Abstract
Early life-history stages of cephalopods are known to play an important role as prey in food webs of the Southern Ocean, but little information is available about their biology and availability to predators. Top predators, such as penguins, are known to feed regularly on coastal juvenile/sub-adult cephalopods. Using eastern rockhopper penguins Eudyptes filholi as coastal biological samplers, we examined in detail the cephalopod component of their diet in Campbell Island (New Zealand) during two consecutive breeding seasons in order to evaluate (1) the relative importance of cephalopods (by frequency of occurrence, by number and by mass) to the diet of both adult and chick penguins, (2) the habitat and trophic levels of the cephalopods in the region and (3) the status of the juvenile/sub-adult cephalopod community in the waters around Campbell Island. Our results show that eastern rockhopper penguins feed on eight species of juvenile and sub-adult cephalopods, with Onykia ingens, Martialia hyadesi and Octopus campbelli being the most important species by frequency of occurrence, number and mass. Differences between the diets of adult and chick penguins and between breeding seasons were found. Habitat (δ13C) and trophic level (δ15N) information also showed that all cephalopod species (and all studied stages) occupy similar habitat on the Campbell shelf, with M. hyadesi showing lower δ15N values than O. ingens and O. campbelli. This study indicates that eastern rockhopper penguins can be valuable biological samplers of local juvenile/sub-adult cephalopods (including poorly known cephalopod species) around Campbell Island when breeding, that these cephalopods were likely to be caught naturally (not from fisheries), providing relevant information for the conservation of these penguins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal marine zones are complex ecosystems influenced by physical, chemical and biological processes under anthropogenic pressure (Halpern et al. 2008), with resources (e.g. exploitable marine organisms) that require careful management (Ngoile and Horrill 1993). It is increasingly important to have a regional and global effort to identify resources that require conservation, to develop and implement ecosystem-based management and to carry out marine basic research to address gaps in ecosystem knowledge (Halpern et al. 2008). Within the Southern Ocean, the current or most immediate threats to the conservation of species, ecosystems and resources are regional ocean warming, acidification, changes in sea-ice distribution and human activities (such as fishing) (Anderson et al. 2011; Tuck et al. 2011; Kennicutt II et al. 2014; Xavier et al. 2016a).
New Zealand and its adjacent sub-Antarctic islands are strongly influenced by the Southern Ocean (Gordon et al. 2010), and some marine keystone species of this region, including fish and seabirds, have already been affected by climate change and by fisheries (Cunningham and Moors 1994; Hilton et al. 2006; Scott et al. 2008; Gordon et al. 2010; Last et al. 2011; Morrison et al. 2015). Yet, there is a paucity of information on how cephalopods might be affected despite an increase of basic ecological and taxonomic knowledge of cephalopods from the Southern Ocean recently (Jackson et al. 1998b; Cherel and Duhamel 2003; Collins and Rodhouse 2006; Guerreiro et al. 2015; Xavier et al. 2015, 2016c). Cephalopods are known to be an important prey of numerous predators, to have a large biomass and may be affected by environmental change (Clarke 1983; Xavier and Cherel 2009; Rodhouse 2013). Indeed, the majority of cephalopod species exhibit a “live fast and die young” life cycle which can result in either positive or negative effects in their response to environmental change (e.g. warming may increase cephalopod growth rate but shorten lifespan that will have implications for their demography and life history) (Pecl and Jackson 2008; Xavier et al. 2015). As cephalopod fauna from the Pacific sector in the Southern Ocean is still poorly known (Xavier et al. 2014), top predators (including penguins) can be used as biological samplers to provide valuable information about local cephalopod fauna (Clarke 1980; Rodhouse et al. 1992; Cherel and Weimerskirch 1995, 1999; Cherel et al. 1999; Xavier and Cherel 2009; Seco et al. 2016), complementing with scientific surveys (Gordon et al. 2010).
Among top predators, albatrosses, petrels, whales and numerous penguin species are known to feed on cephalopods, consuming significant quantities of squid in some regions and seasons (Croxall and Prince 1996; Xavier and Cherel 2009). The eastern rockhopper penguin Eudyptes filholi (Cunningham and Moors 1994; Heather and Robertson 1996; Banks et al. 2006; De Dinechin et al. 2009) breeds in the southern Indian Ocean (Marion, Crozet, Kerguelen and Heard islands) and in the south Pacific Ocean (Auckland, Antipodes, Campbell and Macquarie islands) (BirdLife International 2012; Morrison et al. 2015), and feeds predominantly on fish, crustaceans and cephalopods (Williams and Laycock 1981; Marchant and Higgins 1990; Williams 1995; Hull 1999; Crawford et al. 2003; Sagar et al. 2005; Morrison et al. 2014). Regarding the latter group, the work by P. J. Moors and D. M. Cunningham (Marchant and Higgins 1990) at Campbell Island provided the first preliminary analyses of the cephalopods ingested by eastern rockhopper penguins, but presented no information of estimated body size and mass, and maturity stage. This information is important, not only to the biology/ecology of cephalopods but also for management and conservation of local/regional marine ecosystems. Moreover, cephalopod species may have been misidentified and a review of these identifications may be required.
Rockhopper penguins are known to forage up to 120 km from their breeding island during the breeding season (Sagar et al. 2005) and consequently may allow inferences on spawning grounds, biology and ecology of neritic and oceanic cephalopods and fish (Tremblay and Cherel 2000, 2003). Moreover, the use of stable isotope values of beaks of cephalopods from the diet of their predators can provide valuable information on cephalopod habitat (via δ13C) and trophic level (via δ15N) (Cherel and Hobson 2005) as well as for other prey taxa, such as fish and crustaceans (Barrett et al. 2007; Karnovsky et al. 2012; Stowasser et al. 2012).
The objectives of our study are to use eastern rockhopper penguins as biological samplers of cephalopods around sub-Antarctic islands, because cephalopod-related information is very scarce. More specifically, we aim to:
-
a)
characterise the cephalopod component of the diet of eastern rockhopper penguins at Campbell Island during breeding in two consecutive breeding seasons (1985/86 and 1986/87);
-
b)
study the habitat and trophic level characteristics of cephalopods in the Campbell Island region through stable isotopic analysis and
-
c)
assess how such information is relevant to the ecology and biodiversity studies of juvenile/sub-adult cephalopods in coastal waters around Campbell Island.
Materials and methods
Fieldwork
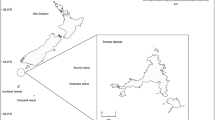
A total of 122 diet samples were obtained from adults (n = 98, via stomach pump lavage, individuals captured en route to the breeding colonies from shoreline landing areas, 1–200 m from colonies, samples collected in approximately 5 min per bird using with seawater) and chicks (n = 24, via autopsy, chicks sampled opportunistically from fresh carcasses encountered in the colonies) of eastern rockhopper penguins from Penguin Bay, Campbell Island (52°32′24″S, 169°8′42″E), New Zealand (Fig. 1), during the breeding seasons of 1985–1986 (adults = 29 (23-12-1985 to 24-01-1986); chicks = 24 (18-12-1985 to 27-01-1986) and 1986–1987 (adults = 69 (13-01-1987 to 07-02-1987), collected randomly each year by P. J. Moors and D. M. Cunningham (Marchant and Higgins 1990). All adult birds (each sampled once only) were released in apparently good condition and all returned to their nesting colony, with the exception of one casualty across the 2 years. Chick carcasses were not collected in the second (1986–1987) breeding season due to fieldwork constraints.
After collecting the samples, liquid was removed from each sample by straining through a muslin bag. Samples were frozen until analysis in mainland New Zealand. Here, we re-examined the cephalopod component of the diet of eastern rockhopper penguins because the taxonomy of cephalopods has undergone considerable revision since the 1980s (e.g. with new species being described or taxonomy updated (O’Shea 1999; Collins and Rodhouse 2006)), additional information on the sizes and maturity stage of some of the cephalopod species caught is now available (Jackson 1997; Jackson et al. 2000; Phillips et al. 2003) and our ability to use stable isotopic analyses to assess the habitat and trophic levels of the main cephalopod species has become well established (Cherel and Hobson 2005; Xavier et al. 2016b). Furthermore, our study incorporates previously unpublished data from adults and analyses of cephalopods in the diet of eastern rockhopper penguins between seasons, information not presented in Marchant and Higgins (1990). The fish and crustacean components in the diet of eastern rockhopper penguins have been published (Marchant and Higgins 1990).
Laboratory work
Cephalopod beaks were identified to species level wherever possible, following Xavier and Cherel (2009) and beak reference collections at the National Institute of Water and Atmospheric Research (NIWA, see below) and at the Marine and Environmental Sciences Centre, University of Coimbra (Portugal). The lower rostral length (LRL; for squid) and lower hood length (LHL; for octopods) were measured using a stereo microscope (Wild Heerbrugg, objective: 1.5 ×; ocular: 15 ×) and maturity stage estimated (according to the darkening of the beaks: juvenile beaks were undarkened or with only the tip of the rostrum darkened; sub-adult beaks exhibited darkened tip and wings or adult beaks with wings totally darkened) (Clarke 1986; Xavier and Cherel 2009). Allometric equations from published sources were used to convert LRL/LHL to mantle length (ML, in mm) and mass (M, in grams (g)) (Clarke 1986; Brown and Klages 1987; Rodhouse and Yeatman 1990; Jackson 1995; Piatkowski et al. 2001; Lalas and McConnell 2012; Roberts and Lalas 2015). Octopod beaks were identified using a reference collection (that includes beaks from known species in the region, including the octopod Enteroctopus zealandicus) held at NIWA in Wellington, New Zealand. For the most important cephalopod species (i.e. by frequency of occurrence, by number and/or by mass) that allowed comparisons of sizes (i.e. LRL/LHL; see below) between years, statistical tests were also performed to assess differences between old and fresh beaks.
For each cephalopod species, frequency of occurrence (F: number of samples with a certain cephalopod species present divided by the total number of samples analysed), number (N: number of lower beaks of a certain species divided by the total number of lower beaks) and contribution to mass (estimated mass of all individuals of a certain cephalopod species divided by the total estimated mass for all cephalopod individuals) were estimated following previous diet studies (Xavier et al. 2002). To complement these analyses, a re-calculation of the contribution of each species by mass was carried out, by adding the mean mass of all individuals of a given species to each beak unmeasured of that species (e.g. the mean mass of 13.5 g was multiplied by the 18 unmeasurable lower beaks of O. ingens in 1985–1986).
For the most numerous species, after double-checking identifications, all lower beaks for each species that were in good condition (i.e. fresh, with no signs of erosion) were stored in a glass jar containing 75% ethanol, and beaks were randomly selected for stable isotopic analysis (O. ingens: 16 lower beaks; M. hyadesi: 10 lower beaks; O. campbelli: 20 lower beaks) following previous studies (Seco et al. 2016; Xavier et al. 2016b). After taking measurements (see above), beaks were prepared for stable isotopic analysis after the beaks being transported from New Zealand to Portugal by plane (kept in 70% ethanol). Each beak was cleaned with 80% ethanol, stored in a microtube and dried in an oven at 60 °C. Dried beaks were smashed into very small fragments inside the microtube using a cleaned tweezer to decrease the chance of contamination. Using a Mettler Toledo® UMX2 ultra-microbalance, samples were weighed, approximately 0.35 mg (0.27 ± 0.10 mg, mean ± standard deviation) into a tin capsule. Values of δ13C and δ15N were obtained using a continuous flow isotope ratio mass spectrometer (Delta V™ Advantage—Thermo Scientific®) interfaced with an organic elemental analyser (Flash™ EA 1112—Thermo Scientific®) at MARE—Figueira da Foz, following previous published work (Seco et al. 2016). We present the results in standard δ notation in ‰ (parts per thousand), following the equation δX = [(Rsample − Rstandard −1) × 1000], where X represents 13C or 15N and R the 13C/12C and 15N/14N ratios, respectively. Data are presented relative to Vienna-Pee Dee Belemnite (V-PDB) limestone and Atmospheric N2 for C and N, respectively. Reference material [acetanilide (Thermo Scientific®)] was measured to determine machine internal analytical errors (< 0.2‰ both for δ13C and δ15N). Carbon-to-Nitrogen (C:N) ratios of the samples were estimated to assess the potential effect of lipids on the results (i.e. C:N < 3.5) (Post et al. 2007).
Statistical analyses
All statistics were carried out using STAT PLUS. In order to maximise the number of beaks analysed to assess the widest range of cephalopods ingested (rather than fresh items only), accumulated old and fresh beaks were combined for statistical analyses (Tables 1, 2), following previous studies (Cherel and Weimerskirch 1999; Xavier et al. 2003). Comparisons between the beak sizes were performed, i.e. between adults and chicks; between seasons for species; between habitat and trophic levels between the most numerous cephalopod species (i.e. O. ingens, M. hyadesi and O. campbelli) using non-parametric tests (Mann–Whitney U test, between two species/samples; Kruskal–Wallis, between more than two species/samples), after confirming that the data were not normally distributed (using STAT PLUS normality test). Result are provided as mean ± standard deviation, unless stated otherwise. A result was significant when p < 0.05.
Results
Cephalopod component of the diet of eastern rockhopper penguins
A total of 939 lower beaks were identified and measured from the 122 samples of eastern rockhopper penguins at Campbell Island in the 1985–1986 and 1986–1987 breeding seasons (Table 1). All the beaks were from juveniles for the squid species and from juveniles/sub-adults for the octopus O. campbelli. Photographs were taken of the key cephalopod species that have not been described before (Fig. 2). No adult beaks (i.e. with wings fully darkened) were encountered in the samples. Eight species of cephalopods were recorded, with O. ingens, M. hyadesi and O. campbelli being the most important species by frequency of occurrence, number and by mass (Table 1). The cephalopods consumed ranged from < 1 g (an individual of O. campbelli) to 129 g (an individual of O. ingens) of estimated mass (Table 2).
Adults versus chicks
Comparing diet data between adults and chicks was only possible in the 1985–1986 breeding season. O. ingens was the most important cephalopod in the diet of adult eastern rockhopper penguins (F: 36.6%; N = 64.9%; M = 50.0–64.6%), whereas M. hyadesi was the most important species in the diet of chicks (F: 58.3%; N = 49.5%; M = 55.6–59.6%: Table 1). The sizes of lower beaks of O. ingens (the only species with sufficient beaks for a statistical test) were different between adults and chicks, with beaks from penguin chicks slightly larger than those from adults (Mann–Whitney U test; U(33,27) = 595.50; p = 0.025; O. ingens from adults: 1.3 ± 0.5 mm (n = 33); from chicks: 1.5 ± 0.4 mm (n = 27)).
Inter-annual variations
Using only comparable adult diet data between years, O. ingens was the most important cephalopod species in the diet (by F, N and M) in both 1985–1986 and 1986–1987 breeding seasons (Table 1). Even when recalculating the mass (by the inclusion of a mean mass for unmeasurable lower beaks), O. ingens continued to be the most important species (by F, N and M) but its relative contribution to the diet by mass in the 1986–1987 season decreased (from 49.6 to 39.7%), while the contribution to the diet by mass of M. hyadesi increased (from 13.0 to 38.0%: Table 1).
A comparison of beak sizes between the main cephalopod species and between seasons (combining all measurements from adults and chicks between years) revealed that there was a significant difference between the beak sizes of O. campbelli between the two seasons (but still juvenile/sub-adult specimens), with larger beaks in the 1985–1986 season (Mann–Whitney U test; U(63,248) = 11,062.00; p < 0.0001; Table 2; Fig. 3).
Frequency of occurrence of the squid Onykia ingens (family Onychoteuthidae), Martialia hyadesi (family Ommastrephidae) and the octopod Octopus campbelli (family Octopodidae) and by lower rostral length in the diet of eastern rockhopper penguins at Campbell island in seasons 1985–1986 (white bars) and 1986–1987 (black bars). (X-axis legend correspond to the following 0.5 size (e.g. 0 = 0.0–0.5 mm, 0.5 = 0.5–1.0 mm,..., 4= 4.0–4.5 mm))
Habitat and trophic levels of cephalopods around Campbell Island
Carbon-to-nitrogen (C:N) ratios of beaks showed no effect of lipids on the results, with values ranging from 3.2 to 4.0 across all beaks analysed (Table 3). All the main species (O. ingens, O. campbelli and M. hyadesi) occupy a very similar habitat based on δ 13C values (Kruskal–Wallis test H(2,46) = 0.08; p = 0.962), with O. ingens showing a wider range of values compared to the other two species (Table 3; Fig. 4). δ15N values of M. hyadesi were significantly lower than those of O. ingens (Kruskal–Wallis test H(1,24) = 10.95; p = 0.0009) and O. campbelli (Kruskal–Wallis test H(1,32) = 9.09; p = 0.002). The latter two species had similar δ15N values (Kruskal–Wallis test H(1,36) = 0.23; p = 0.633).
Discussion
Cephalopod component of penguins’ diet
This study aimed, in part, to compare our results with those obtained by D. M. Cunningham and P. J. Moors from the same diet samples (Marchant and Higgins 1990) collected in the mid-1980s. Cephalopods previously reported comprised a total of three species (Moroteuthis ingens, Alluroteuthis antarcticus and Octopus dofleini) in adult penguins and four species (M. ingens, Kondakovia longimana, M. hyadesi and O. dofleini) in chicks (Marchant and Higgins 1990).
Octopus dofleini [now known as the giant Pacific octopus E. dofleini (Bouchet 2010)] was misidentified in Marchant and Higgins (1990) with the small octopod O. campbelli, which was the most important (and only) octopod species in the diet of eastern rockhopper penguins in our study (Table 1). E. dofleini is only known to be distributed in the northern Pacific Ocean from Japan to southern California (Nesis 1987). Within the genus Enteroctopus, the octopus E. zealandicus occurs in New Zealand sub-Antarctic waters (Bouchet 2010), and has been recorded in the diet of New Zealand sea lions Phocarctos hookeri at Campbell Island (Roberts and Lalas 2015), but beaks of E. zealandicus are considerably larger than the beaks from O. campbelli for the same maturity stage (i.e. small beaks from juvenile/sub-adult of O. campbelli were already considerably more darkened than would be the case for undarkened juvenile/sub-adult beaks from E. zealandicus).
Our study also identified O. ingens (previously known as Moroteuthis ingens) as the most important species (see Tables 1, 2, and Marchant and Higgins 1990) but also identified other cephalopod species in the diet of eastern rockhopper penguin adults [namely A. antarcticus, Gonatus antarcticus, M. hyadesi, Moroteuthis sp. B (Imber 1992) and Oegopsida sp. A (Cherel et al. 2004)] and chicks (G. antarcticus), which may be attributed to better reference collections and cephalopod identification guides (Imber 1992; O’Shea 1999; Cherel et al. 2004; Xavier and Cherel 2009), information not available in the 1980s. Consequently, this study identified four additional squid species from the waters around Campbell Island that were present in the 1980s (see Table 1), providing valuable information on their circumpolar distribution and the use of the Campbell shelf area for their early life stages in the 1980s. G. antarcticus and K. longimana have a circumpolar distribution reaching as far south as the Antarctic continent (Xavier et al. 2016c), with our study providing evidence of their distribution extending also to the Campbell Island shelf (at least in the 1980s), in South Pacific waters. M. sp. B (Imber 1992) is a poorly known species previously reported from New Zealand waters (West and Imber 1986; Imber 1992), and occurring in the diet of predators around the Southern Ocean: (1) Patagonian toothfish Dissostichus eleginoides, (2) wandering albatross Diomedea exulans and king penguin Aptenodytes patagonicus in the south Indian Ocean (Crozet and Kerguelen islands) (Cherel et al. 2004, 2017; Xavier and Cherel 2009) and (3) Antipodean albatross Diomedea antipodensis antipodensis and Gibson’s albatross D. a. gibsoni from south Pacific waters (Xavier et al. 2014). Similarly, Oegopsida sp. A (Cherel) is a rare species that occurs infrequently in the diet of Patagonian toothfish and wandering albatrosses in the south Indian Ocean (at Crozet Island) (Cherel et al. 2004, 2017) and South Georgia (Imber 1992), and in Antipodean and Gibson’s albatross diets from south Pacific waters (Xavier et al. 2014).
Overall, our results show that out of the eight species of cephalopods found in the diet of eastern rockhopper penguins, O. ingens, M. hyadesi and O. campbelli were the most important species by frequency of occurrence, number and by mass (Table 1). Surprisingly, there were differences between adults and chicks, not only in terms of the most important cephalopod species (O. ingens in adults and M. hyadesi in chicks; Table 1) but also chicks being fed slightly larger O. ingens than those taken by adults (although the differences in size are unlikely to be ecologically relevant). As prey found in the diet of chicks is provided by adults, adult eastern rockhopper penguins perhaps preferentially feed their chicks slightly larger squid.
Differences in cephalopod component of penguins’ diet between breeding seasons
Other than comparing the biodiversity of cephalopods occurring in the diet of eastern rockhopper penguins at Campbell Island (Marchant and Higgins 1990), our detailed study allowed us to compare penguin diet across two consecutive breeding seasons. O. ingens was the most frequent cephalopod species in both breeding seasons (1985–1986 and 1986–1987), with both O. ingens and M. hyadesi also playing an important role by mass. O. ingens and M. hyadesi are known to occur in the diet of a wide range of predators across the Southern Ocean (Xavier and Cherel 2009), with penguins (and some smaller albatross species, e.g. black-browed albatross Thalassarche melanophrys and grey-headed albatross T. chrysostoma) generally taking juveniles (examples across the Southern Ocean in Appendix Online Resource) and larger predators, such as whales, great albatrosses (e.g. wandering albatross) and toothfish generally taking sub-adults and adults (Clarke 1980; Cherel et al. 1996, 2004, 2017; Cherel and Weimerskirch 1999; Cherel and Duhamel 2004; Xavier et al. 2014), with larger predators consuming correspondingly larger squid specimens. The most important (and only) octopod species found in the diet of eastern rockhopper penguins was O. campbelli, exhibiting significantly larger sizes in the 1985–1986 breeding season than in the 1986–1987 breeding season (Table 2), despite all beaks being representative of a similar life stage (i.e. juvenile/sub-adult specimens; Fig. 2). As warmer waters are most likely to affect primary productivity negatively, and affect the population of eastern rockhopper penguins through changes in the distribution, availability or abundance of their food supply (Cunningham and Moors 1994; Hilton et al. 2006), sea temperatures may have potentially affected the sizes of O. campbelli. With cooler sea surface temperature conditions in 1985–1986 than in 1986–1987 (Morrison et al. 2015), O. campbelli may have exhibited higher growth in 1985–1986. Indeed with cooler sea surface temperature, waters stay more oxygenated, allowing animals to grow bigger (Chapelle and Peck 1999). However, as the dataset is only from the diet of a selective predator (i.e. rockhopper penguins) that may not represent the overall size distribution of O. campbelli around Campbell Island, and there was no independent evaluation of the abundance of O. campbelli in the area in the breeding seasons included here, it is difficult to draw any firm conclusions.
Octopus campbelli and O. ingens also occurred as relatively minor components in the diet of P. hookeri breeding at Campbell Island (Roberts and Lalas 2015) and at the Auckland Islands (Childerhouse et al. 2001). New Zealand sea lions also feed on E. zealandicus and the pelagic squid Todarodes filippovae (Roberts and Lalas 2015), both of which are absent in rockhopper penguin diets (Table 1). Such different diets could be attributed to E. zealandicus being able to reach large sizes (and therefore hard to handle by the penguins), with larger, mature individuals occurring in deeper waters (up to c. 530 m deep) where this octopus could be caught by New Zealand sea lions (O’Shea 1999). Surprisingly, juvenile E. zealandicus are common in littoral sub-Antarctic waters (including Campbell Island) but do not occur in the diet of eastern rockhopper penguins, which could be attributed to temporal (e.g. juveniles of E. zealandicus are only available during other periods of the year outside the rockhopper penguin breeding season), spatial (e.g. juveniles of E. zealandicus are distributed outside the penguins’ foraging area) or ecological (e.g. juveniles of E. zealandicus are capable of avoiding capture by rockhopper penguins) reasons but more research is needed to address this issue.
Biodiversity, habitat and trophic level of cephalopods in the Campbell Island region through stable isotopic analysis
As biological samplers of juvenile and sub-adult cephalopods around Campbell Island, eastern rockhopper penguins fed on early stages of eight different cephalopod species, demonstrating the importance of the waters around Campbell Island for the life cycle of both benthic and pelagic cephalopods. Although the spawning area of these species is unknown, the presence of juvenile cephalopods in the diet of eastern rockhopper penguins, supported with the habitat (δ 13C) stable isotopic values of sub-Antarctic/subtropical waters (Jaeger et al. 2010; Ceia et al. 2015; Guerreiro et al. 2015) and of the studied cephalopods (see Fig. 4), suggests that some spawning may occur relatively near to Campbell Island in shelf or slope waters. Moreover, these results also suggest that eastern rockhopper penguins are foraging (at least, during the breeding season in the 1980s) close to Campbell Island at a specific or similar isotopic related-habitat type and feeding on some early stages of cephalopods (as supported by the stable isotopic values of the cephalopod prey; see below).
Octopus campbelli occurs locally to Campbell Island (O’Shea 1999), so the isotopic proxy values of habitat (δ 13C) reflect the waters around Campbell Island. Furthermore, our study provides isotopic evidence that juvenile and pelagic O. ingens and M. hyadesi occur in similar habitat as benthic Octopus campbelli around Campbell Island during the breeding season of eastern rockhopper penguins. Consequently, our results suggest eastern rockhopper penguins at Campbell Island perform benthic dives, as already described at Kerguelen Islands (Tremblay and Cherel 2000), although elsewhere they perform mostly pelagic dives (Hull 2000). Onychoteuthis (such as O. ingens) and Ommastrephids (such as M. hyadesi) are known to occur in oceanic waters but can also be found in shelf/slope waters in Antarctic and sub-Antarctic regions (Roper et al. 1985; Jackson et al. 1998a, 2000; Xavier et al. 1999, 2016c; Cherel and Duhamel 2003; Rodhouse et al. 2014; Rosas-Luis et al. 2016). As large juvenile and immature O. ingens occur near the seabed in shelf environments before gradually moving into deeper waters as adults (Jackson 1993; Cherel and Weimerskirch 1999; Cherel and Duhamel 2003), it is likely that eastern rockhopper penguins fed on this species close to the bottom, where O. campbelli is also likely to be encountered. While eastern rockhopper penguins appear to perform mainly pelagic dives (perhaps foraging on fish and crustaceans) (Thompson, unp. data from Campbell Island during the chick-rearing phase) (Hull 2000), our data are indicative that some cephalopod prey are taken at or near the bottom, as well as pelagically.
Juvenile M. hyadesi is more likely to have been caught in the water column within the pelagic environment (Rodhouse 1997). O. ingens and M. hyadesi appear to have a life span of 1–2 years (Rodhouse et al. 1994; Jackson 1997) and juveniles may be a regular annual prey for eastern rockhopper penguins, at least during the breeding season.
The trophic level (δ15N) of the most important cephalopod species show that M. hyadesi has a lower trophic value than O. ingens and O. campbelli (Fig. 4). Consequently, it can be hypothesised that M. hyadesi could form part of the diet of O. ingens and O. campbelli, and that beaks of M. hyadesi found in the diet of rockhopper penguins are a result of being initially captured by O. ingens or O. Campbelli, rather than rockhopper penguins preying directly on M. hyadesi. However, there are no records of M. hyadesi in the diet of O. ingens (see below) (Cherel and Duhamel 2003), nor is it likely that a benthic octopod such as O. campbelli would venture into the water column to feed on a mobile, pelagic squid such as M. hyadesi (however, studies on the diet of O. campbelli would be required to clarify this statement).
In terms of conservation, it is worth noting that the cephalopod prey of breeding eastern rockhopper penguins is likely to be naturally caught as there are no cephalopod fisheries around Campbell Island and the cephalopod bycatch/discards from the southern blue whiting Micromesistius australis trawl fishery around Campbell Island are relatively small [squid and octopods are < 0.01% of the total catch (Anderson 2009)]. Furthermore, O. ingens collected from New Zealand waters, including specimens collected from around Campbell Island, revealed a diet that is mostly composed of small teleost fish (< 100 mm standard length; principally: Lampanyctodes hectoris (59% F), Stomias boa/Chauliodus sloani (46% F) (Jackson et al. 1998b)), with no presence of southern blue whiting. Therefore, the cephalopods preyed upon by breeding eastern rockhopper penguins at Campbell Island are unlikely to have been obtained through local commercial fishing activities, although overlap with commercial fishing may occur outside the breeding season, as rockhopper (and other) penguins are known to disperse during the non-breeding period (Pütz et al. 2006; Ratcliffe et al. 2014).
This study was conducted in the 1980s, and it would be interesting to investigate the current diet of eastern rockhopper penguins, to assess temporal dietary differences across several decades. Rockhopper penguin population fluctuations at Campbell Island appear to follow trends in local sea surface temperature, with declining population trajectories during periods of relatively warm water and recovering populations during relatively cooler periods (Morrison et al. 2015). This thermally dynamic marine system is additionally very likely to affect the cephalopod prey of rockhopper penguins. Indeed, with stable isotope analyses used in this study, it would be possible to evaluate whether there have been changes in habitat and trophic levels of the cephalopod community present in the diet of eastern rockhopper penguins around Campbell Island. As cephalopods may be sensitive to environmental change, which may in turn impact their distribution and abundance (see introduction), such research could provide valuable information on changes in sub-Antarctic marine ecosystems, and contribute information towards the conservation of this penguin species.
References
Anderson OF (2009) Fish and invertebrate bycatch and discards in southern blue whiting fisheries, 2002–2007, vol 43. Ministry of Fisheries, New Zealand
Anderson OR, Small CJ, Croxall JP, Dunn EK, Sullivan BJ, Yates O, Black A (2011) Global seabird bycatch in longline fisheries. Endangered Species Res 14:91–106
Banks J, Van Buren A, Cherel Y, Whitfield JB (2006) Genetic evidence for three species of rockhopper penguins, Eudyptes chrysocome. Polar Biol 30:61–67
Barrett RT et al (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691
BirdLife International (2012) Eudyptes chrysocome. The IUCN Red List of Threatened Species 2012: eT22735250A37849176 http://doi.org/102305/IUCNUK2012-1RLTST22735250A37849176en. Accessed 13 Sept 2016
Bouchet P (2010) Enteroctopus dofleini (Wülker, 1910). In: 2017-06-26 MAtWRoMSahwmoapptio (ed). MolluscaBase (2017). http://www.marinespecies.org/aphia.php?p=taxdetails&id=342305. Accessed 26 Jun 2017
Brown CR, Klages NT (1987) Seasonal and annual variation in diets of Macaroni (Eudyptes chrysolophus) and southern rockhopper (E. chrysocome) penguins at sub-Antarctic Marion Island. J Zool Lond 212:7–28
Ceia FR, Ramos JA, Phillips RA, Cherel Y, Jones DC, Vieira RP, Xavier JC (2015) Analysis of stable isotope ratios in blood of tracked wandering albatrosses fails to distinguish a δ13C gradient within their winter foraging areas in the southwest Atlantic Ocean. Rapid Commun Mass Spectrom 29:2328–2336
Chapelle G, Peck LS (1999) Polar gigantism dictated by oxygen availability. Nature 399:114–115. https://doi.org/10.1038/20099
Cherel Y, Duhamel G (2003) Diet of the squid Moroteuthis ingens (Teuthoidea: Onychoteuthidae) in the upper slope waters of the Kerguelen Islands. Mar Ecol Prog Ser 250:197–203. https://doi.org/10.3354/meps250197
Cherel Y, Duhamel G (2004) Antarctic jaws: cephalopod prey of sharks in Kerguelen waters. Deep-Sea Res I 51:17–31
Cherel Y, Hobson K (2005) Stable isotopes, beaks and predators: a new tool to study the trophic ecology of cephalopods, including giant and colossal squids. Proc R Soc Lond B 272:1601–1607
Cherel Y, Weimerskirch H (1995) Seabirds as indicators of marine resources: black-browed albatrosses feeding on ommastrephid squids in Kerguelen waters. Mar Ecol Prog Ser 129:295–300
Cherel Y, Weimerskirch H (1999) Spawning cycle of onychoteuthid squids in the southern Indian Ocean: new information from seabird predators. Mar Ecol Prog Ser 188:93–104
Cherel Y, Ridoux V, Rodhouse PG (1996) Fish and squid in the diet of king penguin chicks Aptenodytes patagonicus during winter at sub-Antarctic Crozet Islands. Mar Biol 126:559–570
Cherel Y, Waugh S, Hanchet S (1999) Albatross predation of juvenile southern blue whiting (Micromesistius australis) on the Campbell Plateau. NZ J Mar Freshw Res 33:437–441
Cherel Y, Duhamel G, Gasco N (2004) Cephalopod fauna of subantarctic islands: new information from predators. Mar Ecol Prog Ser 266:143–156. https://doi.org/10.3354/meps266143
Cherel Y, Xavier JC, de Grissac S, Trouvé C, Weimerskirch H (2017) Feeding ecology, isotopic niche, and ingestion of fishery-related items of the wandering albatross Diomedea exulans at Kerguelen and Crozet Islands. Mar Ecol Prog Ser 565:197–215
Childerhouse S, Dix B, Gales N (2001) Diet of New Zealand sea lions (Phocarctos hookeri) at the Auckland Islands. Wildl Res 28:291–298
Clarke MR (1980) Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. In: Discovery Reports, vol 37, p 1–324
Clarke MR (1983) Cephalopod biomass-estimation from predation. Memoirs of the National Museum, Victoria 44:95–107
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Collins MA, Rodhouse PGK (2006) Southern ocean cephalopods. Adv Mar Biol 50:191–265
Crawford RJ et al (2003) Decrease in numbers of the eastern rockhopper penguin Eudyptes chrysocome filholi at Marion Island, 1994/95–2002/03. Afr J Mar Sci 25:487–498
Croxall JP, Prince PA (1996) Cephalopods as prey: seabirds. Philos Trans R Soc B 351:1023–1043
Cunningham DM, Moors PJ (1994) The decline of rockhopper penguins Eudyptes chrysocome at Campbell Island, Southern Ocean and the influence of rising sea temperatures. Emu 94:27–36
De Dinechin M, Ottvall R, Quillfeldt P, Jouventin P (2009) Speciation chronology of rockhopper penguins inferred from molecular, geological and palaeoceanographic data. J Biogeogr 36:693–702
Gordon DP, Beaumont J, MacDiarmid A, Robertson DA, Ahyong ST (2010) Marine biodiversity of Aotearoa New Zealand. PLoS ONE 5:e10905
Guerreiro M, Richard A, Phillips RA, Cherel Y, Ceia FR, Alvito P, Rosa R, Xavier JC (2015) Habitat and trophic ecology of Southern Ocean cephalopods from stable isotope analyses. Mar Ecol Prog Ser 530:119–134
Halpern BS et al (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Heather BD, Robertson HA (1996) The field guide to the birds of New Zealand. Viking, Auckland
Hilton GM, Thompson DR, Sagar PM, Cuthbert RJ, Cherel Y, Bury SJ (2006) A stable isotopic investigation into the causes of decline in a sub-Antarctic predator, the rockhopper penguin Eudyptes chrysocome. Glob Chang Biol 12:611–625
Hull CL (1999) Comparison of the diets of breeding royal (Eudyptes schlegeli) and rockhopper (Eudyptes chrysocome) penguins on Macquarie Island over 3 years. J Zool 247:507–529. https://doi.org/10.1111/j.1469-7998.1999.tb01013.x
Hull CL (2000) Comparative diving behaviour and segregation of the marine habitat by breeding royal penguins, Eudyptes schlegeli, and eastern rockhopper penguins, Eudyptes chrysocome filholi, at Macquarie Island. Can J Zool 78:333–345. https://doi.org/10.1139/z99-192
Imber MJ (1992) Cephalopods eaten by wandering albatrosses Diomedea exulans L. breeding at six circumpolar localities. J R Soc NZ 22:243–263
Jackson GD (1993) Growth zones within the statolith microstructure of the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae): evidence for a habitat shift? Can J Fish Aquat Sci 50:2366–2374
Jackson GD (1995) The use of beaks as tools for biomass estimation in the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) in New Zealand waters. Polar Biol 15:9–14
Jackson GD (1997) Age, growth and maturation of the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) in New Zealand waters. Polar Biol 17:268–274
Jackson GD, George MJA, Buxton NG (1998a) Distribution and abundance of the squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) in the Falkland Islands region of the South Atlantic. Polar Biol 20:161–169
Jackson GD, McKinnon JF, Lalas C, Ardern R, Buxton NG (1998b) Food spectrum of the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) in New Zealand waters. Polar Biol 20:56–65
Jackson G, Shaw A, Lalas C (2000) Distribution and biomass of two squid species off southern New Zealand: Nototodarus sloanii and Moroteuthis ingens. Polar Biol 23:699–705
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460
Karnovsky NJ, Hobson KA, Iverson SJ (2012) From lavage to lipids: estimating diets of seabirds. Mar Ecol Prog Ser 451:263–284
Kennicutt MC II et al (2014) Six priorities for Antarctic Science (and supplementary material). Nature 512:23–25
Lalas C, McConnell HM (2012) Prey of Auckland Island shags (Leucocarbo colensoi) in winter. Notornis 59:130–137
Last PR, White WT, Gledhill DC, Hobday AJ, Brown R, Edgar GJ, Pecl G (2011) Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practises. Glob Ecol Biogeogr 20:58–72
Marchant S, Higgins P (1990) Handbook of Australian, New Zealand and Antarctic birds. In: Ratites to Ducks, Part A-Ratites to Petrels, Part B-Australian Pelican to Ducks, vol 1. Oxford University Press, Melbourne
Moore GJ, Robertson G, Wienecke B (1998) Food requirements of breeding king penguins at Heard Island and potential overlap with commercial fisheries. Polar Biol 20:293–302
Morrison KW, Bury SJ, Thompson DR (2014) Higher trophic level prey does not represent a higher quality diet in a threatened seabird: implications for relating population dynamics to diet shifts inferred from stable isotopes. Mar Biol 161:2243–2255. https://doi.org/10.1007/s00227-014-2502-y
Morrison KW, Battley PF, Sagar PM, Thompson DR (2015) Population dynamics of Eastern Rockhopper Penguins on Campbell Island in relation to sea surface temperature 1942–2012: current warming hiatus pauses a long-term decline. Polar Biol 38:163–177
Nesis K (1987) Cephalopods of the world. T. F. H., Neptune City
Ngoile MAK, Horrill CJ (1993) Coastal ecosystems, productivity and ecosystem protection: coastal ecosystem management. Ambio 22:461–467
O’Shea S (1999) The marine fauna of New Zealand: Octopoda (Mollusca: Cephalopoda). NIWA Biodiversity Memoir 112, Wellington
Pecl GT, Jackson GD (2008) The potential impacts of climate change on inshore squid: biology, ecology and fisheries. Rev Fish Biol Fish 18:373–385
Phillips KL, Nichols PD, Jackson GD (2003) Dietary variation of the squid Moroteuthis ingens at four sites in the Southern Ocean: stomach contents, lipid and fatty acid profiles. J Mar Biol Assoc UK 83:523–534
Piatkowski U, Pütz K, Heinemann H (2001) Cephalopod prey of king penguins (Aptenodytes patagonicus) breeding at Volunteer Beach, Falkland Islands, during austral winter 1996. Fish Res 52:79–90
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Pütz K, Rey AR, Schiavini A, Clausen AP, Lüthi BH (2006) Winter migration of rockhopper penguins (Eudyptes chrysocome) breeding in the Southwest Atlantic: is utilisation of different foraging areas reflected in opposing population trends? Polar Biol 29:735–744
Ratcliffe N et al (2014) Love thy neighbour or opposites attract? Patterns of spatial segregation and association among crested penguin populations during winter. J Biogeogr 41:1183–1192
Roberts J, Lalas C (2015) Diet of New Zealand sea lions (Phocarctos hookeri) at their southern breeding limits. Polar Biol 38:1483–1491
Rodhouse PG (1997) Precautionary measures for a new Martialia hyadesi (Cephalopoda, Ommastrephidae) fishery in the Scotia Sea: an ecological approach. CCAMLR Sci 4:125–140
Rodhouse PG (2013) Role of squid in the Southern Ocean pelagic ecosystem and the possible consequences of climate change. Deep-Sea Res II 95:129–138
Rodhouse PG, Yeatman J (1990) Redescription of Martialia hyadesi Rochbrune and Mabille, 1889 (Mollusca: Cephalopoda) from the Southern Ocean. Bull British Mus Nat Hist 56:135–143
Rodhouse PG, Arnbom TR, Fedak MA, Yeatman J, Murray AWA (1992) Cephalopod prey of the southern elephant seal, Mirounga leonina L. Can J Zool 70:1007–1015
Rodhouse PG, Robinson K, Gajadatsy SB, Daly HI, Ashmore MJS (1994) Growth, age structure and environmental history in the cephalopod Martialia hyadesi (Teuthoidea: Ommastrephidae) at the Antarctic Polar Frontal Zone and on the Patagonian Shelf edge. Antarct Sci 6:259–267
Rodhouse PG, Griffiths H, Xavier JC (2014) Southern Ocean squid. In: De Broyer C et al (eds) The CAML/SCAR-MarBIN biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 284–289
Roper C, Sweeney M, Clarke M (1985) Cephalopods. In: Fisher W, JC H (eds) FAO species identification sheets for fishery purposes: Southern Ocean (Fishing areas 48,58 and 88) (CCAMLR Convention Area), vol 1. FAO, Rome
Rosas-Luis R, Navarro J, Sánchez P, Del Río JL (2016) Assessing the trophic ecology of three sympatric squid in the marine ecosystem off the Patagonian Shelf by combining stomach content and stable isotopic analyses. Mar Biol Res 12:402–411
Sagar PM, Murdoch R, Sagar MW, Thompson DR (2005) Rockhopper penguin (Eudyptes chrysocome filholi) foraging at Antipodes Islands. Notornis 52:75–80
Scott D, Scotfield P, Hunter C, Fletcher D (2008) Decline of sooty shearwaters, Puffinus griseus, on The Snares, New Zealand. Pap Proc R Soc Tas 142:185–196
Seco J, Roberts J, Ceia F, Baeta A, Ramos J, Paiva V, Xavier J (2016) Distribution, habitat and trophic ecology of Antarctic squid Kondakovia longimana and Moroteuthis knipovitchi: inferences from predators and stable isotopes. Polar Biol 39:167–175
Stowasser G, Atkinson A, McGill RAR, Phillips RA, Collins MA, Pond DW (2012) Food web dynamics in the Scotia Sea in summer: a stable isotope study. Deep-Sea Res II 59–60:208–221
Tremblay Y, Cherel Y (2000) Benthic and pelagic dives: a new foraging behaviour in rockhopper penguins. Mar Ecol Prog Ser 204:257–267
Tremblay Y, Cherel Y (2003) Geographic variation in the foraging behaviour, diet and chick growth of rockhopper penguins. Mar Ecol Prog Ser 251:279–297. https://doi.org/10.3354/meps251279
Tuck GN et al (2011) An assessment of seabird–fishery interactions in the Atlantic Ocean. ICES J Mar Sci 68:1628–1637
West JA, Imber M (1986) Some foods of Buller’s mollymawk Diomedea bulleri. NZ J Zool 13:169–174
Williams TD (1995) The penguins: Spheniscidae. Oxford University Press, Oxford
Williams AJ, Laycock PA (1981) Euphausiids in the diet of some sub-Antarctic Eudyptes penguins. S Afr J Antarct Res 10(11):27–28
Xavier JC, Cherel Y (2009) Cephalopod beak guide for the Southern Ocean. British Antarctic Survey, Cambridge
Xavier JC, Rodhouse PG, Trathan PN, Wood AG (1999) A geographical information system (GIS) atlas of cephalopod distribution in the Southern Ocean. Antarct Sci 11:61–62. https://doi.org/10.1017/S0954102099000097
Xavier JC, Rodhouse PG, Purves MG, Daw TM, Arata J, Pilling GM (2002) Distribution of cephalopods recorded in the diet of Patagonian toothfish (Dissostichus eleginoides) around South Georgia. Polar Biol 25:323–330. https://doi.org/10.1007/s00300-001-0343-x
Xavier JC, Croxall JP, Trathan PN, Rodhouse PG (2003) Inter-annual variation in the cephalopod component of the diet of wandering albatrosses Diomedea exulans breeding at Bird Island, South Georgia. Mar Biol 142:611–622. https://doi.org/10.1007/s00227-002-0962-y
Xavier JC, Walker K, Elliot G, Cherel Y, Thompson D (2014) Cephalopod fauna of South Pacific waters: new information from breeding New Zealand wandering albatrosses. Mar Ecol Prog Ser 513:131–142. https://doi.org/10.3354/meps10957
Xavier JC et al (2015) Future challenges in cephalopod research. J Mar Biol Assoc UK 95:999–1015
Xavier JC et al (2016a) Future challenges in Southern Ocean ecology research. Front Mar Sci 3:94
Xavier JC et al (2016b) The significance of cephalopod beaks in marine ecology studies: can we use beaks for DNA analyses and mercury contamination assessment? Mar Pollut Bull 103:220–226
Xavier JC, Raymond B, Jones DC, Griffiths H (2016c) Biogeography of cephalopods in the Southern Ocean using habitat suitability prediction models. Ecosystems 19:220–247. https://doi.org/10.1007/s10021-015-9926-1
Acknowledgements
We thank Tiago Jesus for his attempt to obtain DNA from the samples. Bruno Boto for producing the photos of the beaks. Isabel Mendes of the Centre for Marine and Environmental Research of the University of Algarve (CIMA) for the usage of photographic equipment to take photos of the beaks. This work is an international effort under the Scientific Committee on Antarctic Research (SCAR) associated programs, expert and action groups, namely SCAR AnT-ERA, SCAR EGBAMM and ICED. JX was supported by the Investigator FCT program (IF/00616/2013) and PROPOLAR, and FRC by a post-doc grant (SFRH/BPD/95372/2013) by the Foundation for Science and Technology (Portugal) and the European Social Fund (POPH, EU). This study benefited from the strategic program of MARE, financed by FCT (MARE- UID/MAR/04292/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xavier, J.C., Cherel, Y., Ceia, F.R. et al. Eastern rockhopper penguins Eudyptes filholi as biological samplers of juvenile and sub-adult cephalopods around Campbell Island, New Zealand. Polar Biol 41, 1937–1949 (2018). https://doi.org/10.1007/s00300-018-2333-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2333-2