Abstract
The thermal sensitivity of Arctic fish species is poorly understood, yet such data are a critical component of forecasting and understanding ecosystem impacts of climate change. In this study, we experimentally measured temperature-dependent growth and routine swim activity in the juvenile stage of two Arctic gadids (Arctic cod, Boreogadus saida and saffron cod, Eleginus gracilis) and two North Pacific gadids (walleye pollock, Gadus chalcogrammus and Pacific cod, Gadus macrocephalus) over a 6-week growth period across five temperatures (0, 5, 9, 16 and 20 °C). Arctic cod demonstrated a cold-water, stenothermic response in that there was relatively high growth at 0 °C (0.73 % day−1), near-maximal growth at 5 °C (1.35 % day−1) and negative impacts on activity, growth and survival at 16 °C. In contrast, saffron cod demonstrated a warmer-water, eurythermic response, and temperature had a positive effect on growth and condition beyond 16 °C. However, despite these distinct thermal responses, walleye pollock and Pacific cod grew 2–3 times faster than Arctic gadids across a relatively broad temperature range above 5 °C. These results, coupled with possible northward expansion by both Pacific cod and walleye pollock, suggest Arctic cod are highly vulnerable to continued climate change in the Arctic, especially in coastal areas of the Beaufort and Chukchi Seas where temperatures already exceed 14 °C in the summer growth period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is arguably the most important environmental component driving development, growth and survival of marine fish. In cold-water marine systems, small fluctuations in temperature can have profound effects on an individual’s vital rates (i.e., growth and energetic condition), which at the population level can mediate connectivity patterns (O’Connor et al. 2007), genetic structure (Bradbury et al. 2010), cohort survival and eventual recruitment to the adult fish population (Houde 2008). In the Arctic, atmospheric warming is occurring at a more rapid rate than temperate zones (IPCC 2014), and observational data indicate Arctic Ocean surface temperatures are increasing in every region, e.g., 0.5 °C per decade in the Chukchi Sea (Jeffries et al. 2014). Such changes are predicted to impact the entire ecosystem by way of species invasion and changes in productivity (Smetacek and Nicol 2005; Cheung et al. 2009), but accuracy in forecasting (e.g., niche-level modeling) is hindered by the availability of species-specific physiological data. The temperature sensitivity of Arctic fish species has been labeled ‘woefully underrepresented’ within the general knowledge base of cold-water physiology in animals (Farrell and Steffensen 2005). Therefore, due to uncertainty in input data, current forecasting models can only serve as a null hypothesis for future theoretical and empirical studies.
In Alaskan waters, the Arctic region extends northward from the Eastern Bering Sea to the Chukchi Sea, and from the Chukchi eastward to include the Beaufort Sea. Arctic cod (Boreogadus saida) and saffron cod (Eleginus gracilis) are recognized as ecologically important prey species in the Chukchi and Beaufort Sea (hereon referred to as ‘Arctic gadids’), whereas walleye pollock Gadus chalcogrammus and Pacific cod Gadus macrocephalus occupy similar roles in the Bering Sea and further south in the Gulf of Alaska (hereon referred to as ‘North Pacific gadids’). Both Arctic cod and walleye pollock have a pelagic life history and associate with ice-edge blooms (Peck et al. 2004; Barber et al. 2008; Coyle et al. 2008), whereas saffron cod and Pacific cod are more demersal and more commonly found in shallow, nearshore regions as juveniles (Laurel et al. 2007; Fechhelm et al. 2008; Hurst et al. 2015). All four gadid species are abundant and form an essential component of cold-water food webs by transferring energy from the plankton to upper-trophic-level animals such as marine mammals, birds, and other fish (Bain and Sekerak 1978; Lowry et al. 1980; Craig et al. 1982; Springer et al. 1986). However, Arctic cod are particularly rich in energy (Harter et al. 2013), and there is concern that changes in their distribution or abundance as the result of climate change could have profound effects on Arctic ecosystems (Moore and Huntington 2008). This, coupled with a northern expansion of North Pacific gadids (Rand and Logerwell 2011), could result in a complete reorganization of community structure as a result of warming in the Arctic and adjacent seas (Bluhm and Gradinger 2008).
Population-level response of fish to the environment is generally determined during the early life history when mortality potential is highest (Houde 2008). Fish are poikilotherms, and the temperature of the surrounding water regulates a number of physiological processes (e.g., biochemical homeostasis, energy conversion efficiency, muscle performance, etc.) that are manifested collectively in terms of growth, condition, survival and swimming performance of the animal. Juvenile gadids must develop, grow and store lipid reserves rapidly during their first year to minimize predation and maximize overwintering survival (Laurel et al. 2003, 2007; Heintz and Vollenweider 2010). This is especially important in the Arctic where the summers are short and temperatures are <0 °C during the prolonged winter–spring spawning period (Bouchard and Fortier 2011). Food availability is also episodic during the larval–juvenile period in late spring by way of freshwater runoff events and ice-edge blooms (Wassmann 2006). Therefore, the combined ability to grow and store energy under changing thermal environments provides some indication whether populations will persist in regions undergoing rapid climate change.
In general, high-latitude fish species grow faster at colder temperatures than fish from lower latitudes, but have a narrower range of temperature preference and tolerance (stenothermic). In a relative sense, stenothermic fish species will likely be greatly affected by small changes in temperature resulting from climate change, whereas species with wide thermal tolerance (eurythermic) will likely be more able to accommodate increased regional warming without significant impact to their productivity in the central part of their range (Golovanov 1996). As such, warm water gadid populations are thought to be more growth sensitive to changes in food availability, whereas cold-water populations are thought to be more sensitive to changes in temperature (Hutchings et al. 2007). Whether these patterns are consistent across other life history stages is uncertain, but these data indicate the need to gather species-specific physiological data, as it can be highly variable across related species within the same region.
We conducted a series of comparative laboratory experiments on the temperature-dependent growth, condition and activity of similarly sized juvenile gadids from the Arctic and North Pacific (Arctic cod, saffron cod, walleye pollock, and Pacific cod). A priori we assumed Arctic and saffron cod would have physiology adapted for their cold environment, whereas walleye pollock and Pacific cod would perform better in warmer conditions. The goal of this study was to test this assumption as well as to identify temperature ‘tipping points’ where growth conditions become more favorable for one species over another. We discuss these results in the context of hypothesized biogeographical changes among gadid populations resulting from global warming at the North Bering–Chukchi Sea interface.
Methods
All husbandry and experiments were conducted at the Alaska Fisheries Science Center’s laboratory at the Hatfield Marine Science Center in Newport, OR, USA. Live juvenile fish of similar size were collected by either fyke net (Arctic and saffron cod), beach seine (Pacific cod), or lift net (walleye pollock) and shipped from a variety of locations to Newport, OR in 2012 (see Table 1 for details). Before use in laboratory experiments, fish were maintained in 1-m-diameter round tanks with flow-through seawater maintained at 6–8 °C. Holding temperatures were selected on the basis that they were within 3° of the temperature at capture for all species across regions. Fish were fed thawed krill and herring before being weaned onto a gelatinized combination of squid, krill, herring, commercial fish food, amino acid supplements and vitamins (‘gel food’; 52 % protein, 14 % fat). The gel food was prepared in batches every week, frozen and then thawed just prior to feeding.
Growth experiments were conducted in a series of experimental tanks (66 × 46 × 38 cm) supplied with flow-through, temperature-controlled seawater. For each species, fish were initially measured and stocked into experimental tanks at a density of three individuals/tank. The size range of fish used in the experiments was purposely restricted to focus on the effects of temperature rather than size-dependent growth (Peck et al. 2003; Hurst et al. 2012; See Table 1). However, small size differences among individual fish (2–4 mm SL) within each tank were purposefully established in lieu of marking individuals to track individual growth. After establishment of experimental groups, temperatures were adjusted to treatment temperatures (0, 5, 9, and 16 °C) at a rate of 1.5 °C per day. Replicate tanks (n = 2) of each temperature treatment (n = 4) were assigned for each species (n = 4) to comprise a total of 32 tanks. At week 2 following the acclimation period, fish were weighed to the nearest 0.01 g (wet mass) and measured to the nearest 1 mm (SL) to establish ‘time 0’ for the growth experiment. Fish were quickly blot-dried on absorbent paper and weighed in a container of seawater to minimize stress to the animal. After measurement, all fish were returned to their original tank and remeasured in the same manner at week 4 (period week 2–week 4) and again at week 6 (period week 4–week 6) of the experiment to assess growth. Fish were fed daily by hand to apparent satiation using the gelatinized diet. Apparent satiation was determined as the point where excess food remained in the tank 1 h after feeding. Daily feedings were conducted in this manner across the entire acclimation and experimental period. Lights were maintained on a 12:12 light/dark photoperiod for all experiments. Tanks were checked daily for mortalities, and dead fish were removed, weighed and measured.
At the end of the experiment, an additional 20 °C trial was conducted for species able to survive and maintain growth at 16 °C during the initial experiment. Fish used for the 20 °C trial were sourced from general holding tanks and had not been used in the original growth experiment. The experimental design (n = 2 tanks; 3 fish/tank), 2-week acclimation period, daily feeding and growth measurements were identical to those conducted for the initial growth experiment conducted at 0–16 °C.
Swimming activity was measured every 1–2 weeks following the 2-week acclimation period. Observations were conducted in each tank just prior to daily feeding. Activity was scored by bisecting the tanks and scoring the number of times fish crossed the bisection during a 1-min period. Activity scores were averaged to the number of fish in the tank and across all time periods to develop an overall activity score for each replicate tank.
Data analysis
Specific growth rates based on mass of juvenile fish were determined using the following equations:
where g is the instantaneous growth coefficient obtained by the equation:
where WW i is the wet weight of an individual fish at time t i . SGR values were calculated at two time periods (week 2–4 and week 4–6) for each individual in each tank and averaged to develop a tank mean growth rate. However, only week 2–4 growth was used for species experiencing mortality later in the experiment from prolonged exposure to high temperatures. The same approach was used to calculate mean tank growth based on total tank biomass. Notable deviations in the two growth metrics can be an indicator of growth suppression due to social interactions within the tank. Temperature-dependent growth models in wet mass were developed for each species using a three-parameter polynomial function. The third parameter of the model was maintained for consistency in each species, regardless of its statistical significance. Tank replicates (mean SGR) were used as the level of observation for the temperature-dependent growth model of each species. Tank replicates were also used to test for statistical differences in mean SGR between species and temperature using a two-way ANOVA.
Condition for individual fish at the end of the growth experiment was based on length–weight residuals. A log–log transformed length–weight regression model was developed for each species prior to the start of the experiment using 40–50 fish in the size range used for the growth experiment (see Table 1). Departures in observed mass from those predicted from the fish’s length using the regression model provided an index of condition. Condition values were averaged for each tank and analyzed by way of two-way ANOVA to examine the effects of temperature and species.
Results
Saffron cod survived at all temperatures, whereas mortality occurred in the highest temperature treatment (either 16 or 20 °C) for the other gadids. Arctic cod were visibly stressed (i.e., reduced activity, dark in color, cranial hemorrhaging) after 2 weeks of exposure to 16 °C, suggesting that such conditions can only be tolerated for short periods of time, and mortality (n = 3) was observed at 4–5 weeks. In the 20 °C treatment, Pacific cod mortality started after 4 weeks, with only two fish surviving to week 5. The 20 °C treatment for walleye pollock was discontinued after the first growth measurement due to mortalities shortly after acclimation (n = 2 in week 3 and n = 2 just after week 4).
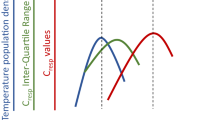
Growth metrics based on tank biomass and individual averages did not differ more than 0.2 % mass day−1 within any treatment (Table 2), suggesting relatively even growth among tank individuals. For the sake of simplicity, all growth results reported hereon are based on tank averages of mean individual growth. The two-way ANOVA on growth indicated a significant effect in the species × temperature interaction term (F 9, 16 = 4.192, P = 0.006), and the three-parameter growth model explained >95 % of the temperature-dependent growth for each species (Table 2; Fig. 1). Arctic cod were capable of highest growth at low temperatures compared to the other gadids. However, walleye pollock had a clear growth advantage at temperatures >2.5 °C, as did Pacific cod at temperatures >5 °C. At modeled temperatures between 10 and 11 °C, Pacific cod and walleye pollock had 2.5–3.0 times more growth than Arctic cod. Interestingly, saffron cod were most eurythermic in their growth response in that they were able to: (1) grow across all thermal conditions with 100 % survival and (2) maintain 80 % maximum growth over the broadest temperature range (10 vs 9 °C in Pacific cod and 8 °C in walleye pollock and Arctic cod).
Temperature-dependent growth models for four species of gadids: Arctic cod B. saida, saffron cod E. gracilis, Pacific cod G. macrocephalus and walleye pollock G. chalcogrammus. The three-parameter model predicts growth (G, % wet weight day−1) at a maximum food ration. Dotted arrows indicate the temperature of maximum growth (T max) for each species. Mean values are based on duplicate tanks (n = 2) of three fish per tank for each species at each temperature. Model and parameter values for each species are listed in Table 2
The two-way ANOVA detected a significant effect of temperature on swim activity (F 3, 16 = 9.183, P = 0.001), and no effect of species either in the interaction term (F 9, 16 = 1.212, P = 0.353) or separately in the model (F 3, 16 = 1.093, P = 0.381). A two-parameter quadratic model illustrated the positive increase in swim activity with temperature up to a maximum (S max) for each species (Fig. 2). Arctic cod were most active at lower temperatures and decreased activity at higher temperatures. The three other gadid species demonstrated a similar behavioral response to temperature, with peak activity between ~15 and 16 °C but higher variability at warmer temperatures. In the case of saffron cod, activity was highly variable across all temperature treatments. As such, the model of swim activity poorly fit the data for saffron cod (r 2 = 0.29) compared to Arctic cod (r 2 = 0.71), walleye pollock (r 2 = 0.59) and Pacific cod (r 2 = 0.54; Fig. 2).
Temperature-dependent swim activity of four species of gadids: Arctic cod B. saida, saffron cod E. gracilis, Pacific cod G. macrocephalus and walleye pollock G. chalcogrammus. Values represent averaged group swimming activity (n = 3 fish) in a tank observed every 1–2 weeks over a 6-week period. Activity was scored by bisecting the tanks and scoring the number of times fish crossed the bisection during a 1-min period. Dotted arrows indicate the temperature of maximum swimming activity (S max) based on two-parameter model-fits
A significant interaction was detected between species and temperature on the condition of fish at the end of the experiment (general linear model; F 3, 27 = 18.55, P < 0.001). The nature of the interaction is shown in Fig. 3. Arctic cod condition was negatively impacted at higher temperatures, whereas saffron cod were negatively impacted at 0°, but steadily increased in condition with increasing temperature. Pacific cod showed a weak, but negative, association of condition with temperature, whereas walleye pollock showed an opposite trend.
Temperature-dependent condition of four species of gadids; Arctic cod B. saida, saffron cod E. gracilis, Pacific cod G. macrocephalus and walleye pollock, G. chalcogrammus. Condition is based on the length–weight residuals of fish following 6 weeks of growth at each temperature. Mean values are based on duplicate tanks (n = 2) of three fish per tank for each species at each temperature. Model and parameter values for each species is listed in Table 2
Discussion
This study is one of the first examinations of temperature-dependent growth in Arctic and saffron cod, while providing additional information on temperature response in walleye pollock and Pacific cod (Hurst et al. 2010). Physiological studies of polar fish largely stem from the Antarctic Ocean, and few studies to date have focused on species from the Arctic (Peck et al. 2004; Farrell and Steffensen 2005). In addition, much of our understanding of temperature response in gadids is based on Atlantic studies of Gadus morhua (e.g., Otterlei et al. 1999; Peck et al. 2003; Folkvord 2005). While this study does not provide data to fully parameterize a bioenergetic model for these species at all life stages (e.g., consumption rates, growth efficiencies), the growth rates observed in this study were similar to published studies of Pacific gadids (discussed below) and within 5–7 % of growth rates observed in identical treatments at 0° and 9° for Arctic and saffron cod in a companion study examining the effects of food ration (Laurel, Iseri, Spencer and L. Copeman unpub. data). Therefore, this study provides both a starting point for modeling temperature-dependent growth in the field (under food-replete conditions) and identifying ‘optimum’ temperatures for growth in species with overlapping geographical range.
Arctic gadids
Arctic cod outperformed the other gadids at colder temperatures (<2 °C), but all metrics from our study (T max, condition factor and S max) indicate Arctic cod should seek >2 °C water (up to 7–8 °C) when food is abundant. Although such thermal habitat is generally not available in Arctic offshore waters, Arctic cod generally avoid intermediate depth regions where temperatures are below 0 °C, instead associating with near-surface waters or deeper Atlantic water where temperatures range between 0 and 2 °C (Crawford et al. 2012). In Lancaster Sound (Nunavut, Canada), Arctic cod generally move from lower and mid-water depths into the warmer upper water column (>2 °C) when waters become seasonally stratified (Crawford and Jorgenson 1996). In nearshore regions of the Beaufort Sea, Arctic cod are found in high abundance at 2–9 °C along thermal-salinity fronts (Moulton and Tarbox 1987). Assuming adequate food is present, Arctic cod could achieve maximum growth potential in these boundary zones, possibly making them important nursery habitat for age-0 and age-1 fish. However, surface and coastal waters can approach 14 °C in August (Craig et al. 1982), with evidence of even higher temperatures more recently (R. Fechhelm and K. McCain pers. comm.). Arctic cod are caught episodically in these extremely warm conditions, usually following onshore winds, which initially deliver colder, higher salinity water from local upwelling (Moulton and Tarbox 1987). Based on our results, Arctic cod can only briefly survive warm, nearshore environments due to reduced growth potential and condition. Therefore, the use of thermal-salinity fronts may be ‘big risk, big reward’ habitat for Arctic cod, especially with continued warming in the Arctic.
In contrast, growth and condition factors indicated saffron cod are a eurythermic species, a surprising result given they are year-round residents in the Arctic. However, saffron cod are routinely caught in high abundance in the nearshore around Prudhoe Bay, AK, where August temperatures exceed 14 °C (Fechhelm and K. McCain pers. comm.). Saffron cod growth in the 20 °C treatment slowed, but there were no mortalities in the experimental tanks, and condition of individual fish was relatively high compared to those in the other experimental treatment. This was in sharp contrast to the other gadids which were showing strong evidence of thermal stress above 16 °C, and even to walleye pollock collected from the lower latitudes of Puget Sound, WA. However, summer surface temperatures seldom range above 14 °C in the region juvenile walleye pollock were collected for this study. Saffron cod have a clear growth advantage over Arctic cod in water >10 °C. Recent observed increases of Saffron cod as far south as Prince William Sound and Kodiak Island have made them a species of interest in the Gulf of Alaska (Laurel et al. 2007; Johnson et al., 2009). Saffron cod have also been linked to low-salinity conditions in the Arctic (Wong et al. 2013). Together, these data and our study suggest that saffron cod may be more physiologically capable of surviving forecasted warming and freshening in the polar seas (Bluhm and Gradinger 2008).
Pacific gadids
Unlike Atlantic cod, for which the influence of temperature on growth has been studied for many years (Jobling 1988), experimental data for Pacific gadids have been relatively scarce thus far, with a greater reliance on rates derived from the field (e.g., Mazur et al. 2007). For walleye pollock, experimental growth data were limited to larger juvenile stages (30–60 g) grown over a narrow temperature range (3–7.5 °C; Smith et al. 1986), single- or bi-temperature studies (Sogard and Olla 2000, 2002) or populations from the Western Pacific (Kooka et al. 2007). Juvenile growth data for Pacific cod are scarcer, but were recently experimentally measured across a relatively broad range of temperatures (2–11 °C, Hurst et al. 2010; 2–13 °C, Hurst et al. 2012). The growth models from this study are unique in that they are parameterized across a broad range of temperatures, nearly capturing the full range of thermal conditions these species could presently experience during both the overwintering and summer growth period in Alaskan waters. Where temperature ranges overlapped, the growth rates from our study agreed with growth rates measured in earlier studies. For example, maximum growth of juvenile walleye pollock and Pacific cod (~2.1 % day−1) at ~8 °C was nearly identical to juvenile growth rates reported in earlier studies for these species (Smith et al. 1986; Kooka et al. 2007; Hurst et al. 2010). Similar growth rates are reported for age-0 juvenile Atlantic cod (1–10 g) grown at 8 °C under experimental conditions (e.g., Brown et al. 1989; Peck et al. 2003). However, T max of walleye pollock (13 °C) was slightly higher than that for Pacific cod (11.5 °C), and pollock had ~1.3× more growth potential at 16 °C than Pacific cod. These data suggest walleye pollock were slightly more warm-adapted than Pacific cod. However, juvenile pollock collected from the Western Pacific were more similar to Pacific cod in our study, with maximum growth at 11.5 °C and rapid decrease in growth at 16 °C (Kooka et al. 2007). While these growth differences are relatively subtle, it is likely that the Puget Sound population of walleye pollock used in our study were more warm-adapted than walleye pollock in Alaskan waters. In an earlier study of the same life stage, juvenile walleye pollock from the Puget Sound had ~10 % higher growth at 12 °C compared to the Gulf of Alaska population (Laurel, Spencer and Hurst unpub. data). In Atlantic cod, high-latitude fish populations tend to grow faster at colder temperatures than fish from lower latitudes, yet have a narrower range of temperature tolerance (Hutchings et al. 2007). The mechanisms for these differences are often assumed to be genetic, but the role of non-genetic effects (e.g., developmental phenotypic plasticity) could equally explain such differences since fish were collected from the field with unknown environmental histories (Hurst et al. 2012).
Biogeography of gadids and consequences of climate change
Gadid species have high commercial and ecological importance in the North Pacific and adjacent polar seas around Alaska, and there is concern how these species will cope with increased warming resulting from climate change. It is clear that temperature plays a dominant role in determining growth rate in each of the fish species examined. While these species may have thermal refuge in the offshore during the adult phase, juvenile stages are especially vulnerable because (1) they tend to be in surface or nearshore regions prone to atmospheric warming, (2) juvenile fish need to achieve rapid growth to avoid predation and survive overwintering environments and (3) early stages of fish generally cannot migrate long distances to more favorable habitats (Sogard 1997). Reductions in optimal thermal habitat can also lead to increased spatial overlap between juvenile and adults, resulting in higher rates of cannibalism (e.g., Cold Pool Hypothesis, Mueter et al. 2006). These mechanisms support the observed links between temperature and first year survival, adult recruitment and population persistence in gadids (Campana 1996; Drinkwater et al. 2010).
The T max values and the shape of the temperature-dependent growth curves from this study give some indication of the vulnerability of each species to changes in thermal habitat resulting from climate change. For example, the lower T max and more apparent stenothermic shape of the growth curve for Arctic cod suggest that continued climate change will eventually lead to reduced thermal habitat for this species while increasing thermal habitat for the Pacific gadids. Broadly speaking, these are the mechanisms by which marine fish populations are expected to shift northward (Cheung et al. 2009), resulting in changes in regional species assemblages (Attrill and Power 2002; Albouy et al. 2012). However, despite indications that gadids from the Pacific and Atlantic are becoming more abundant in the Arctic (Sundby and Nakken 2008; Rand and Logerwell 2011), there is debate whether some gadids will migrate through cold shelf water (<2 °C) following spring–summer ice-melt (Wyllie-Echeverria and Wooster 1998; Hollowed et al. 2012) or can establish new spawning populations under the ice (Hollowed et al. 2013). Still, influxes of warm water into Arctic regions have contributed to species overlap among Arctic and Boreal gadids, and there remains a great deal of uncertainty as to whether all species can coexist in the same region under changing environments (Renaud et al. 2012; Fossheim et al. 2015).
It is also important to recognize that temperature-dependent growth models are highly dependent on environmental conditions. Food limitation will likely lower the optimal temperatures for growth compared to T max values reported in our study (Björnsson et al. 2001), and food sensitivity will likely vary among species under common thermal environments. Similarly, metabolic costs are generally higher in the field than the laboratory due to predator avoidance, foraging and migratory behavior (Jobling 1988). Therefore, the growth models from this study should be considered best case scenarios and just a starting point in forecasting and developing hypotheses on biogeographical changes among these species. For example, the intersection of each species’ temperature-dependent growth curve may represent a ‘tipping point’ when thermal conditions could potentially favor survival in one species over another. Future experimental studies should focus on further developing bioenergetic models for these species.
The timing and extent of changes among these gadids have important ramifications to the higher trophic animals that depend on them. Gadids have broadly varying energetic value, both within and across species depending on geographical and ontogenetic state (e.g., Copeman et al. 2015). Perhaps more concerning are the changes in food conversion efficiency associated with each species at higher temperatures. Arctic cod are reported to convert more than 80 % of the energy from the prey biomass to fish biomass at 0 °C (Hop et al. 1997). Such high assimilation efficiency and an ability to maintain growth at low temperatures is a fundamental component of Arctic food webs (Bluhm and Gradinger 2008). At temperatures beyond those optimal for growth, conversion efficiencies generally decrease (Jobling 1988), and could be a significant factor in energy flow and ecosystem characteristics in Arctic. Continued work on the comparative physiology on these species should further our understanding of ecosystem impacts in the Arctic due to climate change.
References
Bain H, Sekerak AD (1978) Aspects of the biology of Arctic cod, Boreogadus saida, in the Central Canadian Arctic. Report prepared by LGL Limited for Polar Gas Project, Environmental Research Associates, Toronto
Albouy C, Guilhaumon F, Araujo MB, Mouillot D, Leprieur F (2012) Combining projected changes in species richness and composition reveals climate change impacts on coastal Mediterranean fish assemblages. Glob Change Biol 18:2995–3003
Attrill MJ, Power M (2002) Climatic influence on a marine fish assemblage. Nature 417:275–278
Barber DG, Lukovich JV, Keogak J, Baryluk S, Fortier L, Henry GHR (2008) The changing climate of the Arctic. Arctic 61:7–26
Björnsson B, Steinarsson A, Oddgeirsson M (2001) Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.). ICES J Mar Sci 58:29–38
Bluhm BS, Gradinger R (2008) Regional variability in food availability for Arctic marine mammals. Ecol Appl 18:77–96
Bouchard C, Fortier L (2011) Circum-arctic comparison of the hatching season of polar cod Boreogadus saida: a test of the freshwater winter refuge hypothesis. Prog Oceanogr 90:105–116
Bradbury IR, Hubert S, Higgins B, Borza T, Bowman S, Paterson IG, Snelgrove PVR, Morris CJ, Gregory RS, Hardie DC, Hutchings JA, Ruzzante DE, Taggart CT, Bentzen P (2010) Parallel adaptive evolution of Atlantic cod on both sides of the Atlantic Ocean in response to temperature. R Proc Soc B 277:3725–3734
Brown JA, Pepin P, Methven DA, Somerton DC (1989) The feeding, growth and behaviour of juvenile cod, Gadus morhua L., in cold environments. J Fish Biol 35:373–380
Campana SE (1996) Year-class strength and growth rate in young Atlantic cod Gadus morhua. Mar Ecol Prog Ser 135:21–26
Cheung WL, Lam VWL, Sarmiento JL, Kearney K, Watson R, Pauly D (2009) Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish 10:235–251
Copeman LA, Laurel BJ, Heintz R, Vollenweider J, Boswell K (2015) Ontogenetic variability in the lipid content of saffron cod (Eleginus gracilis) and Arctic cod (Boreogadus saida) from the Western Arctic and Northern Chukchi. Polar Biol (this issue)
Coyle KO, Pinchuk AI, Eisner LB, Napp JM (2008) Zooplankton species composition, abundance and biomass on the eastern Bering Sea shelf during summer: the potential role of water-column stability and nutrients in structuring the zooplankton community. Deep Sea Res Part II 55:1775–1791
Craig PC, Griffiths WB, Haldorson L, McElderry H (1982) Ecological studies of Arctic cod (Boreogadus saida) in Beaufort Seas coastal waters, Alaska. Can J Fish Aquat Sci 39:395–406
Crawford RE, Jorgenson JK (1996) Quantitative studies of Arctic cod (Boregoadus saida) schools; important energy stores in the Arctic food web. Arctic 49:181–193
Crawford RE, Vagle S, Carmack EC (2012) Water mass and bathymetric characteristics of polar cod habitat along the continental shelf and slope of the Beaufort and Chukchi seas. Polar Biol 35:179–190
Drinkwater KF, Beaugrand G, Kaeriyama M, Kim S, Ottersen G, Perry RI, Pörtner HO, Polovina JJ, Takasuka A (2010) On the processes linking climate to ecosystem changes. J Mar Sys 79:374–388
Farrell AP, Steffensen JF (2005) The physiology of Polar fishes. Elsevier Academic Press, San Diego
Fechhelm RG, Williams BW, Daigneault, MJ Link MR (2008) Year 25 of the long-term monitoring of nearshore Beaufort Sea fishes in the Prudhoe Bay region, 2007. Report for BP Exploration (Alaska) Inc. by LGL Alaska Research Associates, Inc, Anchorage, Alaska
Folkvord A (2005) Comparison of size-at-age of larval Atlantic cod (Gadus morhua) from different populations based on size- and temperature-dependent growth models. Can J Fish Aquat Sci 62:1037–1052
Fossheim M, Primicerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV (2015) Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat Clim Chan. doi:10.1038/nclimate2647
Golovanov VK (1996) The ecological and evolutionary aspects of thermoregulation behavior on fish. J Ichthyol 46:180–187
Harter BB, Elliott KH, Divoky GJ, Davoren GK (2013) Arctic cod (Boreogadus saida) as prey: fish length-energetics relationships in the Beaufort Sea and Hudson Bay. Arctic 66:191–196
Heintz RA, Vollenweider JJ (2010) Influence of size on the sources of energy consumed by overwintering walleye pollock (Theragra chalcogramma). J Exp Mar Biol Ecol 393:43–50
Hollowed AB, Barbeaux SJ, Cokelet ED, Kotwicki S, Ressler PH, Spital C, Wilson CD (2012) Effects of climate variations on pelagic ocean habitats and their role in structuring forage fish distributions in the Bering Sea. Deep Sea Res II 65–70:230–250
Hollowed AB, Planque B, Loeng H (2013) Potential movement of fish and shellfish stocks from the sub-Arctic to the Arctic Ocean. Fish Oceanogr 22:355–370
Hop H, Tonn WM, Welch HE (1997) Bioenergetics of Arctic cod (Boreogadus saida) at low temperatures. Can J Fish Aquat Sci 54:1772–1784
Houde ED (2008) Emerging from Hjort’s shadow. J Northwest Atl Fish Sci 41:53–70
Hurst TP, Laurel BJ, Ciannelli L (2010) Thermal dependence of growth rates of early life stages of Pacific cod (Gadus macrocephalus). Fish Bull 108:382–392
Hurst TP, Munch SB, Lavelle KA (2012) Thermal reaction norms for growth vary among cohorts of Pacific cod (Gadus macrocephalus). Mar Biol 159:2173–2183
Hurst TP, Cooper DW, Duffy-Anderson JT, Farley EV (2015) Contrasting coastal and shelf nursery habitats of Pacific cod in the southeastern Bering Sea. ICES J Mar Sci 72:515–527
Hutchings JA, Swain DP, Rowe S, Eddington JD, Puvanendran V, Brown JA (2007) Genetic variation in life-history reaction norms in a marine fish. Proc R Soc B 274:1693–1699
IPCC (2014) Climate change 2014: impacts, adaptation, and Vulnerability. Part B: regional aspects. In: Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jeffries MO, Richter-Menge J, Overland JE (2014) Arctic Report Card 2014. http://www.arctic.noaa.gov/reportcard
Jobling M (1988) A review of the physiological and nutritional energetics of cod, Gadus morhua L., with particular reference to growth under farmed conditions. Aquaculture 70:1–19
Johnson SW, Thedinga JF, Neff AD (2009) Invasion by saffron cod Eleginus gracilis into nearshore habitats of Prince William Sound, Alaska, USA. Mar Ecol Prog Ser 389:293–312
Kooka K, Yamamura O, Nishimura A, Hamatsu T, Yanagimoto T (2007) Optimum temperature for growth of juvenile walleye pollock Theragra chalcogramma. J Exp Mar Biol Ecol 347:69–76
Laurel BJ, Gregory RS, Brown JA (2003) Settlement and distribution of age 0 juvenile cod, Gadus morhua and Gadus ogac, following a large-scale habitat manipulation. Mar Ecol Prog Ser 262:241–252
Laurel BJ, Stoner AW, Ryer CH, Hurst TP, Abookire AA (2007) Comparative habitat associations in juvenile Pacific cod and other gadids using seines, baited cameras and laboratory techniques. J Exp Mar Biol Ecol 351:42–55
Lowry LF, Frost KJ, Burns JJ (1980) Variability in the diet of ringed seals, Phoca hispida, in Alaska. Can J Fish Aquat Sci 37:2254–2261
Mazur MM, Wilson MT, Dougherty AB, Buchheister A, Beauchamp DA (2007) Temperature and prey quality effects on growth of juvenile walleye pollock Theragra chalcogramma (Pallas): a spatially explicit bioenergetics approach. J Fish Biol 70:816–836
Moore SE, Huntington HP (2008) Arctic marine mammals and climate change: impacts and resilience. Ecol App 18:S157–S165
Moulton LL, Tarbox KE (1987) Analysis of Arctic cod movements in the Beaufort Sea nearshore region, 1978–79. Arctic 40:43–49
Mueter FJ, Ladd C, Palmer MC, Norcross BL (2006) Bottom-up and top-down controls of walleye pollock (Theragra chalcogramma) on the eastern Bering Sea shelf. Prog Oceanogr 68:152–183
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci 104:1266–1271
Otterlei E, Nyhammer G, Folkvord A, Stefansson SO (1999) Temperature- and size-dependent growth of larval and early juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and northeast Arctic cod. Can J Fish Aquat Sci 56:2099–2111
Peck MA, Buckley LJ, Caldarone EM, Bengston DA (2003) Effects of food consumption and temperature on growth rate and biochemical-based indicators of growth in early juvenile Atlantic cod Gadus morhua and haddock Melanogrammus aeglefinus. Mar Ecol Prog Ser 251:233–243
Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630
Rand KM, Logerwell EA (2011) The first demersal trawl survey of benthic fish and invertebrates in the Beaufort Sea since the late 1970s. Polar Biol 34:475–488
Renaud PE, Berge J, Varpe Ø, Lønne OJ, Nahrgang J, Ottesen C, Hallanger I (2012) Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida? Pol Biol 35:401–412
Smetacek V, Nicol S (2005) Polar ocean ecosystems in a changing world. Nature 437:362–368
Smith RL, Paul AJ, Paul JM (1986) Effect of food intake and temperature on growth and conversion efficiency of juvenile walleye pollock (Theragra chalcogramma (Pallas)): a laboratory study. J Cons Int Explor Mer 42:241–253
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Sogard SM, Olla BL (2000) Endurance of simulated winter conditions by age-0 walleye pollock: effects of body size, water temperature and energy stores. J Fish Biol 56:1–21
Sogard SM, Olla BL (2002) Contrasts in the capacity and underlying mechanisms for compensatory growth in two pelagic marine fishes. Mar Ecol Prog Ser 243:165–177
Springer AM, Roseneau DG, Lloyd DS, McRoy CP, Murphy EC (1986) Seabird responses to fluctuating prey availability in the eastern Bering Sea. Mar Ecol Prog Ser 32:1–12
Sundby S, Nakken O (2008) Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. ICES J Mar Sci 65:953–962
Wassmann P (2006) Structure and function of contemporary food webs on Arctic shelves: an introduction. Prog Ocean 71:123–128
Wong S, Walksuz W, Hanson M, Papst MH (2013) The influence of the Mackenzie River plume on distribution and diversity of marine larval fish assemblages on the Canadian Beaufort Shelf. J Mar Sci 127:36–45
Wyllie-Echeverria T, Wooster WS (1998) Year-to-year variations in Bering Sea ice cover and some consequences for fish distributions. Fish Oceanogr 7:159–170
Acknowledgments
We thank C. Ryer, T. Hurst, and I. Bradbury for reviewing earlier drafts of this manuscript. Thanks also to Bill Kopplin, Robert Fechhelm, Kyle McCain, Bill Streever and the LGL field crew for their assistance in the collection of Arctic and saffron cod in Prudhoe Bay as well as to Scott Haines, Michele Ottmar, and Eric Hanneman for their assistance in the fish transport and animal husbandry. This project was supported with funding from the North Pacific Research Board (NPRB) Grant #R1228 and 2014 Essential Fish Habitat funding from NOAA–AFSC. This study is NPRB contribution no. 560. The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue on the “Ecology of Arctic Gadids”, coordinated by Franz Mueter, Jasmine Nahrgang, John Nelson, and Jørgen Berge.
Rights and permissions
About this article
Cite this article
Laurel, B.J., Spencer, M., Iseri, P. et al. Temperature-dependent growth and behavior of juvenile Arctic cod (Boreogadus saida) and co-occurring North Pacific gadids. Polar Biol 39, 1127–1135 (2016). https://doi.org/10.1007/s00300-015-1761-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1761-5