental care, the chicks do not make foraging trips, so they have a similar diet as adults. Thus, it may be argued that in both skuas species, the antioxidant status was determined by age and the type of diet.
Considering the multiple factors that affect the S. maccormicki and S. antarcticus antioxidant status, the information reported, herein, is a first promising dataset that would allow giving reference values for these species. Oxidative stress is considered the main physiological mechanism involved in short- and long-term life histories, operating through the progressive deterioration of tissues, with consequences in the reproductive performance, growth patterns, aging, and survival (Costantini 2014). Therefore, if studies are continued during the development of these seabirds, a more complete set of data will be obtained, which can be reliably related to growth, reproductive stage, and aging. Also, they will be fundamental for comparing them with observations developed in other skuas´ populations and in hybrid individuals. Besides, future sex-specific studies are necessary because differences between sexes can influence the oxidative state parameters during the breeding season (Alonso-Alvarez et al. 2004; Costantini 2010).
Under the present Antarctic environmental change and in agreement with what was pointed out by Beaulieu and Costantini (2014), the markers of oxidative status may be a priori informative tool to evaluate individual perspectives of reproduction and survival. In this way and according to the Antarctic Treaty (2009), it will be possible to optimize the actual management plans for protecting Antarctic species and wildlife in general.
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Akaike H (1992) Information theory and an extension of the maximum likelihood principle. In: Kotz S, Johnson NL (eds) Breakthroughs in statistics. Springer, New York
Akerboom T, Sies H (1981) Assay of glutathione disulfide and glutathione mixed disulfides in biological samples. Method Enzymol 77:373–382. https://doi.org/10.1016/s0076-6879(81)77050-2
Alan RR, McWilliams SR (2013) Oxidative stress, circulating antioxidants, and dietary preferences in songbirds. Comp Biochem Phys B 164:185–193. https://doi.org/10.1016/j.cbpb.2012.12.005
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G (2004) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett 7:363–368. https://doi.org/10.1111/j.1461-0248.2004.00594.x
Alonso-Alvarez C, Pérez-Rodríguez L, García J, Viñuela J, Mateo R (2010) Age and breeding effort as sources of individual variability in oxidative stress markers in a bird species. Physiol Biochem Zool 83:110–118. https://doi.org/10.1086/605395
Ansaldo M, Repetto MG, Montalti D (2005) Comparison of blood antioxidant responses among Catharacta maccormicki (South Polar Skua) and Catharacta antarctica lönnbergi (Brown Skua). In: Evolution of Antarctic fauna: extended abstracts of the IBMANT/ANDEEP International Symposium and Workshop in 2003. Thatje S, Calcagno J, Arntz W (Eds). Berichte zur Polar und Meeresforschung (Reports on Polar and Marine Research), Bremerhaven, Alfred Wegener Institute for Polar and Marine Research, 507:200. https://doi.org/10.2312/BzPM_0507_2005
Ansaldo M, Sacristán H, Wider EA (2007) Does starvation influence the antioxidant status of the digestive gland of Nacella concinna in experimental conditions? Comp Biochem Phys C 146:118–123. https://doi.org/10.1016/j.cbpc.2006.11.004
Antarctic Treaty (2009) Annex II to the Protocol on Environmental Protection. Conservation of Antarctic fauna and flora. https://www.ats.aq/e/protocol.html
Beaulieu M, Costantini D (2014) Biomarkers of oxidative status: missing tools in conservation physiology. Conserv Physiol 2:1–16. https://doi.org/10.1093/conphys/cou014
Beaulieu M, Ropert-Coudert Y, Le Maho Y, Ancel A, Criscuolo F (2010) Foraging in an oxidative environment: relationship between δ13C values and oxidative status in Adélie penguins. Proc R Soc B 277:1087–1092. https://doi.org/10.1098/rspb.2009.1881
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Method Enzymol 52:302–3010. https://doi.org/10.1016/s0076-6879(78)52032-6
Cohen A, Klasing K, Ricklefs R (2007) Measuring circulating antioxidants in wild birds. Comp Biochem Phys B 147:110–121. https://doi.org/10.1016/j.cbpb.2006.12.015
Cohen A, McGraw K, Robinson WD (2009) Serum antioxidant levels in wild birds vary in relation to diet, season, life history strategy, and species. Oecologia 161:673–683. https://doi.org/10.1007/s00442-009-1423-9
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251. https://doi.org/10.1111/j.1461-0248.2008.01246.x
Costantini D (2010) Effects of diet quality on serum oxidative status and body mass in male and female pigeons during reproduction. Comp Biochem Phys A 156:294–299. https://doi.org/10.1016/j.cbpa.2010.02.021
Costantini D, Dell’Ariccia G, Lipp HP (2008) Long flights and age affect oxidative status of homing pigeons (Columba livia). J Exp Biol 211:377–381. https://doi.org/10.1242/jeb.012856
Costantini D, Monaghan P, Metcalfe NB (2013) Loss of integration is associated with reduced resistance to oxidative stress. J Exp Biol 216:2213–2220. https://doi.org/10.1242/jeb.083154
Costantini (2014) Oxidative stress and hormesis in evolutionary ecology and physiology. A marriage between mechanistic and evolutionary approaches. Springer, Berlin. ISBN 978-3-642-54663-1 (eBook). https://doi.org/10.1007/978-3-642-54663-1
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2015) InfoStat Software v2015. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL https://www.infostat.com.ar.
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Sci 7:129–142. https://doi.org/10.1198/10857110260141193
Flohé L, Gunzler WA (1984) Assays of glutathione peroxidase. Method Enzymol 105:114–121. https://doi.org/10.1016/s0076-6879(84)05015-1
Graña Grilli M, Libertelli M, Montalti D (2011) Diet of South Polar Skua chicks in two areas of sympatry with Brown Skua. Waterbirds 34:495–498. https://doi.org/10.1675/063.034.0412
Graña Grilli M, Montalti D (2012) Trophic interactions between Brown and South Polar Skuas at Deception Island, Antarctica. Polar Biol 35:299–304. https://doi.org/10.1007/s00300-011-1054-6
Graña Grilli M, Montalti D (2015) Variation in diet composition during the breeding cycle of an Antarctic seabird in relation to its breeding chronology and that of its main food resource. Polar Biol 38:643–649. https://doi.org/10.1007/s00300-014-1627-2
Graña Grilli M, Pari M, Ibañez A (2018) Poor body conditions during the breeding period in a seabird population with low breeding success. Mar Biol 165:142–150. https://doi.org/10.1007/s00227-018-3401-4
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-s-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hall ME, Blount JD, Forbes S, Royle NJ (2010) Does oxidative stress mediate the trade-off between growth and self-maintenance in structured families? Funct Ecol 24:365–373. https://doi.org/10.1111/j.1365-2435.2009.01635.x
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Ibañez AE, Graña Grilli M, Figueroa A, Pari M, Montalti D (2018) Declining health status of Brown Skua (Stercorarius antarcticus lonnbergi) parents and their offspring during chick development. Polar Biol 41:193–200. https://doi.org/10.1007/s00300-017-2181-5
Isaksson C, Örnborg J, Stephensen E, Andersson S (2005) Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. EcoHealth 2:138–146. https://doi.org/10.1007/s10393-005-3869-5
Kim S-Y, Noguera JC, Morales J, Velando A (2010) Heritability of resistance to oxidative stress in early life. J Evol Biol 23:769–775. https://doi.org/10.1111/j.1420-9101.2010.01942.x
Kopp M, Peter HU, Mustafa O, Lisovski S, Ritz MS, Phillips RA, Hahn S (2011) South Polar Skuas from a single breeding population overwinter in different oceans though show similar migration patterns. Mar Ecol Prog Ser 435:263–267. https://doi.org/10.3354/meps09229
Krietsch J, Hahn S, Kopp M, Phillips RA, Peter HU, Lisovski S (2017) Consistent variation in individual migration strategies of Brown Skuas. Mar Ecol Prog Ser 578:213–225. https://doi.org/10.3354/meps11932
López Arrabé J, Cantarero A, Pérez-Rodríguez L, Palma A, Moreno J (2016) Oxidative stress in early life: associations with sex, rearing conditions, and parental physiological traits in nestling pied flycatchers. Physiol Biochem Zool 89:83–92. https://doi.org/10.1086/685476
Lowry OM, Rosenbrough NJ, Farr L, Randall RJ (1951) Protein measurement with the phenol reagent. J Biol Chem 193:265–275
Malzof S, Quintana RD (2008) Diet of the South Polar Skua Catharacta maccormicki and the Brown Skua C. antarctica lonnbergi at Cierva Point, Antarctic Peninsula. Polar Biol 31:827–835. https://doi.org/10.1007/s00300-008-0421-4
Meister A, Anderson ME (1983) Glutathione. Ann Rev Biochem 52:711–760. https://doi.org/10.1146/annurev.bi.52.070183.003431
Metcalfe NB, Alonso-Alvarez C (2010) Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol 24:984–996. https://doi.org/10.1111/j.1365-2435.2010.01750.x
Misra HP, Fridovich Y (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Monaghan P, Metcalfe N, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92. https://doi.org/10.1111/j.1461-0248.2008.01258.x
Montalti D, Casaux R, Coria NR, Soave GE, Graña Grilli M (2009) The importance of fish in the diet of the South Polar Skua (Stercorarius maccormicki) at the South Shetland Islands, Antarctica. Emu 109:305–309. https://doi.org/10.1071/mu09042
Nikolaidis MG, Kyparos A, Spanou C, Paschalis V, Theodorou AA, Vrabas IS (2012) Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J Exp Biol 215:1615–1625. https://doi.org/10.1242/jeb.067470
Norwegian Polar Institute (2018). Quantarctica 3 (Quantum GIS [Since 2.0 just QGIS] + Antarctica = "Quantarctica"). The following data sets were used: SCAR. Antarctic Digital Database, British Antarctic Survey, Cambridge (2017); Reference for the use of the material is 'COMNAP 2017'; Terauds A (2016) An update to the Antarctic Specially Protected Areas (ASPAs), Australian Antarctic Data Centre. https://quantarctica.npolar.no/.
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Özkan S, Basmacioğlu Malayoğlu H, Yalçin S, Karadaş F, Koçtürk S, Çabuk M, Oktay G, Özdemir S, Özdemir E, Ergül M (2007) Dietary vitamin E (α-tocopherol acetate) and selenium supplementation from different sources: performance, ascites-related variables and antioxidant status in broilers reared at low and optimum temperatures. Brit Poult Sci 48:580–593. https://doi.org/10.1080/00071660701593951
Phillips RA, Catry P, Silk JRD, Bearhop S, McGill R, Afanasyev V, Strange IJ (2007) Movements, winter distribution and activity patterns of Falkland and Brown Skuas: insights from loggers and isotopes. Mar Ecol Prog Ser 345:281–291. https://doi.org/10.3354/meps06991
Reinhardt K, Hahn S, Peter HU, Wemhoff H (2000) A review of the diets of Southern Hemisphere skuas. Mar Ornithol 28:7–19
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Method Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Ritz MS, Steffen H, Janickle T, Peter HU (2006) Hybridisation between South Polar Skua (Catharacta maccormicki) and Brown Skua (C. antarctica lonnbergi) in the Antarctic Peninsula region. Polar Biol 29:153–159. https://doi.org/10.1007/s00300-005-0034-0
Romero Haro AA, Alonso-Alvarez C (2015) The level of an intracellular antioxidant during development determines the adult phenotype in a bird species: a potential organizer role for glutathione. Am Nat 185:390–405. https://doi.org/10.1086/679613
Schreiber EA, Burger J (2001) Biology of marine birds. CRC Press, Boca Raton
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464. https://doi.org/10.1214/aos/1176344136
Sies H (1985) Oxidative stress: introductory remarks. Academic Press Inc., London
Smith SM, Nager RG, Costantini D (2016) Meta-analysis indicates that oxidative stress is both a constraint on and a cost of growth. Ecol Evol 6:2833–2842. https://doi.org/10.1002/ece3.2080
Surai PF, Bortolotti GR, Fidgett AL, Blount JD, Speake BK (2001) Effects of piscivory on the fatty acid profiles and antioxidants of avian yolk: studies on eggs of the gannet, skua, pelican and cormorant. J Zool 255:305–312. https://doi.org/10.1017/s0952836901001406
Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
Acknowledgements
The authors acknowledge the Instituto Antártico Argentino staff that collaborated in sampling. This work was totally funded by the PICTO 0091 (FONCyT/DNA-IAA) project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Fonzo, C., Montalti, D. & Ansaldo, M. Blood antioxidant status of Stercorarius maccormicki and Stercorarius antarcticus from Potter Peninsula, 25 de Mayo (King George) Island, Antarctica. Polar Biol 43, 745–754 (2020). https://doi.org/10.1007/s00300-020-02676-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02676-5