Abstract
Mitochondrial respiration is a multi-step pathway that involves matrix and membrane-associated enzymes and plays a key role in acclimation to variable environmental conditions, but until now it has not been clear which of these steps would be most important in acclimation to changing temperatures and CO2 levels. Considering scenarios of ocean warming and acidification we assessed the role and limitation to phenotypic plasticity in the hearts of two Gadoid species adapted to different thermal ranges: the polar cod (Boreogadus saida), an Arctic stenotherm, and the Northeast Arctic population of Atlantic cod (NEAC, Gadus morhua), a cold eurytherm. We analysed the capacity of single enzymes involved in mitochondrial respiration [citrate synthase (CS), succinate dehydrogenase (SDH), cytochrome c oxidase (CCO)], the capacity of the electron transport system and the lipid class composition of the cellular membranes. Juveniles of the two species were held for four months at four temperatures (0, 3, 6, 8 °C for polar cod and 3, 8, 12, 16 °C for NEAC), at both ambient and elevated \(P_{{{\text{CO}}_{2} }}\) (400 µatm and 1170 µatm, respectively). Polar cod showed no changes in mitochondrial enzyme capacities and in the relative lipid class composition in response to altered temperature or elevated \(P_{{{\text{CO}}_{2} }} .\) The lack of cardiac cellular plasticity together with evidence at the whole-animal level coming from other studies is indicative of little or no ability to overcome stenothermy, in particular during acclimation to 8 °C. In contrast, eurythermal NEAC exhibited modifications of membrane composition towards a more rigid structure and altered enzyme capacities to preserve functionality at higher temperatures. Furthermore, in NEAC, the capacities of SDH, CCO and CS were increased by high levels of CO2 if combined with high temperatures (12 and 16 °C), suggesting the compensation of an inhibitory effect. These results indicate that the cold eurythermal species (NEAC) is able to alter its mitochondrial function to a far greater extent than the Arctic stenotherm (polar cod), indicating greater resilience to variable environmental conditions. This difference in plasticity may underpin differences in the resilience to climate change and affect future species distributions and, eventually, survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature has a defining role in the life of ectotherms, especially those living in an aquatic environment given their much-reduced ability to alter body temperatures through behavioural means. In some cases, species have developed mechanisms to compensate for temperature effects, notably visible in cold water fish which show partial compensation for the suppressing effect of cold temperatures on metabolic rates, for example through enhanced enzyme activities and/or adjustments to the density and capacity of mitochondria (Torres and Somero 1988; Crockett and Sidell 1990; Lannig et al. 2003). However, these adjustments are suggested to be different between cold stenothermal and cold eurythermal fish (Pörtner et al. 2000; Pörtner 2002). While cold stenotherms tend to have high mitochondrial densities but low-mitochondrial capacity in aerobic tissues (Pörtner et al. 2000, 2008), eurythermal cold adaptation rather involves maximized mitochondrial capacities (Pörtner et al. 2008; Blier et al. 2013). This reveals the presence of alternative strategies to cope with an extreme conditions depending on the level of environmental variability. The on-going increase in oceanic temperature, coupled with elevated \(P_{{{\text{CO}}_{2} }}\) (causing ocean acidification), raises the question of how cold stenothermal and cold eurythermal fish will respond to the future climate, given their different physiologies.
In a previous study, we analysed the potential for mitochondrial acclimation in permeabilized heart fibres of two Gadoid species living in the waters off the Svalbard archipelago (Leo et al. 2017). We chose polar cod (Boreogadus saida) as a cold (Arctic) stenotherm (thermal range around Svalbard − 2 to + 7 °C) (Laurel et al. 2016; Mark and Rohardt 2016) and the Northeast Arctic population of Atlantic cod (NEAC, Gadus morhua) as a cold eurytherm (experiencing temperatures ranging from − 1.5 °C in winter in the Barents Sea up to 10 °C when moving along the Norwegian coast at spawning time) (Righton et al. 2010; Michalsen et al. 2014). The fish heart is a key organ since it is highly responsive to temperature change (Farrell 2007; Ekström et al. 2016). Most of the energy demand of the fish heart is supplied by mitochondrial metabolism. The increased requirement for energy at higher temperatures is mirrored by shifts in the activity of individual enzymes or even between metabolic pathways (Lucassen et al. 2006; Melzner et al. 2009; Windisch et al. 2011; Strobel et al. 2013a, b). We also found that mitochondria of polar cod subjected to relatively high temperatures (8 °C) displayed increased proton leak, causing decreasing mitochondrial efficiency, and a decreased capacity of cytochrome c oxidase (CCO); they did not respond to changes in \(P_{{{\text{CO}}_{2} }} .\) In contrast, mitochondrial function and efficiency of NEAC was preserved at elevated temperatures (16 °C), but overall mitochondrial capacity was depressed by a combination of high temperature and high \(P_{{{\text{CO}}_{2} }}\) (Leo et al. 2017).
Here, we focus on the cellular and biochemical causes of these differences in mitochondrial response. Mitochondrial respiration is a complex multi-step process that involves several enzymes, some of them embedded in the mitochondrial membranes. Thus, the study of the capacity of specific enzymes involved in mitochondrial metabolism and the analysis of the composition of the membrane in which they are embedded can help us to understand and identify the causes of the difference in acclimation potential of the two species. Many studies have focused on the effect of high temperature on mitochondrial enzyme capacities. For example, warm-acclimated fish of a number of species displayed suppressed citrate synthase (CS) activity while CCO activity remained more or less unchanged (Hardewig et al. 1999; Lannig et al. 2003; Lucassen et al. 2006; Windisch et al. 2011). Few studies have addressed the effect of elevated \(P_{{{\text{CO}}_{2} }}\) on mitochondrial enzymes: Michaelidis et al. (2007) reported a decrease in CS activity in the heart of the temperate sea bream (Sparus aurata) incubated at high \(P_{{{\text{CO}}_{2} }}\) and unchanged temperature. Strobel et al. (2013a) found decreased activities of both CS and CCO in the heart of Antarctic Notothenia rossii acclimated to high temperature and elevated \(P_{{{\text{CO}}_{2} }} .\) It is unclear whether and how these changes in mitochondrial enzyme activity may be paralleled by a change in mitochondrial membrane structure. Long-term exposure to high temperatures alters the structure of cellular membranes, with more rigid membranes in warm-adapted or warm-acclimated individuals to counteract the destabilizing effects of elevated temperature (Dahlhoff and Somero 1993; Kraffe et al. 2007). The remodelling of membrane lipids owing to increasing temperature involves changes in the head group composition (e.g. choline lowers the membrane fluidity and ethanolamine increases it), length and saturation of the acyl chains and where present, changes in cholesterol (Chol) content (Hazel 1995; Kraffe et al. 2007; Hoffmann and Todgham 2010). However, little is known about the effects of elevated \(P_{{{\text{CO}}_{2} }}\) on the lipid composition of cellular membranes (Strobel et al. 2013b).
In this study, we analysed the combined effects of temperature and ocean acidification on the capacity of key enzymes involved in mitochondrial metabolism: CS (located in the matrix) and succinate dehydrogenase (SDH, membrane-bound complex II), being enzymes of the tricarboxylic acid cycle (TCA) cycle, and CCO and NADH:cytochrome c oxidoreductase (CI:CIII) as indicators of the capacity of the electron transport system which are all membrane-associated complexes. We also quantified the lipid composition of the pool of cellular membranes. These mitochondrial traits are measured in the hearts of polar cod and NEAC held for 4 months at either their present ambient or at elevated temperatures (0, 3, 6 or 8 °C and 3, 8, 12 or 16 °C according to the species), and either at current \(P_{{{\text{CO}}_{2} }}\) levels (400 µatm) or at those projected for the year 2100 in the Arctic (1170 µatm; representative concentration pathway, RCP 8.5, Van Vuuren et al. 2011). These measurements allow us to evaluate how adjustments in mitochondrial enzyme capacities and in membrane lipids contribute to the acclimation potential of the two species and when put in relation with higher organismic levels they can help to understand the differences in thermal plasticity.
Materials and methods
Animal collection and incubation
Animal collection and incubation are described in detail in Kunz et al. (2016) and Leo et al. (2017). Briefly, juvenile polar cod were caught by the University of Tromsø (NO) in January 2013 from the inner part of Kongsfjorden (Svalbard, NO). Afterwards, they were kept at 3.3–3.8 °C and 32 PSU in the facilities of the Havbruksstasjonen i Tromsø AS (HiT) in Kårvik (NO) until late April 2013 when they were transported to the Alfred Wegener Institute’s (AWI) facilities in Bremerhaven (Germany) and held at 5 °C and 32 PSU. Juvenile NEAC were caught during the RV Heincke cruise HE408 in August 2013 from various locations of the Svalbard Archipelago and transported to the AWI facilities in Bremerhaven, where they were kept at 5 °C and 32 PSU until the beginning of the incubation.
The incubations of polar cod and NEAC started in June 2013 and in May 2014, respectively. Fish from both species were kept for at least 4 weeks at 5 °C and 32 PSU to acclimate to laboratory conditions, then they were placed in single tanks and randomly allocated to temperature and \(P_{{{\text{CO}}_{2} }}\) treatment groups (n = 12 for each group). Individual polar cod were held at water temperatures of 0, 3, 6 or 8 °C, while NEAC were held at 3, 8, 12 or 16 °C, with each temperature treatment being combined with a \(P_{{{\text{CO}}_{2} }}\) of 400 µatm (control \(P_{{{\text{CO}}_{2} }}\)) or 1170 µatm (high \(P_{{{\text{CO}}_{2} }}\)) in a 4 × 2 cross design for both species. In all cases, fish were held in temperature-controlled rooms on a 12 h:12 h light/dark cycle. The seawater temperature, salinity, DIC and pH (total scale) were measured once to twice a week in triplicates throughout the incubation period; these parameters are reported in the Open Access Library PANGAEA (Schmidt et al. 2016). The animals were held under their treatment conditions for 4 months and were fed ad libitum with commercial pellet feed (Amber Neptun, 5 mm, Skretting AS, Norway) every fourth day.
Sampling
Sampling took place after four days of fasting at the end of the 4-month treatment period. Half of the fish present at the end of the incubation time were used in the study by Leo et al. (2017), the second half was used in this study. Fish were anaesthetized with 0.2 g L−1 tricaine methane sulphonate (MS222) and killed by a spinal cut behind the head plate. Hearts were rapidly excised and frozen in liquid nitrogen. Mean length, weight and number of the specimens used this study are given in Table 1.
Enzymatic activity
Frozen heart samples were homogenized in 9 volumes (w:v) of ice cold extraction buffer (Tris–HCl 20 mM, Na–EDTA 1 mM, Triton X-100 0.1%, pH 7.4 at 4 °C) with a tissue homogenizer (Precellys 24, Bertin Technologies, France) at 5000 rpm and 4 °C, 3 times for 15 s. The homogenate was then centrifuged at 1000 g and 4 °C for 10 min. The supernatant was used for analysis of enzymatic activities. Enzyme activities of each sample were measured at all four treatment temperatures for the species using a UV/Vis spectrophotometer (Specord S600, Analytik Jena AG, Germany) equipped with a thermostatted cell holder. The buffer pH was set to be constant at the acclimation temperatures to simulate the acclimation and to change according to temperature variation during the acute test. CS activity was detected in a buffer containing Tris–HCl 75 mM pH 8.0, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) 0.25 mM, acetyl-CoA 0.4 mM, oxaloacetate 0.5 mM. The activity was determined from the increase in absorbance at λ = 412 nm, due to the transfer of sulfhydryl groups from coenzyme A to DTNB, using the extinction coefficient ε412 = 13.61 mol−1 cm−1 (Sidell et al. 1987).
CCO activity was detected in a buffer containing Tris–HCl 20 mM pH 7.8, cytochrome cred 0.057 mM, Tween 20 0.5%. The activity was determined from the decrease in extinction at λ = 550 nm due to the oxidation of cytochrome c, using the extinction coefficient ε550 = 19.1 mol−1 cm−1 (modified after Moyes et al. 1997).
SDH activity was detected in a buffer containing imidazole/HCl buffer 100 mM pH 8.0, MgCl2 5 mM, succinate 20 mM, sodium azide 4 mM, antimycin A 0.04 mM, rotenone 0.005 mM, 2,6-dichlorphenolindophenol (DCPIP) 0.1 mM, ubiquinone Q1 0.1 mM. The activity was determined from the decrease in extinction at λ = 600 nm due to the reduction of DCPIP, using the extinction coefficient ε600 = 19.2 mol−1 cm−1 (modified after Lemieux et al. 2010).
CI:CIII was determined in a buffer containing imidazole/HCl buffer 25 mM pH 7.4, MgCl2 2 mM, sodium azide 4 mM, cytochrome cox 0.08 mM, NADH 0.2 mM. The activity was determined from the increase in extinction at λ = 550 nm due to the reduction of cytochrome c, using the extinction coefficient ε550 = 19.1 mol−1 cm−1 (modified after Möller and Palmer 1982). All chemicals were obtained by Sigma-Aldrich (Germany).
Lipid class determination
The lipid class composition of heart cellular membranes was determined for the polar cod and NEAC from the lowest and highest temperature treatments (0 vs. 8 °C and 3 vs. 16 °C, respectively) and both \(P_{{{\text{CO}}_{2} }}\) treatments. Membrane lipids were extracted after Folch et al. (1957). The frozen hearts were homogenized in a glass homogenizer containing 6 mL dichloromethane:methanol (v 2:1) for three times at 1200 U min−1 for 2 min. The extract was then diluted with 2 mL of 0.88% KCl and centrifuged for 5 min at 1000 rpm. The lipid phase was carefully separated and the aqueous phase was diluted again with 2 mL dichloromethane and centrifuged three times for five minutes at 1000 rpm to completely separate the lipid from the aqueous phase. After complete evaporation of the lipid phase with nitrogen, the raw extract was suspended in 50 µL chloroform. The lipid classes were separated and identified according to Graeve and Janssen (2009) on a monolithic silica column (ChromolithPerformance-Si) using high-performance liquid chromatography (HPLC, LaChromElite HPLC system) with an evaporative light-scattering detector (ELSD). A gradient program with combination of three solvent mixtures was used: eluent A consisted of isooctane:ethylacetate (99.8:0.2, v/v), eluent B was a mixture of acetone and ethyl acetate [2:1, v/v) containing acetic acid (0.02% (v/v)] and eluent C 2-propanol–water (85:15, v/v) [with acetic acid and ethanolamine, each 0.05% (v/v)].
Data analysis
Values are given as mean ± SEM if not stated otherwise. Data analysis was performed using R 3.2.0 (R Core Team 2015), with the level of statistical significance for all statistical tests set at p < 0.05.
Enzymatic activity
Enzyme activities were calculated per mg fresh-weight (U mg FW−1) of heart tissue. The normality and the homoscedasticity of the data were tested by Shapiro–Wilk test and Bartlett’s test, respectively. The differences in enzymatic activities were evaluated by two-way ANOVA followed by Tukey-HSD post hoc test. The temperature coefficients Q10 of the enzyme activities were calculated according to the equation:
where R is the enzyme capacity and T is the temperature in “°C” at which the enzyme capacities were measured. Q10 was calculated for the enzymes CS and CCO for the following temperature ranges: 0–6 °C and 6–8 °C for polar cod and 3–12 °C and 12–16 °C for NEAC.
The CCO:CS ratio was calculated for both species.
Lipid class composition
After the assessment of normality (Shapiro–Wilk test) and homoscedasticity (F test), the differences in lipid class compositions between CO2 treatments were tested by Student’s t test with Welch correction in the case of non-homogeneous variances. Since there was no significant effect of CO2 treatment, the data from the same temperature groups were pooled to examine effects of temperature alone. The Chol, phosphatidylethanolamine (PE) and phosphatidylcholine (PC) contents were calculated as percentages of total lipid content (polar and neutral lipids). In addition, the PE:PC ratio was calculated. The differences between temperatures in the lipid class compositions were tested using Student’s t test with the Welch correction if needed.
Results
Enzymatic capacity
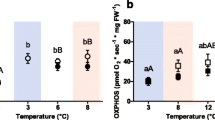
The capacity of the four enzymes analysed in this study (CS, CCO, SDH, CI:CIII) is illustrated in Fig. 1 for polar cod and Fig. 2 for NEAC. Q10 values are presented in Table S1 of the Supplementary Material.
Enzymatic capacities of heart tissue of polar cod (Boreogadus saida) acclimated and measured at the respective acclimation temperature. a Citrate synthase (CS), b cytochrome c oxidase (CCO), c NADH:cytochrome c oxidoreductase (CI:CIII), and d succinate dehydrogenase (SDH). Within panels: *indicates the difference between CO2 treatments at the same incubation temperature. Lower case letters indicate significant differences across temperature at control \(P_{{{\text{CO}}_{2} }} ,\) upper case letters indicate the difference across temperature treatments at high \(P_{{{\text{CO}}_{2} }} .\) White bars: control \(P_{{{\text{CO}}_{2} }}\) (400 µatm), grey bars: high \(P_{{{\text{CO}}_{2} }}\) (1170 µatm). Values are given as means ± SEM
Capacities of mitochondrial enzymes from heart tissue of NEAC (Gadus morhua) measured at the respective acclimation temperatures. a Citrate synthase (CS), b cytochrome c oxidase (CCO), c NADH:cytochrome c oxidoreductase (CI:CIII), and d succinate dehydrogenase (SDH). Values are presented as means ± SEM. In the panel: *indicates the difference between CO2 treatments at the same incubation temperature. Lower case letters indicate differences between temperature treatments at control \(P_{{{\text{CO}}_{2} }} ,\) upper case letters indicate differences between temperature treatments at high \(P_{{{\text{CO}}_{2} }} .\) White bars: control \(P_{{{\text{CO}}_{2} }}\) (400 µatm), grey bars: high \(P_{{{\text{CO}}_{2} }}\) (1170 µatm)
Polar cod (Fig. 1)
In polar cod CS capacity was unaffected by temperature (F = 1.24, p > 0.05), but in the cold temperature treatments (0 and 3 °C) CS capacity was higher in the groups incubated at high \(P_{{{\text{CO}}_{2} }}\) compared to the control groups (Fig. 1a: 0 °C: p = 0.0471; 3 °C: p = 0.0064). The Q10 values for CS (Table S1) ranged from 0.65 ± 0.25 to 1.91 ± 0.40 for the 0–6 °C range and from 0.25 ± 0.06 to 1.08 ± 0.42 for the 6–8 °C range, and so were significantly higher when measured over the lower temperature range (p = 0.0009). The temperature treatment also had an effect on Q10 (p = 0.0018), with lowered values with increasing acclimation temperature.
CCO capacity was sensitive to both temperature and CO2 treatments (temperature: F3,32 = 4.38, p = 0.0108; CO2: F1,32 = 8.82, p = 0.0056), with thermal sensitivity being influenced by the CO2 treatment (temperature × CO2 interaction: F3,32 = 3.25, p = 0.0345; Fig. 1b). In fact, while CCO capacity followed a bell-shaped thermal reaction norm in the control \(P_{{{\text{CO}}_{2} }}\) groups with a maximum at 3 °C, CCO capacity in the high \(P_{{{\text{CO}}_{2} }}\) groups was high and similar across all three lower test temperatures but decreased at 8 °C. CCO Q10 values did not vary between the tested temperature ranges (p > 0.05), being between 0.27 ± 0.03 and 2.03 ± 0.72 in the 0–6 °C range and between 0.16 ± 0.04 and 1.90 ± 0.54 in the 6–8 °C range. Both treatment temperature and CO2 had an effect on Q10 (temperature: p = 0.0144; CO2: p = 0.0049), with Q10 values generally lower in the high \(P_{{{\text{CO}}_{2} }}\) groups. The CCO:CS ratio was not affected by CO2 treatment (F1,33 = 0.158, p > 0.05) but showed temperature sensitivity (F3,33 = 3.487, p = 0.0265), with higher values at intermediate temperatures (Fig. 3a).
CCO:CS ratios in polar cod (Boreogadus saida, panel a) and NEAC (Gadus morhua, panel b) heart tissue measured at acclimation temperature. Within panels: lower case letters indicate differences between temperatures at control \(P_{{{\text{CO}}_{2} }} ,\) upper case letters indicate differences between temperatures at high \(P_{{{\text{CO}}_{2} }} ,\) *indicates differences between CO2 treatments at the same temperature. Values are reported as means ± SEM. White bars: control \(P_{{{\text{CO}}_{2} }}\) (400 µatm), grey bars: high \(P_{{{\text{CO}}_{2} }}\) (1170 µatm)
CI:CIII capacity exhibited no differences between treatments but an interaction between the two drivers (temperature: F3,33 = 2.24, p > 0.05; CO2: F1,33 = 0.24, p > 0.05; temperature × CO2 interaction: F1,33 = 3.98, p = 0.0158; Fig. 1c). SDH capacity was not significantly affected by CO2 treatment (F1,33 = 3.29; p > 0.05) but was influenced by temperature, being highest at the intermediate (6 °C) treatment (Fig. 1d).
NEAC (Fig. 2)
In NEAC, acclimation to high \(P_{{{\text{CO}}_{2} }}\) increased CS capacity (F1,16 = 30.66, p < 0.0001) compared to the control \(P_{{{\text{CO}}_{2} }}\) at all temperatures except at 8 °C (p > 0.05). Acclimation temperature also affected CS capacity (F3,16 = 23.68, p < 0.0001), with the lowest capacities at intermediate temperatures (Fig. 2a). Q10 values for CS activity ranged between 0.47 ± 0.04 and 1.32 ± 0.17 for the 3–12 °C temperature range and between 0.06 ± 0.02 and 4.42 ± 0.14 for the 12–16 °C temperature range (Table S1). The values varied according to temperature range (p = 0.0409) and to acclimation temperature (p < 0.0001), but with an interaction between these two factors (p < 0.0001). Q10 values were thus highest at temperatures farthest away from the acclimation temperature. For instance, for the groups acclimated at 3 °C the Q10 values were higher in the 12–16 °C range (control CO2: p < 0.0001; high CO2: p = 0.0408), while in the groups acclimated at 12 and 16 °C the Q10 values were higher in the 3–12 °C range (12 °C/control CO2: p < 0.0001; 12 °C/high CO2: p = 0.0230; 16 °C/control CO2: p = 0.0434; 16 °C/high CO2: p > 0.05).
CCO capacity increased with rising acclimation temperatures (F3,20 = 31.64, p < 0.0001; Fig. 2b). CO2 acclimation decreased the activity of CCO (F1,20 = 8.402, p = 0.0089), but this effect was primarily driven by differences at the highest temperatures (temperature × CO2 interaction: F3,20 = 3.62, p = 0.0310). Q10 values were statistically similar between temperature ranges (p > 0.05), temperature treatments (p > 0.05) and CO2 treatment (p > 0.05; Table S1). They ranged between 1.01 ± 0.22 and 1.96 ± 0.17 for the 3–12 °C range and between 0.51 ± 0.14 and 1.92 ± 0.84 for the 12–16 °C range. The CCO:CS ratio was sensitive to both temperature and CO2 (temperature: F3,16 = 35.10, p < 0.0001; CO2: F1,16 = 4.518, p = 0.0456), increasing with temperature but at each temperature tending to be lower for the high \(P_{{{\text{CO}}_{2} }}\) groups (Fig. 3b).
As with polar cod, CI:CIII in NEAC did not vary with treatment (p > 0.05; Fig. 2c). SDH capacity was unaffected by temperature treatment (F3,21 = 2.22, p > 0.05) but was higher in the fish held under high \(P_{{{\text{CO}}_{2} }}\) (3 °C: p > 0.05, 8 °C: p = 0.0501, 12 °C: p = 0.0629, 16 °C: p = 0.0064; Fig. 2d).
Comparison between species
NEAC had greater CS capacities than did polar cod at 3 °C (control CO2: p < 0.0001; high CO2: p = 0.0355), but this difference became smaller at 8 °C (control CO2: p = 0.0500; high CO2: p < 0.0001) due to a reduction in the CS capacity of NEAC. However, the two species did not differ in activities for the other mitochondrial enzymes (CCO, CI:CIII and SDH) at either of the two common test temperatures (p > 0.05).
Lipid classes
The Chol, PE and PC composition and the PE:PC ratio are shown in Table 2 for both polar cod and NEAC. In polar cod, none of the lipid classes changed in relative abundance with rising incubation temperatures (Chol: p > 0.05, PE: p > 0.05, PC: p > 0.05, PE:PC: p > 0.05). In NEAC, the percentage of Chol and PE did not change with temperature (Chol: p > 0.05, PE: p > 0.05), however, the PC content tended to be higher in the 16 °C group compared to the 3 °C group, although not significantly (p = 0.0626).
Discussion
This study highlights the effects of current levels and future scenarios of ocean acidification and warming on the activity levels and capacity of key mitochondrial enzymes, and on the lipid composition of cellular membranes. We studied an Arctic stenotherm (polar cod) and a cold eurytherm (the NEAC) currently living in the same region (the water off the Svalbard Archipelago) but both being at the limit of their thermal range (upper limit for polar cod, lower limit for NEAC). Unlike earlier studies on the effects of temperature on mitochondrial enzymes of cold-adapted fish (Lucassen et al. 2003, Lucassen et al. 2006; Strobel et al. 2013a, b), which analysed only CS and CCO capacity, we chose four enzymes (CS, CCO, SDH and CI:CIII) to broaden our view on the mitochondrial respiration processes. Moreover, while many previous studies used limited acclimation time or unrealistically high \(P_{{{\text{CO}}_{2} }}\) levels (e.g. Lucassen et al. 2003, Lucassen et al. 2006; Michaelidis et al. 2007), our study includes long-term acclimation (4 months) as well as temperature and \(P_{{{\text{CO}}_{2} }}\) values projected for the year 2100 (RCP 8.5, Van Vuuren et al. 2011).
In general, the data reflect the importance of looking at the capacity of a more complete set of enzymes involved in mitochondrial metabolism, as they varied in their responses to temperature and \(P_{{{\text{CO}}_{2} }} .\) Among those measured, CS and SDH were the most sensitive to CO2, while CCO was mainly affected by temperature (as well as the lipid layer in which it is embedded) and CI:CIII showed little acclimation to either temperature or \(P_{{{\text{CO}}_{2} }} .\) Furthermore, data will be discussed in the light of the previous results obtained from the same acclimation project by Kunz et al. (2016) and Leo et al. (2017). The enzymes analysed in this study were selected to be the same or to be directly linked with the features examined in the above-mentioned studies.
Enzymatic capacity
Polar cod exhibited very little modification of enzyme capacities after being held for several months under elevated temperatures and high \(P_{{{\text{CO}}_{2} }}\). CS capacity did not change between polar cod held at different temperatures, although the Q10 decreased upon warming, indicating a slight warm acclimation. CS capacity was higher in fish held under elevated \(P_{{{\text{CO}}_{2} }}\) but the Q10 values were similar to the control \(P_{{{\text{CO}}_{2} }}\) groups suggesting that incubation under high levels of CO2 does not affect the acute thermal tolerance of this enzyme. CCO showed different thermal trends in response to the \(P_{{{\text{CO}}_{2} }}\) incubation levels (Fig. 1b; Table 3), indicating an interaction between these two drivers. Under control levels of \(P_{{{\text{CO}}_{2} }}\) the thermal reaction norm highlights 3 °C as the optimum temperature for this enzyme, whereas in fish held at high \(P_{{{\text{CO}}_{2} }}\) levels the marked decrease at 8 °C paralleled by a very low Q10 for this group may suggest the onset of thermal constraints.
In NEAC, the CS capacity of the control \(P_{{{\text{CO}}_{2} }}\) groups follows the thermal trajectory well described for cold eurythermal fish, including other studies on NEAC (Lannig et al. 2003; Lucassen et al. 2003, 2006; Khan et al. 2014), with decreased capacity at warmer temperatures; the Q10 values indicate warm acclimation through downregulation in the 12 and 16 °C groups. However, the increased capacity upon CO2 acclimation suggests compensatory upregulation.
Unlike in polar cod, the CCO capacity of NEAC increased with increasing acclimation temperature, with each acclimation group showing similar Q10 values, indicating that NEAC is able to modulate the capacity of this enzyme, following an increased respiratory demand. An increased CCO capacity and decreased CS capacity during warm acclimation in cold-adapted fish has previously been reported (Windisch et al. 2011). Windisch et al. (2011) suggested that the alteration of the CCO:CS ratio was due to changes in respiratory capacity (through CCO) as well as a change in the multiple functions of CS, especially a reduction in the provision of intermediates from the TCA cycle, e.g. to fatty acids and amino acid anabolism. In our study, the CCO:CS ratio showed little modification in polar cod but increased with temperature in NEAC, highlighting the greater potential for warm acclimation in NEAC compared to polar cod.
The differences in enzymatic capacities of polar cod and NEAC could be explained by different fuel preferences in their heart tissues. Cold-adapted fish show a preference towards fatty acids metabolism rather than carbohydrates (Guderley and Gawlicka 1992; Rodnick and Sidell 1994; Driedzic et al. 1996). The β-oxidation of fatty acids produces acetyl-CoA for the TCA cycle as well as FADH2 and NADH + H+, which feed directly into the Electron Transport System. This could explain why polar cod showed lower capacity of CS (a key enzyme of the TCA cycle) at 3 and 8 °C compared to NEAC despite similar capacities of CI:CIII and CCO (directly fed by FADH2 and NADH + H+).
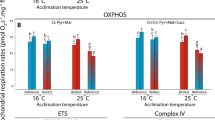
The suggested preference of polar cod heart for catabolizing fatty acids and the subsequent excess of CCO and CI:CIII enzymes compared to CS could explain the relative thermostability in mitochondrial function for this species. However, combining this study with earlier studies examining other aspects of the same acclimation experiment (Kunz et al. 2016; Leo et al. 2017; summarized in Table 3) suggests an inability to increase enzymatic capacity and/or switching to other metabolic pathways at 8 °C. At this temperature the mitochondrial proton leak (LEAK) increased exponentially but this was not paralleled by an increase in oxidative phosphorylation capacity (OXPHOS, Table 3). At the whole-animal level, the 8 °C groups were characterized by increased SMR (Table 3) and increased mortality (Kunz et al. 2016), indicating that the metabolic restrictions evident at the mitochondrial level at 8 °C could be translated into long-term constraints on whole-animal performance. A caveat might still be that the common source of data may lead to interpretations that are limited by the lack of independence between the features analysed in this study.
The performance of NEAC was affected more by the combination of temperature and high \(P_{{{\text{CO}}_{2} }} .\) In the groups incubated at 16 °C, CS, CCO and SDH had a higher capacity in the fish incubated under high \(P_{{{\text{CO}}_{2} }} .\) However, we previously showed that the OXPHOS of permeabilised heart tissue from the animals incubated under these conditions was lower than in the control \(P_{{{\text{CO}}_{2} }}\) group (Leo et al. 2017; Table 3). Exposure to elevated levels of CO2 in the water can cause an increase in intracellular bicarbonate in fish (Brauner et al. 2004; Michaelidis et al. 2007; Strobel et al. 2012, 2013b). High bicarbonate levels can competitively inhibit citrate oxidation in mammalian kidney mitochondria, with a resulting increase in intracellular citrate concentration (Simpson 1967) that can inhibit CS by feedback mechanisms, thus reducing the activity of the whole TCA cycle. An increase in the capacity of CS, like the one seen in this study on NEAC, may compensate for the initial inhibition by bicarbonate. In a previous study, Strobel et al. (2013b) found a decreased flux through SDH under high \(P_{{{\text{CO}}_{2} }}\) levels in the Antarctic fish N. rossii, suggesting an inhibition by elevated CO2 (Wanders et al. 1983). To overcome this inhibition (seen in respiring mitochondria), the enzymatic capacity of SDH may be enhanced, e.g. by increasing the enzyme abundance, and this enhancement will result in an increased capacity of the enzyme in vitro as seen in the present study.
Lipid classes
The relative composition of lipid classes in cellular membranes differed only in relation to species and incubation temperature but not in relation to \(P_{{{\text{CO}}_{2} }} .\) While in polar cod there were no changes in the relative composition of the lipid classes, in NEAC there were signs of modulation of the membrane composition that may maintain membrane fluidity and properties at high temperatures. This modulation, termed homeoviscous adaptation (Hazel 1995), is mainly present in eurythermal fish (Cossins and Bowler 1987; Hazel 1995; Kraffe and et al. 2007; Grim et al. 2010; Hoffmann and Todgham 2010). Such modifications of membrane composition in warm-adapted or warm-acclimated fish imply an increased proportion of saturated fatty acids, increased Chol content and changes in the PE:PC ratio to counteract the destabilizing effects of elevated temperature (Dahlhoff and Somero 1993; Kraffe et al. 2007). In our study, the NEAC groups incubated at 16 °C had a similar Chol content but a higher PC content and a lower PE:PC ratio compared to the cod kept at 3 °C. This is in line with the study of Kraffe et al. (2007) on rainbow trout (Oncorhynchus mykiss), in which the PE:PC ratio was the most responsive marker to increases in acclimation temperature. As PC is considered a bilayer stabilizer, a ratio in favour of PC would lower membrane fluidity (Hazel 1995; Kraffe et al. 2007; Hoffmann and Todgham 2010), causing the membrane to be more rigid, and therefore to maintain fluidity at high temperatures (Dahlhoff and Somero 1993; Kraffe et al. 2007). Although the determination of the relative lipid class composition was conducted on the entire pool of cellular membranes, we assume that the same pattern of adjustments is reflected in the mitochondrial membranes alone. Mitochondrial membranes have a lower Chol content and PC and PE are their main constituents, accounting together for about 80% of the total phospholipid composition (Daum 1985). During thermal adaptation and acclimation, the PE:PC ratio changes in the mitochondrial membranes in a similar way as in other cellular membranes (Wodtke 1978, 1981) and as presented in this study. Since mitochondrial membrane lipids play a role in the modulation of mitochondrial processes (Schlame et al. 2000; Kraffe et al. 2007), the inability of polar cod to adjust the properties of the membranes may contribute to the decrease in capacity of the membrane-associated enzymes SDH and CCO in the groups incubated at 8 °C and the abrupt increase in proton leak in the heart mitochondria from the same acclimation group compared to the colder groups (Table 3). On the other hand, the trend in NEAC towards membrane modification in response to temperature could in part explain the lack of thermal sensitivity of the membrane-bound enzymes SDH and CI:CIII and the increase in CCO capacity at high temperature (Table 3).
Conclusions
In this study, we analysed how temperature and \(P_{{{\text{CO}}_{2} }}\) levels alter the enzymatic capacities of four enzymes involved in mitochondrial respiration and the lipid composition of the cellular membranes, comparing an Arctic stenotherm and a cold eurytherm fish. Furthermore, we discussed how the differences in plasticity between the two species could affect their relative response to future ocean acidification and warming.
The Arctic stenotherm polar cod showed little acclimation potential at the level of mitochondrial enzymes as well as in terms of membrane composition. The inability of polar cod to modify the capacity of their mitochondrial enzymes upon warm acclimation may have little impact at temperatures below 6 °C, but could set long-term limitations to performance at 8 °C since a less efficient mitochondrial respiration is paired with increased SMR (Table 3) and mortality (Kunz et al. 2016). Moreover, the lack of modification of membrane composition could become detrimental through compromising the functionality of membrane-associated enzymes such as CCO and SDH at higher temperatures. In contrast, NEAC displays all the features of a cold eurytherm, showing thermal adjustments in CCO and CS capacities, and in membrane composition. Even if mitochondrial respiration in the heart of NEAC is negatively affected by the combination of high temperature and CO2 (Table 3), this happens only at temperatures far above the projections for the end of the century for the Arctic region. Therefore, cardiac mitochondrial metabolism of polar cod appears to be more sensitive to future Arctic Ocean conditions than NEAC and only a profound change in the cardiac mitochondrial function could generate the plasticity needed by polar cod to cope with future temperatures.
Abbreviations
- NEAC:
-
Northeast Arctic population of Atlantic cod
- RCP:
-
Representative concentration pathway
- CS:
-
Citrate synthase
- CCO:
-
Cytochrome c oxidase
- SDH:
-
Succinate dehydrogenase
- CI:CIII:
-
NADH:cytochrome c oxidoreductase
- Chol:
-
Cholesterol
- PE:
-
Phosphatidylethanolamine
- PC:
-
Phosphatidylcholine
- TCA cycle:
-
Tricarboxylic acid cycle
References
Blier PU, Lemieux H, Pichaud N (2013) Holding our breath in our modern world: will mitochondria keep the pace with climate changes? Can J Zool 92:591–601
Brauner CJ, Wang T, Wang Y, Richards JG, Gonzalez RJ, Bernier NJ, Xi W, Patrick M, Val AL (2004) Limited extracellular but complete intracellular acid–base regulation during short-term environmental hypercapnia in the armoured catfish, Liposarcus pardalis. J Exp Biol 207:3381–3390
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, London
Crockett EL, Sidell BD (1990) Some pathways of energy metabolism are cold adapted in Antarctic fishes. Physiol Zool 63(3):472–488
Dahlhoff E, Somero GN (1993) Effect of temperature on mitochondria from abalone (Genus: Haliotis): adaptive plasticity and its limits. J Exp Biol 185:151–168
Daum G (1985) Lipids of mitochondria. Biochim Biophys Acta 822:1–42
Driedzic WR, Bailey JR, Sephton DH (1996) Cardiac adaptations to low temperature in non-polar teleost fish. J Exp Zool 275:186–195
Ekström A, Brijs J, Clark TD, Gräns A, Jutfelt F, Sandblom E (2016) Cardiac oxygen limitation during an acute thermal challenge in the European perch: effects of chronic environmental warming and experimental hyperoxia. Am J Physiol Reg Integr Comp Physiol 311:R440–R449
Farrell AP (2007) Cardiorespiratory performance during prolonged swimming tests with salmonids: a perspective on temperature effects and potential analytical pitfalls. Philos Trans R Soc B 362:2017–2030
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Graeve M, Janssen D (2009) Improved separation and quantification of neutral and polar lipid classes by HPLC-ELSD using a monolithic silica phase: application to exceptional marine lipids. J Chromatogr B 877:1815–1819
Grim JM, Miles DRB, Crockett EL (2010) Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J Exp Biol 213:445–452
Guderley H, Gawlicka A (1992) Qualitative modification of muscle metabolic organization with thermal acclimation of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem l0:123–132
Hardewig I, Peck LS, Pörtner H-O (1999) Thermal sensitivity of mitochondrial function in the Antarctic Notothenioid Lepidonotothen nudifrons. J Comp Physiol B 169:597–604
Hazel JR (1995) Thermal adaptation in biological membranes: is homeoviscous adaptation an explanation? Annu Rev Physiol 57:19–42
Hoffmann GE, Todgham AE (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72:127–145
Khan JR, Iftikar FI, Herbert NA, Gnaiger E, Hickey AJR (2014) Thermal plasticity of skeletal muscle mitochondrial activity and whole animal respiration in a common intertidal triplefin fish, Forsterygion lapillum (Family: Tripterygiidae). J Comp Physiol B 184:991–1001
Kraffe E, Marty Y, Guderley H (2007) Changes in the mitochondrial oxidative capacities during thermal acclimation of rainbow trout Oncorhynchus mykiss: roles of membrane proteins, phospholipids and their fatty acid compositions. J Exp Biol 210:149–165
Kunz KL, Frickenhaus S, Hardenberg S, Johansen T, Leo E, Pörtner H-O, Schmidt M, Windisch HS, Knust R, Mark FC (2016) New encounters in Arctic waters: a comparison of metabolism and performance of polar cod (Boreogadus saida) and Atlantic cod (Gadus morhua) under ocean acidification and warming. Polar Biol 39:1137–1153
Lannig G, Eckerle LG, Serendero I, Sartoris FJ, Fischer T, Knust R, Johansen T, Pörtner H-O (2003) Temperature adaptation in eurythermal cod (Gadus morhua): a comparison of mitochondrial enzyme capacities in boreal and Arctic populations. Mar Biol 142:589–599
Laurel BJ, Spencer M, Iseri P, Copeman LA (2016) Temperature-dependent growth and behavior of juvenile Arctic cod (Boreogadus saida) and co-occurring North Pacific gadids. Polar Biol 39:1127–1135
Lemieux H, Tardif JC, Dutil JD, Blier PU (2010) Thermal sensitivity of cardiac mitochondrial metabolism in an ectothermic species from a cold environment, Atlantic wolfish (Anarhichas lupus). J Exp Mar Bio Ecol 384:113–118
Leo E, Kunz KL, Schmidt M, Storch D, Pörtner H-O, Mark FC (2017) Mitochondrial acclimation potential to ocean acidification and warming of polar cod (Boreogadus saida) and Atlantic cod (Gadus morhua). Front Zool 14:21
Lucassen M, Schmidt A, Eckerle LG, Pörtner H-O (2003) Mitochondrial proliferation in the permanent vs. temporary cold: enzyme activities and mRNA levels in Antarctic and temperate zoarcid fish. Am J Physiol Regul Integr Comp Physiol 285:R1410–R1420
Lucassen M, Koschnick N, Eckerle LG, Pörtner H-O (2006) Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J Exp Biol 209:2462–2471
Mark FC, Rohardt G (2016) Continuous thermosalinograph oceanography along HEINCKE cruise track HE451-1. PANGAEA, Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/PANGAEA.863418
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner H-O (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
Michaelidis B, Spring A, Pörtner H-O (2007) Effects of long-term acclimation to environmental hypercapnia on extracellular acid–base status and metabolic capacity in Mediterranean fish Sparus aurata. Mar Biol 150:1417–1429
Michalsen K, Johansen T, Subbey S, Beck A (2014) Linking tagging technology and molecular genetics to gain insight in the spatial dynamics of two stocks of cod in Northeast Atlantic waters. ICES J Mar Sci 71:1417–1432
Möller IM, Palmer JM (1982) Direct evidence for the presence of a rotenone resistant NADH dehydrogenase on the inner surface of the inner membrane of plant mitochondria. Physiol Plant 54:267–274
Moyes CD, Mathieu-Costello OA, Tsuchiya N, Filburn C, Hansford RG (1997) Mitochondrial biogenesis during cellular differentiation. Am J Physiol Cell Physiol 272:C1345–C1351
Pörtner H-O (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761
Pörtner H-O, van Dijk PLM, Hardewig I, Sommer A (2000) Levels of metabolic cold adaptation: tradeoffs in eurythermal and stenothermal ectotherms. In: Davison W, Williams HC (eds) Antarctic ecosystems: models for wider ecological understanding. Caxton Press, Christchurch, pp 109–122
Pörtner H-O, Bock C, Knust R, Lannig G, Lucassen M, Mark FC, Sartoris FJ (2008) Cod and climate in a latitudinal cline: physiological analyses of climate effects in marine fishes. Clim Res 37:253–270
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 4 April 2017
Righton DA, Haste Andersen K, Neat F, Thorsteinsson V, Steingrund P, Svedäng H, Michalsen K, Hinrichsen H-H, Bendall V, Neuenfeldt S, Wright P, Jonsson P, Huse G, van der Kooij J, Mosegaard H, Hüssy K, Metcalfe J (2010) Thermal niche of Atlantic cod Gadus morhua: limits, tolerance and optima. Mar Ecol Prog Ser 420:1–13
Rodnick KJ, Sidell BD (1994) Cold acclimation increases carnitine palmitoyltransferase I activity in oxidative muscle of striped bass. Am J Physiol 266:R405–R412
Schlame M, Rua D, Greenberg ML (2000) The biosynthesis and functional role of cardiolipin. Prog Lipid Res 39:257–288
Schmidt M, Leo E, Kunz KL, Lucassen M, Windisch HS, Storch D, Bock C, Pörtner H-O, Mark FC (2016) Time series of seawater carbonate chemistry calculated throughout incubation periods of Boreogadus saida and Gadus morhua during exposure to different CO2 and temperature conditions. PANGAEA, Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven. https://doi.org/10.1594/PANGAEA.866369
Sidell BD, Driedzic WR, Stowe DB, Johnston IA (1987) Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol Zool 60:221–232
Simpson DP (1967) Regulation of renal citrate metabolism by bicarbonate ion and pH: observations in tissue slices and mitochondria. J Clin Investig 46:225
Strobel A, Bennecke S, Leo E, Mintenbeck K, Pörtner H-O, Mark FC (2012) Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and PCO2. Front Zool 9:28
Strobel A, Leo E, Pörtner H-O, Mark FC (2013a) Elevated temperature and PCO2 shift metabolic pathways in differentially oxidative tissues of Notothenia rossii. Comp Biochem Physiol B 166:48–57
Strobel A, Graeve M, Pörtner H-O, Mark FC (2013b) Mitochondrial acclimation capacities to ocean warming and acidification are limited in the Antarctic nototheniid fish, Notothenia rossii and Lepidonotothen squamifrons. PLoS ONE 8:e68865
Torres JJ, Somero GN (1988) Metabolism, enzymic activities and cold adaptation in Antarctic mesopelagic fishes. Mar Biol 98:169–180
Van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F et al (2011) The representative concentration pathways: an overview. Clim Change 109:5–31
Wanders RJA, Meijer AJ, Groen AK, Tager JM (1983) Bicarbonate and the pathway of glutamate oxidation in isolated rat-liver mitochondria. Eur J Biochem 133:245–254
Windisch HS, Kathöver R, Pörtner H-O, Frickenhaus S, Lucassen M (2011) Thermal acclimation in Antarctic fish: transcriptomic profiling of metabolic pathways. Am J Physiol Regul Integr Comp Physiol 301:1453–1466
Wodtke E (1978) Lipid adaptation in liver mitochondrial membranes of carp acclimated to different environmental temperatures. Phospholipid composition, fatty acid pattern and cholesterol content. Biochim Biophys Acta 529:280–291
Wodtke E (1981) Temperature adaptation of biological membranes. The effects of acclimation temperature on the unsaturation of the main neutral and charged phospholipids in mitochondrial membranes of the carp (Cyprinus carpio L.). Biochim Biophys Acta 640:698–709
Acknowledgements
We thank Silvia Hardenberg, Timo Hirse, Isabel Ketelsen, Nils Koschnick, Kristina Kunz, Matthias Schmidt and Heidrun Windisch for their help during the incubation and sampling and Dieter Janssen and Valeria Adrian for their assistance during the lipid extraction and analysis. Furthermore, we acknowledge the Project Polarisation (Norwegian Research Council, 214184/F20) for providing polar cod specimens and the crews of RV Heincke (HE 408) and RV Helmer Hanssen (University of Tromsø) for the animal collection. This study was supported by the German Federal Ministry of Education and Research (BMBF, FKZ 03F0655B, FKZ 03F0728B) within the Research Program BIOACID Phase II and by the PACES Program of AWI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The handling of the specimens of polar cod and NEAC was carried out according to the Ethical Permission Number AZ522-27–22/02–00 (113) released by the Senator for Healthcare, Bahnhofsplatz 29, 28195 Bremen on February 21st, 2013 (permit valid until February 21st, 2018).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the special issue on the “Arctic Gadids in a Changing Climate”, coordinated by Franz Mueter, Haakon Hop, Benjamin Laurel, Caroline Bouchard, and Brenda Norcross.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leo, E., Graeve, M., Storch, D. et al. Impact of ocean acidification and warming on mitochondrial enzymes and membrane lipids in two Gadoid species. Polar Biol 43, 1109–1120 (2020). https://doi.org/10.1007/s00300-019-02600-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02600-6