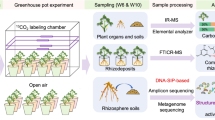
Abstract
Antarctic vascular plants such as Deschampsia antarctica (Da) could generate more suitable micro-environmental conditions for the establishment of other plants like Colobanthus quitensis (Cq). Although positive plant–plant interactions have been shown to contribute to plant performance and establishment, little is known about how microorganisms might modulate those interactions, particularly in stressful environmental conditions. Several reports have focused on the possible ecological roles of microorganisms on vascular plants, but if rhizospheric microorganisms can impact positive interactions among Antarctic plants has been seldom studied. Here, we assessed the physical–chemical characteristics of rhizospheric soils from Cq growing alone or associated with Da (Cq + Da). In addition, we compared the rhizosphere microbiomes associated with Cq, either growing alone or associated with Da (Cq + Da), using a shotgun metagenomic DNA sequencing approach and using eggNOG for comparative and functional metagenomics. Overall, there were no differences among rhizospheric soils in terms of physical–chemical characteristics. On the other hand, our results show significant differences in terms of taxonomic diversity between rhizospheric soils. Functional annotation and pathway analysis showed that microorganisms from rhizospheric soil samples also have significant differences in gene abundance associated with several functional categories related to environmental tolerance and in metabolic pathways linked to osmotic stress, among others. Overall, this study provides foundational information which will allow to explore the biological impact of the rhizobiome and its functional mechanisms and molecular pathways on plant performance and help explain the concerted strategy deployed by Cq to inhabit and cope with the harsh conditions prevailing in Antarctica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diverse mutualistic bacteria and fungi thrive on plant surfaces and inhabit most plant tissues. Many of these microorganisms interact with their plant hosts intimately; they can influence plant metabolism and hormonal pathways in addition to providing novel nutritional or biosynthetic capacities, stimulating plant growth, and conferring enhanced resistance to different stressors (Lugtenberg and Kamilova 2009; de Zelicourt et al. 2013). Several studies have shown that microorganisms can have a direct effect on plants capacity to resist biotic and abiotic stress such as herbivory, drought, extreme temperatures and high salinity (Redman et al. 2002; Márquez et al. 2007; Giauque and Hawques 2013; Acuña-Rodríguez et al. 2019). Many bacteria and fungi have been found in association with plant roots, facilitating the establishment, spread and/or increasing plant fitness in stressful environments (Frey-Klett et al. 2007; Bano and Fatima 2009; Hoffman and Arnold 2010; Torres-Díaz et al. 2016). On the other hand, it has been documented that some microorganisms can modulate the interaction between plants or filter the establishment of new species in a given community (Amsellem et al. 2017). Thus, if microorganisms can impact plant–plant interactions, studying the diversity and composition of microbial communities is key for understanding how vascular plants interact and survive. This could be especially relevant in stressful environmental conditions, such as those found in Antarctic habitats, where positive interactions, in particular, could be essential for survival (He et al. 2013; Atala et al. 2019).
The Antarctic ecosystem is one of the most stressful natural habitats, especially for terrestrial plants (Convey et al. 2014; Pointing et al. 2015). In such harsh environment, only two vascular plants; Colobanthus quitensis (Cq, Caryophyllaceae) and Deschampsia antarctica (Da, Poaceae), have colonized the ice-free zones (Moore 1970). Although both plants are found in the Antarctic Peninsula, Cq is mainly found growing in association with Da in more stressful areas, and alone in more favorable sites (Atala et al. 2019). Da, on the other hand, is capable of growing alone in areas with higher abiotic stress (Alberdi et al. 2002; Atala et al. 2019). Da is a grass that form tussocks where micro-environmental conditions above and below their canopy could be milder than outside, acting like a “nurse species” for other less tolerant species (e.g., Cq) in Antarctica (see Molina-Montenegro et al. 2013). In fact, some native and invasive species increase its physiological performance and fitness-related traits when growing in association with Da compared with those growing alone (Atala et al. 2019). Although it is clear that positive interactions can determine the performance and survival for some less tolerant species, the underlying mechanisms are not clear and whether microorganisms mediate this positive interaction remains unknown.
It has been proposed that positive inter-specific interactions between plants and microorganisms play a pivotal role in the structure and functioning of several plant communities (i.e. Smith and Read 2008; Sielaff et al. 2018). Plants harbor a wide diversity of microorganisms, which play a crucial role in their growth, survival and establishment by conferring enhanced resistance to abiotic stress, allowing plants to grow in extreme conditions (De Zelicourt et al. 2013; Louca et al. 2018) such as those from polar environments (Hughes et al. 2015). Despite microorganisms having positive impacts on plant fitness in stressful environmental conditions (Vandenkoornhuyse et al. 2015; Torres-Díaz et al. 2016; Ramos et al. 2018), little is known about how Antarctic rhizospheric microorganisms might affect plant performance. Although several reports have focused on the occurrence, type of association, diversity and possible ecological roles of microorganism interactions with vascular plants (Upson et al. 2009; Torres-Díaz et al. 2016), there are very few studies regarding microorganisms’ interactions with Antarctic vascular plants (but see Rosa et al. 2009; Ramos et al. 2018). For example, Teixeira et al. (2010) showed no clear differences in terms of diversity between rhizospheric bacterial communities from the rhizospheres of Cq and Da in Admiralty Bay, by 16S rRNA analysis. However, and to the best of our knowledge, there is no evidence regarding the metabolic capacity and functional traits of the rhizospheric microbiomes related to Cq and Da, nor if the rhizospheric microorganisms could modulate positive interactions among these vascular Antarctic plants.
In this study, we compare the rhizosphere microbiomes associated with Cq, either growing alone or in association with Da (Cq + Da) by using an approach based on shotgun metagenomic DNA sequencing technology. This strategy allowed us to gain insight into the rhizospheric microbial taxonomic diversity (including non-culturable organisms), and to compare similarities and differences in terms of the observed functional attributes (Jovel et al. 2016). Specifically, we asked if the rhizosphere microbiome of Cq when grow associated to Da have higher taxonomic and functional diversity than when grow alone. Finally, we analyzed the physical–chemical characteristics of the rhizospheric soil in order to assess if differences in microbiome or edaphic characteristics induced by Da can help explain the greater performance and abiotic tolerance of Cq when grow associated to with Da.
Materials and methods
Site description and soil sample processing
Rhizospheric soil samples were collected from Devils Point, Byers Peninsula, Livingstone Island, Antarctica (62°40′S, 61°10′W) during the growing season in the Summer (February) of 2016 (Fig. 1). Colobanthus quitensis’ rhizospheric soil (Cq) and rhizospheric soil of Cq growing associated with Da (Cq + Da) were sampled at sea level. Plants and surrounding soil were dug out at 7 cm of depth using a sterilized shovel, bulk soil and fine roots were discarded by shaking the plants by hand until non-adhering particles were completely removed. Then, for each sample, fine soil particles ( ≤ 2 mm), tightly adhered to the roots (rhizospheric soil), were carefully separated from the roots and stored in 50 mL sterile screw cap tubes at – 20 °C until DNA extraction and processing of soil samples. All plant and soil samples were collected under permission of the Chilean Antarctic Institute (INACH; Authorization Number 1060/2014).
Physical–chemical analysis of rhizospheric soil
To assess whether physical–chemical characteristics of soil can modify the rhizospheric soil biodiversity we measured nitrogen, phosphorus, potassium, organic material, pH, and sand, silt and clay percentages in the rhizospheric soil of Cq growing alone and associated with Da. A rhizospheric soil sample at 5–7 cm depth (ca. 50 g) was taken beneath 12 Cq growing alone and beneath other 12 Cq individuals growing associated with Da. All samples were collected inside of patch of 25 × 25 m and each pair of samples (i.e., Cq and Cq + Da) were distanced no more than 100 cm. Soil samples were stored in hermetic plastic bags at – 20 °C and then sent for analyses to Centro Tecnológico de Suelo y Cultivos at the Universidad de Talca (Order of Analysis R-PT-09–01).
DNA extraction and sequencing
For a total of six rhizospheric soil samples (three replicates per condition, Cq or Cq + Da), total DNA was extracted using the PowerSoil DNA Isolation Kit (MoBio Laboratories, Inc.). DNA integrity was checked with capillary electrophoresis using a Fragment Analyzer (AATI) and DNA quantification was performed using fluorometry (Qubit 2.0; Qubit DNA Broad Range Assay Kit, Invitrogen). After QC, all samples were subjected to library construction for Illumina sequencing. Briefly, DNA was fragmented by Covaris ultrasonicator (average fragment size of 550 bp), and size-selected using AMPure XP purification beads. Libraries were constructed using the TruSeq LT kit following manufacturer instructions (Illumina), ligated to indexed adapters for cluster generation and sequenced using the Illumina MiSeq reagent kit (v3) in an Illumina MiSeq sequencer (600 cycle; 300 bp, Paired-End sequencing) (Illumina, San Diego, CA). Demultiplexing and fastq generation were performed automatically using Illumina’s built-in software. Raw metagenomic datasets were deposited in NCBI’s Sequence Read Archive database under BioProject ID PRJNA419970.
Statistical and bioinformatics analyses
To assess the differences in physical–chemical characteristics of rhizospheric soils beneath C. quitensis plants growing alone (Cq) and C. quitensis associated with Da (Cq + Da) a t-test was used.
Raw sequences obtained from six metagenomic samples were subjected to a quality check using FastQC v0.11.3 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Next, the sequences were run through Trimmomatic 0.36 (Bolger et al. 2014) to remove low-quality base pairs, sequencing adapters and reads shorter than 100 bp; the following parameters were used: SLIDINGWINDOW 4:15 MINLEN: 36. After trimming, all libraries were interleaved for downstream analyses.
For taxonomic assignment, libraries were aligned using DIAMOND BLASTX algorithm ver. 0.9.30.92 (using default parameters; Buchfink et al. 2015) to the NCBI-NR database (April 2018). Only alignments with an e-value of 10−3 or lower were included in our analysis. Alignment result files were imported in MEGAN v6.11.5 (MEGAN6; Huson et al. 2016), which parsed results using the Lowest Common Ancestor Algorithm (LCA) and NCBI’s taxonomy under default values. All samples were normalized by using MEGAN6′s built-in normalization tool. Comparison between Cq and Cq + Da rhizospheric soil samples were carried out using MEGAN6′s Taxonomic abundance estimates (phylum, genera and species levels) and were imported into STAMP (Statistical Analysis of Metagenomic Profiles, version 2.1.3; Parks et al. 2014) for statistical analysis, using a Welch’s t-test with correction for multiple comparisons (Benjamini–Hochberg false discovery rate correction approach, q-value < 0.05). Further, taxonomic diversity analysis was carried out in R using the ggplot2, phyloseq, vegan, and DESeq2 packages (Dixon 2003; McMurdie and Holmes 2013; Love et al. 2014). Finally, comparison between samples was performed using the software STAMP (Statistical Analysis of Metagenomic Profiles, version 2.3.1).
To analyze metabolic profiles and perform functional annotations, all interleaved, filtered libraries per condition (n = 3) were pooled in silico and assembled using MEGAHIT v1.1.3 (minimum contig length 400 bp; default parameters) (Li et al. 2015). Each representative, assembled metagenome was aligned using DIAMOND BLASTX algorithm ver. 0.9.30.92 (using default parameters; Buchfink et al. 2015) to the NCBI-NR database (April 2018). Functional assignment was performed by mapping aligned contigs to eggNOG database using MEGAN6 in long-read mode. For sample comparisons, relative abundance of aligned bases binned to eggNOG clusters between samples (based on aligned contig coverage, long-read mode) were normalized using MEGAN6 built-in normalization tools. Statistical analysis of samples was performed in STAMP, using a Fisher exact test with correction for multiple comparisons (Benjamini–Hochberg false discovery rate correction approach, q-value < 0.05). Additionally, for each assembled metagenome, all protein-encoding genes were predicted with Prodigal v2.6.2 in metagenomics mode (Hyatt et al. 2012) and were used as queries for functional module and pathway analysis [as defined by Kyoto Encyclopedia of Genes and Genomes (KEGG)]. This analysis allows estimating the percentage of module components filled with input genes [module completion ratio (MCR)] by mapping genes to all functional modules/pathways/complexes using the single-directional best-hit method, implemented in the Metabolic and Physiological potential Evaluator system v2.3.0 (MAPLE; Arai et al. 2018). If all genes are assigned to all KEGG orthology IDs in each condition, the MCR is 100%, thus allowing comparison in terms of MCR between metagenomes (Takami et al. 2015).
Results
Physical–chemical analysis of rhizospheric soil
The chemical characteristics of soil samples (nitrogen, phosphorus, potassium, organic material and pH) were not different between rhizospheric soils beneath Cq and Cq + Da (Table 1). Similarly, physical characteristics of soil samples (sand, silt and clay percentages) were not different between rhizospheric soils from Cq and Cq + Da (Table 1).
Summary of sequencing data and de novo assembly
For the Cq metagenomes, a total of 20,081,770 reads were obtained, while for Cq + Da samples, 19,348,212 reads were obtained (Online Resource 1). Read sizes ranged from 35 to 300 bp, although the majority of reads ( ~ 97% of total sequenced reads per library) had a length equal or over 290 bp. Filtering steps reduced library sizes in approximately 18%. After trimming, two representative metagenomes were assembled by combining per-condition libraries (Cq and Cq + Da); a total of 1,013,395 and 676,033 contigs were obtained, respectively (Online Resource 2). Additionally, separate DIAMOND alignments of each trimmed library were used for taxonomic analysis; approximately 56% of reads per library were successfully assigned into a taxonomic category (Online Resource 1).
General prokaryotic taxonomic analysis and rhizospheric soil diversity
Results of the MEGAN taxonomic analysis for both metagenomes revealed that Bacteria, followed by Eukaryota and Archaea dominated both samples (96.8%, 2.8% and 0.2%: Cq, 99.2%, 0.58%, and 0.24%: Cq + Da). The differences in relative abundances for Eukaryota detected between samples may be related to a higher number of sequences aligned to Viridiplantae in Cq compared to Cq + Da (mean difference between samples: 58,542.3; p-value = 1.4e−3), suggesting that plant DNA was also recovered and sequenced from the rhizospheric soil samples. Therefore, all non-bacterial aligned reads were in silico filtered and removed from the following analysis.
Microbiome analyses revealed a diverse microbial community at the phylum level for both soil samples and showed that Proteobacteria was the most abundant phylum (34.1%), followed by Actinobacteria (23.7%) and Bacteroidetes (16.4%) (Fig. 2; Core Microbiome is shown in Online Resource 3). The clustering analysis based on Bray–Curtis dissimilarities calculated from the relative abundance of each genus, indicated higher similarity values within the three sampled replicates per rhizosphere (within Cq and Cq + Da, respectively), but dissimilarities between Cq and Cq + Da rhizospheres, respectively (Online Resource 4). Furthermore, comparative taxonomic analysis at genus-level showed differences in the relative diversity between Cq and Cq + Da rhizospheric soil samples (222 vs. 215 genera; genus listed in Online Resource 4), while significant differences were detected for 89 bacterial genera (Online Resource 5), being Sporosarcina and Chryseobacterium the more represented genus in Cq and Cq + Da, respectively (Fig. 3).
At bacterial species level (considering presence/absence) we found that 46.1% species were shared between rhizospheric soil samples; 25.4% were exclusively found in Cq rhizospheric soil and 28.5% were found only in Cq + Da rhizospheric soil (Online Resource 6). Alpha diversity analysis revealed that all Cq + Da samples were consistently found to be more diverse than Cq samples, both in richness [observed and corrected (Chao1)] and evenness (ACE, Shannon, Simpson, Inverse Simpson) (Online Resource 7). While interquartile ranges did not overlap under any metric, suggesting significant differences, these are sensitive to low sample sizes for which more samples need to be collected and evaluated to obtain precise alpha diversity estimates. However, median values between Observed and Chao1 estimates coincide ( ~ 755 taxa), which suggests that sequencing depth was adequate for sampling these rhizosphere communities. It is worth noting that the taxonomic analyses performed in this study are based on tools relying on sequence alignments using as reference known/classified microorganisms. However, a high proportion of the observed lineages are either from unknown or unclassified bacteria, suggesting that they are unique to Antarctic soils (Bottos et al. 2014) and warrant further study.
Functional categories analysis and functional rhizospheric soil sample comparison
In order to gain functional insights into microorganisms abundantly present in these rhizospheric libraries, soil metagenomics samples were pooled by condition, assembled to construct contigs and aligned against NCBI’s NR database using DIAMOND. A total of 839,980 and 598,467 contigs (82.9–88.5%) had homologous sequences within this database (Cq and Cq + Da assembles, respectively). However, as the alignment results included matches with hypothetical/predicted proteins, not all aligned contigs could be annotated. Thus, 265,414 and 278,276 sequences (26.2–41.2% of all contigs per assembly) were annotated and functionally categorized using the eggNOG database in MEGAN6 and associated with at least one cluster of orthologous genes of the following top hierarchies: cellular processes and signaling, information storage and processing, and metabolism. Subsequent distribution analysis of putative genes with general functions indicated that overall, the most represented functional categories at eggNOG level 2 in both rhizospheres were linked to aminoacid transport and metabolism (11.4%), replication, recombination, and repair (11.1%), energy production and conversion (9.8%) and carbohydrate transport and metabolism (7.9%) (Online Resource 8).
We also compared the number of assigned contigs to eggNOG categories between rhizospheric soil samples, which revealed a varying degree of similarity between the two groups in all categories (Online Resource 8). However, there was significant differences in terms of relative abundance for 16 categories (out of 22), with mean differences ranging between 0.08 and 2.24% (q-value < 0.05; Fig. 4; Online Resource 8). Of these 16 categories, 11 were more represented in Cq (Fig. 4, blue dots) while the other 5 were more represented in Cq + Da (Fig. 4, orange dots). A global, deep comparison of genes binned to different eggNOG categories at level 3 (using MEGAN6 visualization tool) showed that 372 categories displayed differences in terms of relative abundance between both samples (Online Resource 8). These results suggest an overall enrichment of several molecular functions and biological process linked to these eggNOG categories, such as Serine Threonine protein kinases, which in bacteria have been linked to phosphorylation of serine or threonine residues in proteins, thus being considered as a key mechanism in regulation of protein activity and control of cellular functions, such as stress response (Pereira et al. 2011) (Online Resource 8). However, our results also indicate that many categories with similar molecular functions such as “ABC transporter” display opposite patterns of enrichment: for example, “ABC transporter, permease (ENOG410XP9H)” is enriched in Cq + Da, while “ABC transporter, permease (COG0577)” is enriched in Cq. Hence, in this case, evaluation and differentiation of the functional potentials between different metagenomes based on functional annotation of genes and eggNOG clustering/classification tools would be a difficult task (Takami et al. 2015). Thus, as we were interested in characterizing the functional genomic features derived from both rhizospheric metagenomes (“Functionome”, sensu Fujinawa et al. 2016), we comparatively studied the potential functionomes using a method based on the presence and completeness of KEGG functional modules and metabolic pathways (Takami et al. 2015; Fujinawa et al. 2016). The predicted aminoacid sequences from both metagenomes were loaded in MAPLE 2.3.0 (Arai et al. 2018). This analysis revealed that, in both samples, 116 metabolic pathways have 100% MCR, thus were considered as shared among rhizospheres. We also found 76 pathways with differences in their MCR between rhizospheres (Online Resource 9). In the case of Cq, 23 out of 33 pathways linked to secondary metabolism had higher MCR compared to Cq + Da (e.g. biosynthesis of secondary metabolites, aromatics degradation). Contrastingly, in the case of Cq + Da, 33 out of 43 pathways with higher MCR were related to “carbohydrate and lipid metabolism” category, while no pathways related to these categories had a high MCR in Cq (Online Resource 9).
SEED functional categories found in rhizospheric soil bacteria communities. Bar plot shows mean proportion (%) of functional categories found in rhizospheric bacterial communities based on the SEED database level 2 categories. Points indicate the differences between C. quitensis and C. quitensis + D. antarctica soils (blue and orange bars, respectively). Corrected p-values (q-values) were derived from a Fisher’s exact test with Benjamini–Hochberg correction for false discovery rate. (Color figure online)
Discussion
We explored the taxonomic and functional diversity of microbial communities in two rhizospheric soils (Cq and Cq + Da) of the vascular species from Antarctica using shotgun sequencing. Our metagenomic analyses revealed that significant differences were observed between samples in terms of bacterial abundance, although bacterial communities shared some similarities in terms of taxonomic composition. In addition, some functional categories also showed differences in terms of abundance between rhizospheres, suggesting that these microbial communities could have different functional activities. This could ultimately have an effect on colonization and plant growth and environmental tolerance.
Results showed that bacterial species had the highest relative abundance in both habitats (98%) compared to Archaea (0.22%) and Eukaryota (1.77%), suggesting that a highly complex bacterial community is present in these rhizospheric soil samples. Interestingly, the rhizospheric core microbiome (shared Phyla between both samples; Online Resource 6) obtained in this study indicates that the most abundant bacterial Phyla were Proteobacteria, Actinobacteria, Bacteroidetes, Acidobacteria and Verrucomicrobia, accounting for approximately 85% of the sequences in rhizospheric soil samples. Thus, they may constitute a core, functional microbial community structure, capable of performing several biochemical functions related to plant growth promotion, nutrient acquisition, and abiotic stress tolerance, among others (Chen et al. 2017; Louca et al. 2018). However, our findings in terms of bacterial Phyla abundance and diversity were rather unexpected, as they contrast with the previously reported bacterial diversity from Antarctic rhizospheric bacterial communities associated with vascular plants, where Firmicutes were the most abundant phylum in almost all of the samples, and Acidobacteria were rarely found (Teixeira et al. 2010). Congruently, the bacterial Phyla detected in our study are similar to those detected in soil rhizospheres worldwide (Delgado-Baquerizo et al. 2018) and agree with other studies on microbial diversity in Antarctica (Wang et al. 2015). Nevertheless, it must be noted that differences in methodological approaches (i.e., amplicon vs. shotgun) may explain disparities in terms of phylum abundance between our study and those previously reported.
Another possible explanation for the observed differences is that other environmental factors, such as physical/chemical soil characteristics or vegetative cover could participate in shaping soils’ microbial diversity (Teixeira et al. 2010; Bottos et al. 2014; Wang et al. 2015), as bacterial community structures of Antarctic soils are highly heterogeneous (Yergeau et al. 2012; Bottos et al. 2014) and may display unique biochemical adaptations, depending on habitat conditions (González-Rocha et al. 2017). For example, several studies have demonstrated that soil characteristics, like pH or carbon mineralization rate, can help explain the relative abundance of dominant Phyla and/or bacterial community structure (Fierer et al. 2007; Lauber et al. 2009). Previous studies conducted in the Antarctica have shown differences in the presence and concentrations of nutrients in Antarctic soils, but majority of this studies have compared sites spatially distanced or with presence and absence of ornithogenic or mammals effects, suggesting that big vertebrates are the main driver for the variation of nutrient soil amount, more than plants itself (see Bokhorst et al. 2019). On the other hand, other studies have shown that under different type of Antarctic vegetation the nutrient and edaphic characteristics can variety significantly (see Roberts et al. 2009). Nevertheless, this study compared different functional groups (vascular plants vs. mosses or plants vs lichens) but no between vascular plants. In our study, no differences among physical–chemical characteristics of rhizospheric soils from Cq and Cq + Da were found. This result could be explained since we assessed the rhizospheric soil, that is, the portion that is in direct contact with roots. In addition, we compared the rhizospheric soil the same species, although in two conditions, but only in the glued soil to roots of Cq. On the other hand, Beyer et al. (2000) suggested that other factor as moisture, temperature or wind are most important traits to explain colonization and performance in Antarctic plants compared with nutrients. Similar microclimatic modifications as those provided by Da to Cq. In addition, rhizospheric soil samples were taken in a patch of 25 × 25 m, where each pair (Cq and Cq + Da) was less than 1 m away. So, the probability to collect soils samples with external influences rather than the root effect of Cq was low. Thus, the evident variation both in diversity and structure of microorganism community could be mainly determinate by the effects that Da is able to exert on soil microorganisms. Future studies should also consider other aspects of the interaction among plant and microorganisms (e.g., energetic costs, vertebrates or vegetation presence surrounding) in order to explain other ecological and functional aspects of this symbiosis.
Regarding the phylum composition between the rhizospheric communities of Cq growing alone compared to Cq growing with Da, we also found differences in their relative abundance. For example, Bacteroidetes, were higher in Cq compared to Cq + Da samples (19.08% and 13.53%, respectively). These bacteria are involved in degradation of plant material and related organic molecules such as starch and cellulose (Aislabie et al. 2013) and their abundance correlates with soil pH, available nitrogen/phosphorus content and water content (Zhang et al. 2014). We also observed significant differences in the taxonomic distinctiveness of the rhizospheric soil samples observed at the genus level and in terms of unique and shared bacterial species present in both rhizospheres, suggesting the existence of complex microbial assemblages associated with each rhizospheric type. Furthermore, the Bray–Curtis dissimilarity index suggests that Cq and Cq + Da samples are dissimilar in terms of diversity of bacterial genera. A previous study reported no clear differences between microbial communities from the rhizospheres of Cq and Da (Teixeira et al. 2010). However, a recent study found evidence of different bacterial strains from the rhizospheres of these Antarctic plants (Gallardo-Cerda et al. 2018).
Rhizospheric microorganisms may play a crucial role in growth and stabilization of plants in extreme environmental conditions such as those found in Antarctica (Hughes et al. 2015). Thus, characterizing the number of biochemical functions or genes present in Antarctic rhizospheric communities is an important step towards the understanding of the complex ecological aspects and biotechnological potentials of Antarctic bio-resources (Wang et al. 2015; Molina-Montenegro et al. 2016; Gallardo-Cerda et al. 2018; Louca et al. 2018; Acuña-Rodríguez et al. 2019). Based on functional analysis using eggNOG categories, all categories had at least 3 genes assigned, although mean gene abundance per category varied widely; for example, the most abundant category was “Replication, recombination and repair” (61,814 summed genes) while the least abundant category is “extracellular structures” (3 summed genes). These results in terms of gene abundance indicate a specific enrichment of some eggNOG categories, which could be related to microbial adaptation to cold Antarctic environment. This could include energy production, nutrient transportation, cellular membrane functions and tolerance to abiotic stress (Barrientos-Díaz et al. 2008), suggesting selection of particular functional traits that could be adaptive in a given environment. Therefore, we propose that differences observed both in the completion rates of functional modules and in the eggNOG functional categories from Cq and Cq + Da rhizospheres could be a consequence of microorganism selection by presence of specific functional traits (Yan et al. 2017). Specific genera/species of bacteria, uniquely present in a certain sample/condition (but under- or not-represented in the other sample/condition), could be responsible for these functional traits which, in turn, could explain the observed differences in terms of microbial diversity/abundance. However, it must be noted that the differences/similarities in terms of functional modules/categories described in this study, do not necessarily reflect the actual working probability of each functional module (Takami et al. 2015).
Finally, our results suggest that Antarctic plants may shape their rhizosphere communities, by differentially altering both the abundance and diversity of bacteria. This is especially relevant when comparing sites where Cq grows alone to sites where is found associated with Da, possibly being a key element in the positive effect of the latter on Cq and even on some invasive species (Atala et al. 2019). Furthermore, it is plausible that these different rhizospheric microbial communities encode different molecular mechanisms and express different functional pathways. However, the results in this study do not show the actual working probability of these functional pathways, nor indicate whether they are actually being expressed and/or functional in the soil. Hence, additional research is required to uncover the biological impact of these different microbiomes on plant performance and survival in order to shed lights about the strategy deployed by Cq to inhabit and cope with the harsh abiotic conditions prevailing in Antarctica.
References
Acuña-Rodríguez IS, Hansen H, Gallardo-Cerda J, Atala C, Molina-Montenegro MA (2019) Antarctic extremophiles: biotechnological alternative to crop productivity in saline soils. Front Bioeng Biotechnol 7:22
Aislabie J, Deslippe JR, Dymond JR (2013) Soil microbes and their contribution to soil services. In: Dymond JR (ed) Ecosystem services in New Zealand—condition and trends. Manaaki Whenua Press, Lincoln, pp 143–161
Alberdi M, Bravo LA, Gutierrez A, Gidekel M, Corcuera LJ (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115:479–486
Amsellem L, Brouat C, Duron O, Porter SS, Vilcinskas A, Facon B (2017) Importance of microorganisms to macroorganisms invasions: is the essential invisible to the eye? Adv Ecol Res 57:99–146
Arai W, Taniguchi T, Goto S, Moriya Y, Uehara H, Takemoto K et al (2018) MAPLE 2.3.0: an improved system for evaluating the functionomes of genomes and metagenomes. Biosci Biotechnol Biochem 24:1–3
Atala C, Pertierra LR, Aragón P, Carrasco-Urra F, Lavín P, Gallardo-Cerda J, Ricote-Martínez N, Torres-Díaz C, Molina-Montenegro MA (2019) Positive interactions among native and invasive vascular plants in Antarctica: assessing the “nurse effect” at different spatial scales. Biol Invasions. https://doi.org/10.1007/s10530-019-02016-7
Bano A, Fatima M (2009) Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol Fertil Soils 45:405–413
Barrientos-Díaz L, Gidekel M, Gutiérrez-Moraga A (2008) Characterization of rhizospheric bacteria isolated from Deschampsia antarctica Desv. World J Microbiol Biotechnol 24:2289–2296
Beyer L, Bölter M, Seppelt RD (2000) Nutrient and thermal regime, microbial biomass, and vegetation of Antarctic soils in the Windmill islands region of east Antarctica (Wilkes Land). Arct Antarct Alp Res 32:30–39
Bokhorst S, Convey P, Aerts R (2019) Nitrogen inputs by marine vertebrates drive abundance and richness in Antarctic terrestrial ecosystems. Curr Biol 29:1721–1727.e3
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Bottos EM, Woo AC, Zawar-Reza P, Pointing SB, Cary SC (2014) Airborne bacterial populations above desert soils of the McMurdo Dry Valleys, Antarctica. Microb Ecol 67:120–128
Buchfink B, Xie C, Huson DH (2015) Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60
Chen W, Liu H, Wurihan-Gao Y, Card SD, Ren A (2017) The advantages of endophyte-infected over uninfected tall fescue in the growth and pathogen resistance are counteracted by elevated CO2. Sci Rep 7:28–30
Convey P, Chown SL, Clarke A, Barnes DKA, Bokhorst S, Cummings V et al (2014) The spatial structure of Antarctic biodiversity. Ecol Monogr 80:203–244
De Zelicourt A, Al-Yousif M, Hirt H (2013) Rhizosphere microbes as essential partners for plant stress tolerance. Mol Plant 6:242–245
Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD et al (2018) A global atlas of the dominant bacteria found in soil. Science 359:320–325
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Frey-Klett P, Garbaye J, Tarkka GM (2007) The mycorrhiza helper bacteria revisited. New Phytol 176:22–36
Fujinawa K, Asai Y, Miyahara M, Kouzuma A, Abe T, Watanabe K (2016) Genomic features of uncultured methylotrophs in activated-sludge microbiomes grown under different enrichment procedures. Sci Rep 6:1–9
Gallardo-Cerda J, Levihuan J, Lavín P, Oses R, Atala C, Torres-Díaz C et al (2018) Antarctic rhizobacteria improve salt tolerance and physiological performance of the Antarctic vascular plants. Polar Biol 41:1973–1982
Giauque H, Hawques CV (2013) Climate affects symbiotic fungal endophyte diversity and performance. Am J Bot 7:1435–1444
González-Rocha G, Muñoz-Cartes G, Canales-Aguirre CB, Lima CA, Domínguez-Yévenes M, Bello-Toledo H et al (2017) Diversity structure of culturable bacteria isolated from the Fildes Peninsula (King George Island, Antarctica): a phylogenetic analysis perspective. PLoS ONE 12:e0179390
He Q, Bertness MK, Altieri AH (2013) Global shifts towards positive species interactions with increasing environmental stress. Ecol Lett 16:695–706
Hoffman MT, Arnold AE (2010) Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microbiol 12:4063–4075
Hughes KA, Cowan DA, Wilmotte A (2015) Protection of Antarctic microbial communities—“out of sight, out of mind”. Front Microbiol 6:1–6
Huson DH, Beier S, Flade I, Górska A, El-Hadidi M, Mitra S et al (2016) MEGAN Community edition-interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput Biol 12:e1004957
Hyatt D, LoCascio PF, Hauser LJ, Uberbacher EC (2012) Gene and translation initiation site prediction in metagenomic sequences. Bioinformatics 28:2223–2230
Jovel J, Patterson J, Wang W, Hotte N, Keefe SO, Mitchel T et al (2016) Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol 7:459
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Li D, Liu C-M, Luo R, Sadakane K, Lam T-W (2015) MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:16741676
Lin W, Wu L, Lin S, Zhang A, Zhou M, Lin R et al (2013) Metaproteomic analysis of ratoon sugarcane rhizospheric soil. BMC Microbiol 13:135
Louca S, Polz MF, Mazel F, Albright MBN, Huber JA, O’Connor MI et al (2018) Function and functional redundancy in microbial systems. Nat Ecol Evol 2:936–943
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–546
Mahoney AK, Yin C, Hulbert SH (2017) Community structure, species variation, and potential functions of rhizosphere-associated bacteria of different winter wheat (Triticum aestivum) cultivars. Front Plant Sci 8:132
Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ (2007) A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315:513–515
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217
Molina-Montenegro MA, Ricote-Martínez N, Muñoz-Ramírez C, Torres-Díaz C, Gómez-González S, Gianoli E (2013) Positive interactions between the lichen Usnea antarctica (Parmeliaceae) and the native flora in maritime Antarctica. J Veg Sci 24:463–472
Molina-Montenegro MA, Oses R, Torres-Díaz C, Atala C, Zurita-Silva A, Ruiz-Lara S (2016) Root-endophytes improve the ecophysiological performance and production of an agricultural species under drought condition. AoB Plants 8:plw062
Moore DM (1970) Studies in Colobanthus quitensis (Kunth) Bartl. and Deschampsia antarctica Desv. II Taxonomy, distribution and relationships. Br Antarct Surv Bull 23:63–80
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124
Pereira SFF, Goss L, Dworkin J (2011) Eukaryote-like serine/threonine kinases and phosphatases in bacteria. Microbiol Mol Biol Rev 75:192–212
Pointing SB, Büdel B, Convey P, Gillman LN, Körner C, Leuzinger S et al (2015) Biogeography of photoautotrophs in the high polar biome. Front Plant Sci 6:692
Ramos P, Rivas N, Pollmann S, Casati P, Molina-Montenegro MA (2018) Hormonal and physiological changes driven by fungal endophytes increase Antarctic plant performance under UV-B radiation. Fungal Ecol 34:76–78
Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298:1581
Roberts PR, Newsham KK, Bardgett RD, Farrar JF, Jones DL (2009) Vegetation cover regulates the quantity, quality and temporal dynamics of dissolved organic carbon and nitrogen in Antarctic soils. Polar Biol 32:999–1008
Rosa LH, Vaz ABM, Caligiorne RB, Campolina S, Rosa CA (2009) Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol 32:161–167
Sielaff AC, Upton RN, Hofmockel KS, Xu X, Polley HW, Wilsey BJ (2018) Microbial community structure and functions differ between native and novel (exotic-dominated) grassland ecosystems in an 8-year experiment. Plant Soil 432:359–372
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Elsevier Ltd., London
Takami H, Arai W, Takemoto K, Uchiyama I, Taniguchi T (2015) Functional classification of uncultured “Candidatus caldiarchaeum subterraneum” using the MAPLE system. PLoS ONE 10:1–18
Teixeira LCRS, Peixoto RS, Cury JC, Sul WJ, Pellizari VH, Tiedje J et al (2010) Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay, maritime Antarctica. ISME J 4:989
Torres-Díaz C, Gallardo-Cerda J, Lavín P, Oses R, Carrasco-Urra F, Atala C et al (2016) Biological interactions and simulated climate change modulates the ecophysiological performance of Colobanthus quitensis in the Antarctic ecosystem. PLoS ONE 11:e0164844
Upson R, Newsham KK, Bridge PD, Pearce DA, Read DJ (2009) Taxonomic affinities of dark septate root endophytes of Colobanthus quitensis and Deschampsia antarctica, the two native Antarctic vascular plant species. Fungal Ecol 2:184–196
Vandenkoornhuyse P, Quaiser A, Duhamel M, Van Le A, Dufresne A (2015) The importance of the microbiome of the plant holobiont. N Phytol 206:1196–1206
Wang NF, Zhang T, Zhang F, Wang ET, He JF, Ding H et al (2015) Diversity and structure of soil bacterial communities in the Fildes Region (maritime Antarctica) as revealed by 454 pyrosequencing. Front Microbiol 6:1–11
Yan Y, Kuramae EE, De Hollander M, Klinkhamer PGL, Van Veen JA (2017) Functional traits dominate the diversity-related selection of bacterial communities in the rhizosphere. ISME J 11:56–66
Yergeau E, Bokhorst S, Kang S, Zhou J, Greer CW, Aerts R et al (2012) Shifts in soil microorganisms in response to warming are consistent across a range of Antarctic environments. ISME J 6:692–702
Zhang X, Chen Q, Han X (2014) Soil bacterial communities respond to mowing and nutrient addition in a steppe ecosystem. PLoS ONE 8:e84210
Acknowledgements
We thank Instituto Antártico Chileno (INACH) and the Chilean Navy for logistics and field support. All sampling was performed in accordance to international permits and authorizations given by INACH. ECN was funded by “CONICYT-FONDECYT de iniciación en la investigación 11160905″. MAM-M was funded by FONDECYT 1181034 and PII20150126. We would like to thank The George Washington University’s High-Performance Computing Facility, Colonial-One, for providing data storage, support, and computing power for genomic analyses (https://colonialone.gwu.edu). All supplementary material is available at https://figshare.com/s/5d7961c1859f33067dab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study has been conducted in absence of conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Impact statement
Functional symbiosis can be a key strategy used by plants to cope with the harsh Antarctic environment by activation of functional pathways.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molina-Montenegro, M.A., Ballesteros, G.I., Castro-Nallar, E. et al. A first insight into the structure and function of rhizosphere microbiota in Antarctic plants using shotgun metagenomic. Polar Biol 42, 1825–1835 (2019). https://doi.org/10.1007/s00300-019-02556-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02556-7