Abstract
In the present paper, we compare how the kelp gull, Larus dominicanus, utilizes various nest building materials, particularly vascular plants, bryophytes, lichens and other components, in the Fildes Peninsula area (King George Island) and on the Argentine Islands area. In both areas, nest material primarily consisted of the Antarctic hairgrass (Deschampsia antarctica), bryophytes, lichens, feathers, limpets, and algae. Our study reveals area-specific differences in the utilization of plants for nest building related to local conditions during the nesting season. In the Fildes area, vegetation emerges from under the winter snow cover earlier in the spring, giving the gulls greater choice locally, meaning that the gulls need not resort to long distance material transfer. Here, mosses and lichens dominate in the nest material, likely collected from the nearby vegetation formations. The Antarctic hairgrass in these conditions is mostly found in nests located directly within hairgrass formations. However, on the more southern Argentine Islands, kelp gulls routinely use D. antarctica and some mosses, transferring them from coastal hill tops where snow generally disappears earlier. Here, the gulls appear to be selective still, as they rarely use some mosses, such as Polytrichum strictum, that are abundant near the nesting locations. In the Argentine Islands area, we documented long-range transfer of the Antarctic hairgrass and some other vegetation materials from places of abundance to bare rocks of low islands lacking developed vegetation. This demonstrates the potential of the gulls to serve as dispersal and gene pool exchange agents for the local terrestrial biota in the maritime Antarctic, especially between highly isolated populations from small islands and ice-free areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica’s harsh environmental conditions pose substantial barriers to the dispersal of its resident organisms, especially plants. Antarctic vegetation mostly consists of bryophytes and lichens, the only two vascular plant species being the Antarctic hairgrass, Deschampsia antarctica E. Desv. and the Antarctic pearlwort, Colobanthus quitensis (Kunth) Bartl. (Parnikoza et al. 2007). However, the factors that contribute to the dispersal of these two vascular plants in the maritime Antarctic are poorly understood (Parnikoza et al. 2007, 2015c; Ozheredova et al. 2015). Since the last glacial maximum and the subsequent period of deglaciation, the landscapes of the Antarctic Peninsula have changed dramatically. Relatively low-lying islands close to the Antarctic Peninsula’s coasts are likely to have been ice-covered at glacial maxima, but they have been exposed and redeveloped vegetation cover in the Holocene (Govoruha 1997). Along with such factors as wind (Hołdyński et al. 2003), birds have been suggested as contributors to this spread, potentially transporting adhering seeds or other vascular plant propagules (Hughes et al. 2006; Peklo 2007). Other vegetation components, such as bryophytes and lichens, are also used by both kelp gulls and skuas, Catharacta sp. Brisson, 1760, in their nesting materials depending on the plants’ local abundance (Quintana et al. 2001; de Albuquerque et al. 2012). Based on published reports of plant species utilization by Antarctic birds during nest construction, we have recently proposed three regionally resident candidate bird species that have the potential to transfer vascular plant propagules: the kelp gull, Larus dominicanus Lichtenstein, 1823, the south polar skua, Catharacta maccormicki Saunders, 1893, and the brown skua, Catharacta lonnbergi Mathews, 1912 (Parnikoza et al. 2012). The kelp gull and the south polar skua are the most common of these species in the maritime Antarctic (Peklo 2007).
In a previous study on D. antarctica and other vegetation components dropped by gulls in flight, and the material composition of their nests, conducted at one location and within one season, we provided evidence that the kelp gull may be involved in the dispersal of viable propagules of the Antarctic hairgrass in the Argentine Islands region (Parnikoza et al. 2012, 2014). This study, however, did not address whether the observed behavior was regularly used and more widespread. A further caveat is that then Argentine Islands represent only a tiny part of the central region of the maritime Antarctic. At much larger scale, regional differences in the composition of nest material have been reported both for skua and gull nests, whereby hairgrass appears to be a preferred component by maritime Antarctic skuas and gulls, while South African and Patagonian birds do not show any preferences (Burger and Gochfield 1981; Quintana et al. 2001; Yorio and Borboroglu 2002; Parnikoza et al. 2012). Greater overall vegetation abundance at more northern locations might decrease the dependence of these birds on specific nest building materials.
With this background, a larger survey effort was warranted, spanning several seasons (for confirming regularity) and different regions within the maritime Antarctic. For this extended purpose, we chose two locations separated from each other by 400 km: the Fildes Peninsula (King George Island, South Shetland Islands) and the Argentine Islands. Both locations have broadly comparable hairgrass vegetation cover in areas where vegetation is well developed, although the overall area available for vegetation development is much larger on the Fildes Peninsula. The selected regions are characterized by marked differences in climate, particularly in the earlier timing of snow melt and thus earlier vegetation activity on the Fildes Peninsula. In these two areas, we set out to document and compare the details of the use of different materials in the nests over several seasons.
Materials and methods
Study regions
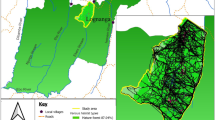
Field survey and sampling was conducted in two regions of the maritime Antarctic: Fildes Peninsula, King George Island, South Shetland Islands, during the three consecutive summer seasons of 2013–2016, and the Argentine Islands during seven seasons between 2009 and 2016 (Fig. 1a). The Fildes Peninsula (62°12′S, 58°58′W, Fig. 1b) is the approximately 7.5 km long south-west extremity of King George Island (the largest island of the South Shetland Islands). The terrain varies substantially, from mountains to broad valleys located on marine terraces. The vegetation-rich Ardley Island is close by (henceforth, reference to ‘the Fildes area’ includes Ardley Island). The South Shetland Islands have a maritime climate with frequent and rapid weather changes, mild temperatures (daily mean temperature in the range − 6.5 to + 1.5 °C) and high precipitation levels (annual average of 687.4 mm for 1969–2005), as well as strong and predominantly westerly winds (Barsch et al. 1985; bas.ac.uk/icd/gjma/bellingshausen.temps.html). The active season for vegetation in the Fildes area according to our observations starts in December and ends in April, connected with the timing of snow recession and mean monthly temperatures above 0 °C for the 4 month period (Lindsay 1971). Vascular plant populations are generally small (up to 100 plants). Larger populations here are only known from Ardley Island, Green Point and Nebles Point (Peter et al. 2008).
General map of localization of study areas (a), map of Deschampsia antarctica populations and studied kelp gull nest locations in Fildes area, King George Island, South Shetland Islands (b). Map of Deschampsia antarctica and kelp gull nest localities on Galindez Island area, Argentine Islands (c). Deschampsia antarctica populations indicated for Galindez Island only. Coverage estimated using data from the 2016 season. The number near each nest location indicates the number of years the nest was active at this location in the study period
The Argentine Islands archipelago, totaling 20 km2 in area, consists of over 40 relatively small and low-lying islands that form four groups separated by shallow (< 50 m) channels (Gozhyk et al. 2002). The largest islands are representatives from the first group—Skua and Galindez Islands (where the Ukrainian Antarctic Akademik Vernadsky station—previously the United Kingdom’s Faraday station—is located; 65° 14.751′S, 64° 15.453′W, Fig. 1c), Uruguay and Irizar Islands, Petermann, Hovgaard, Pleneau and Booth Islands (the Wilhelm Archipelago) are located in the northern part of the Argentine Islands, separated by the 4 km wide French Passage. The Argentine Islands are separated from the mainland of the Antarctic Peninsula (which hosts biological oases, such as Rasmussen oasis, Cape Tuxen, and the small Moot and Rasmussen Islands which have been exposed from retreating glaciers), by the Penola Strait, which is 2–6 km. The Yalour Islands are located in the Penola Strait, 4 km from Galindez Island, and near 9 km south-east of the Argentine Islands are the Berthelot Islands. Henceforth, reference to ‘the Argentine Islands area’ includes all these locations.
The terrain of the inner, larger islands, such as Galindez Island, consists of rocky ridges separated by depressions in which snow banks or patches usually persist throughout the entire summer season. Erosion has created crevices and gaps harboring vegetation, where sedimentation and primary soil formation occurs (Parnikoza et al. 2016). Generally, air temperature only rises above freezing in the short summer season from December to April (Martazinova et al. 2010). An absolute minimum temperature of − 43.3 °C was registered in August 1958, and maximum of + 11.7 °C in February 1960 (Smith and Corner 1973). During the period 1996–2016 an extreme minimum temperature of − 28.6° C and maximum of + 8.2 °C, and a mean summer temperature of + 0.7 °C were experienced. Average annual precipitation is 450 mm water equivalent (Martazinova et al. 2010). Snow melting times (usually late December to early January) and snow-free area can vary widely depending on the conditions in a given year. Unlike on Fildes, many low areas are not reliably released from snow cover, so vegetation is absent. Plant communities are concentrated in elevated areas, on slopes or along the north-facing shorelines of the islands and neighboring Antarctic Peninsula. Galindez, like other islands in the archipelago, harbors fragmented plant communities consisting primarily of bryophytes, lichens, and the two species of vascular plants (Smith and Corner 1973; Fowbert and Smith 1994; Govoruha 1997; Parnikoza et al. 2009b). Relatively large populations of D. antarctica occur within the archipelago, with a few consisting of more than 90 individuals present on Galindez Island (Parnikoza et al. 2015a) (Fig. 1c). However in some coastal oases, and on the slopes of the Berthelot Island we recorded thousands of individuals of D. antarctica in 2014–2016.
Sampling techniques
The nests of the kelp gull in both studied regions are located on exposed coastal rocks, near the shallow littoral zones where the gulls feed (Peter et al. 2008; Parnikoza et al. 2014). Nests were inspected late in the nesting season, carefully within a short time to reduce bird disturbance. At the time of sample collection, GPS coordinates and photographs of each nest were taken. In the field, data nests were recorded on the percentage of each fraction of building material in the nest composition, analyzed for the proportion of the general material fractions in the nest, i.e. percent of D. antarctica, bryophytes, lichens, feathers, limpet shells, and algae. On some occasions bones were noted within nests, which we interpreted as food residues and did not include as nesting material. A sample of nest material of approximately 10 × 10 cm was collected from all or majority of nests. In this sample we also quantified presence of each plant and lichen species as the percentage of nests that contained a particular species. Moss and lichen species identification from sampled material was based on standard taxonomic guides (Øvstedal and Smith 2001; Ochyra et al. 2008).
During the 2013/2014 and 2015/2016 seasons we sampled all accessible active kelp gull nests in the Fildes area (14 and 6 nests, respectively), while in 2014/2015 approximately 50% were sampled (7 nests). All of these nests were analyzed for the proportion of the general material fractions in the nest. All the nests examined in the 2013/2014 and 2014/2015 seasons were included in the sample analysis of presence of each vegetation component. The fraction of nests examined on the Argentine Islands varied between years. The breeding population on Galindez Island varies, but usually consists of up to 10 pairs. The feeding group, however, is 20–80 birds, again varying between seasons. A total of 22 nesting sites that are accessible, depending on the conditions of the season, are present on Galindez Island (Fig. 1c).
In the Argentine Islands archipelago, we also studied nests on Winter, Skua, Grotto, Corner, Barchans, Shelter, Three Little Pigs, and Indicator Islands, as well as Wild Rock (65°14.969′S, 64°16.349′W) and the Buttons. In the 2015/2016 season, the overall nesting population of the Argentine Islands was estimated to be 110 pairs. In some seasons, additional nests from more distant locations were also sampled, including the Yalour Islands, Rasmussen Island, Cape Tuxen, Petermann, Hovgaard and Moot Islands, and the Berthelot Archipelago. In the 2009/2010 season, out of the total 22 nests, 15 nests were analyzed for the proportion of the general material fractions in the nest, i.e. percent of bryophytes, hairgrass or other. All the 22 nests were also analyzed for the presence of each vegetation component (15/22). In following years these numbers were: 2010/2011—24/0, 2011/2012—54/45, 2012/2013—4/0, 2013/2014—73/73, 2014/2015—62/0, 2015/2016—75/0. As shown in Fig. 1c, and using Galindez Island as an example, some nests were located at the same point over several gull breeding seasons. However, a substantial portion of nests, both on Galindez Island and at other locations, were only occupied in a single year.
Visual observations of kelp gull behavior in the nesting period were also carried out from an observation point on the slipway at Vernadsky station. During this period, all instances of plant material transportation by kelp gulls were recorded using binoculars every day from 0900 to 1000 over 30 days in November 2015. Instances of rooted hairgrass tufts or tillers observed in nests were also recorded.
Statistical analyses
Data on nest material composition were first analyzed for normality by the D’Agostino–Pearson method (all samples were negative). Then, comparisons of material proportions were made using the non-parametric Kruskal–Wallis ANOVA with post hoc pairwise comparisons made by Dunn’s method. All analyses were carried out using the GraphPad software (GraphPad Software, Inc., California).
Results
Figures 2 and 3 illustrate the overall proportions of the different nest materials present in kelp gull nests on the Fildes Peninsula and the Argentine Islands, respectively. In both regions, the examined kelp gull nests were constructed primarily from plant material. In the Fildes area, bryophytes and lichens dominated, and the proportion of hairgrass was relatively small and did not differ significantly from other materials, such as limpets and feathers. During the 2013/2014 (Kruskal–Wallis H = 25.96, p < 0.0001) and 2014/2015 (Kruskal–Wallis H = 20.63, p = 0.001) seasons, the material proportions were fairly evenly distributed, while in 2015/16 the share of bryophytes increased considerably (Kruskal–Wallis H = 13.23, p = 0.0213). For pairwise Dunn’s Multiple Comparison Test values for pairwise comparison for all seasons of Fildes area, see Online Resource, Table 1. This annual variability in nest composition might be related to the use of different nesting spots by the same gull pairs within the oasis, or differences in vegetation material availability due to seasonal variability in snow cover.
In the Argentine Islands area, bryophytes again dominated the nest fraction, but with a large proportion of hairgrass, which constituted the second most important material, with both being used in considerably higher amounts than any other materials (Kruskal–Wallis H = 981.9, p < 0.0001). For pairwise Dunn’s Multiple Comparison Test values for pairwise comparison for all seasons of Argentine Islands area, see Online Resource, Table 2.
Feathers, both those of kelp gulls and other species (mainly penguins), as well as limpet shells consistently represented minority nest material fractions in both locations. Algae (both marine and terrestrial) were relatively abundant in nests on Fildes, while being a negligible component in the Argentine Islands area.
The presence of D. antarctica and other vegetation components in nests on Fildes is illustrated in Fig. 4a and on the Argentine Islands in Table 1 and Fig. 4b. The comparison between presence of different materials between the larger Galindez Island and the relatively small Indicator Island is represented on Fig. 5. In total, 20 taxa of vegetation components were collected in nests on Fildes and 25 on the Argentine Islands.
The presence of Deschampsia antarctica and other vegetation components (% of samples in which the material was present): a in the general dataset for three seasons (data for all mosses except Sanionia uncinata are only from seasons 2012/13 and 2014/15), Fildes area. In the season 2014/15 only nests that did not have D. antarctica were studied. 1—Deschampsia antarctica, 2—Sanionia georgicouncinata, 3—Sanionia uncinata, 4—Bryum pseudotriquetrum, 5—Syntrichia magellanica, 6—Syntrichia filaris, 7—Hennediella antarctica, 8—Syntrichia saxicola, 9—Brachythecium austrosalebrosum, 10—Schistidium antarctici, 11—Hennediella heimii, 12—Polytrichastrum alpinum, 13—Chorisodontium aciphyllum, 14—Pohlia cruda, 15—Usnea antarctica, 16—Usnea aurantiacoatra, 17—Ramalina terebrata, 18—Sphaerophorus globosus, 19—Prasiola crispa, 20—Marine algae; b in the general dataset for three seasons, Argentine Islands area: 1—Deschampsia antarctica, 2—Sanionia georgicouncinata, 3—Polytrichum strictum, 4—Bryum pseudotriquetrum, 5—Sanionia uncinata, 6—Syntrichia magellanica, 7—Pohlia nutans, 8—Polytrichum piliferum, 9—Brachythecium austrosalebrosum, 10—Ceratodon purpureus, 11—Polytrichum juniperinum, 12—Warnstorfia fontinaliopsis, 13—Polytrichastrum alpinum, 14—Bryum pallescens, 15—Brachythecium austroglareosum, 16—Bartramia patens, 17—Hypnum revolutum, 18—Pohlia cruda, 19—Bryum archangelicum, 20—Andreaea depressinervis, 21—Chorisodontium aciphyllum, 22—Usnea antarctica, 23—Usnea aurantiacoatra, 24—Prasiola crispa, 25—Marine algae
a Presence of Deschampsia antarctica and other vegetation components (% of samples in which the material was present) in the general dataset for three seasons, Galindez Island, Argentine Islands area: 1—Deschampsia antarctica, 2—Usnea antarctica, 3—Sanionia georgicouncinata, 4—Polytrichum strictum, 5—Bryum pseudotriquetrum, 6—Sanionia uncinata, 7—Syntrichia magellanica, 8—Pohlia nutans, 9—Brachythecium austrosalebrosum, 10—Polytrichum juniperinum, 11—Warnstorfia fontinaliopsis, 12—Polytrichastrum alpinum, 13—Brachythecium austroglareosum, 14—Bartramia patens, 15—Pohlia cruda, 16—Chorisodontium aciphyllum, 17—Prasiola crispa, b Presence of Deschampsia antarctica and other vegetation components (% of samples in which the material was present) in the general dataset for three seasons, Indicator Island, Argentine Islands area: 1—Deschampsia antarctica, 2—Usnea antarctica, 3—Sanionia georgicouncinata, 4—Bryum pseudotriquetrum, 5—Sanionia uncinata, 6—Syntrichia magellanica, 7—Pohlia nutans, 8—Polytrichum piliferum, 9—Brachythecium austrosalebrosum, 10—Ceratodon purpureus, 11—Warnstorfia fontinaliopsis, 12—Polytrichastrum alpinum, 14—Bryum archangelicum, 15—Prasiola crispa
On Fildes in the 2013/2014 season, only 50% (7/14) of the studied nests contained hairgrass, and those where hairgrass was absent also did not include it in 2014/2015. In 2015/2016, the proportion of hairgrass-containing nests was 33% (2/6). The grass fraction in the nest material ranged from 6 to 17%. On the Argentine Islands, hairgrass was found in most nests (Table 1), ranging from 7–42% in proportion. Part of the material collected on Fildes included living and rooted hairgrass plants. These were found in 4/7 nests that contained D. antarctica in the 2013/2014 season, and in 1/2 hairgrass-containing nests identified in 2015/2016. A substantial proportion of hairgrass plants in these nests had seeds (50–100%), which possibly also indicates facilitation of sexual reproduction.
Most of the inspected nests from the Argentine Islands area contained green tillers (which have the potential to root; mean percentage varying from 49 to 100% in different seasons). Additionally a high percentage of plants with seeds (77–95% in different seasons) were observed here. Established hairgrass cushions (on Western Corner in 2016) and seedlings or tillers (e.g., on Grotto Island in 2012, Island Larus (65°14.668′S, 64°14.656′W), Eastern island of Three Little Pigs in 2016) were observed near some nests.
The second Antarctic vascular plant, the pearlwort Colobanthus quitensis, was absent from the Fildes material (it is absent in flora of Fildes area), and was only once recorded in the Argentine Island nest materials (in the season 2015/2016), in a kelp gull nest on Tooth Rock near Finger Point, Skua Island (65°15.267′S, 64°16.485′W), (Fig. 6a). We also registered evidences of C. quitensis collecting by some birds on Finger Point Fig. 6b–c. In the same season on the north coast of Black Island (65°14.668′S, 64°14.656′W) in the zone of kelp gull activity (possibly at nesting place), one mature and three young specimens of C. quitensis were found (Fig. 6d). These facts suggesting that transfer of this plant by birds is possible, but rare.
The Antarctic pearlwort Colobanthus quitensis from nests on the Argentine Islands: a—a pearlwort sample from a kelp gull nest (season 2015/2016), b and c—traces of C. quitensis use by birds at Finger Point (Skua Island); photos from 2016: d—a cushion of pearlwort found on Black Island within the gulls’ activity area
The bryophyte material present in our Fildes collections indicates that the genus Sanionia, primarily S. uncinata (Hedw.) Loeske, was most used for nest building by kelp gulls. Its mean contribution in the bryophyte fraction varied from 88 to 98% in different seasons. A closely-related species, S. georgicouncinata (Müll.Hal.) Ochyra & Hedenäs, occurred in some nests, but much less frequently. However, on the Argentine Islands, S. georgicouncinata was most frequently used by kelp gulls (77–95% of the bryophyte fraction.).
Other moss species represented in nests in at least in one season at > 10% of the bryophyte fraction. Bryum pseudotriquetrum (Hedw.) P.Gaertn., B.Mey. & Scherb. (both regions), Syntrichia magellanica (Mont.) R.H. Zander (both regions), S. filaris (Müll.Hall.) R.H.Zander and S. antarctica (Hampe) R.H. Zander (Fildes area), Ceratodon purpureus (Hedw.) Brid. (Argentine Islands area), Hennediella heimii (Hedw.) R.H. Zander (Fildes area), Polytrichastrum alpinum (Hedw.) G.L. Sm. (Fildes area) Brachythecium austrosalebrosum (Müll.Hal.) Kindb. (both regions), Chorisodontium aciphyllum (Hook.f. & Wilson) Broth. (Fildes area), Polytrichum strictum Brid. (Argentine Island area), Polytrichum juniperinum Hedw. (Argentine Islands area), and Pohlia nutans (Hedw.) Lindb. (Argentine Islands area). The contribution of other moss species to the nest materials in both studied regions was negligible and irregular (Figs. 4, 5).
Lichens constitute another major material in kelp gull nests. On Fildes, lichens were the second most frequent nest material together with mosses, while on the Argentine Islands their fraction was significantly lower. In both regions, fruticose lichens of the genus Usnea dominated the lichen fraction. On Fildes, during 3 study seasons we observed both Usnea antarctica Du Rietz. (with presence of 50–86%) and Usnea aurantiacoatra (Jacq.) Bory (with presence of 33–64%). On the Argentine Islands, the genus was almost exclusively represented by Usnea antarctica (67–93%). Only 2 nests in the 2103/2014 season on the Argentine Islands area had a minor presence of U. aurantiacoatra (3%).
On Fildes, the lichen Ramalina terebrata Hook.f. & Taylor. was also often found in nests. The lichens Umbilicaria antarctica Frey & I.M. Lamb., Parmelia saxatilis (L.) Ach., Cetraria aculeata (Shreb.) Fr., Physcia sp., Cladonia sp., and Coelopogon epiphorellus (Nyl.) Brusse & Kärnefelt were found only as minor components in nests on the Argentine Islands.
The algae fraction in the nest material of both studied regions included the terrestrial foliose alga Prasiola crispa (Lightf.) Kütz. and some marine algae. Both on Fildes and the Argentine Islands, P. crispa represented a negligible fraction of the nest materials. However, it occurred in the nests on a regular basis in both regions (Fig. 4, 5).
On Fildes, significant fractions of marine algae were recorded in all study seasons, being the main algal fraction in gull nests. Marine algae were present in 28% of nests in 2013/2014 and in 100% of nests in 2014/2015 and 2015/2016 (see Fig. 4a), and were mainly represented by Desmarestia sp., Plocamium sp. and Curdiea sp. Only 14% of nest samples from the Argentine Islands from the 2009/10 season contained identifiable marine algae (see Fig. 4b).
Our direct observations confirm that the birds collect and transfer the material in the nesting period from some points with rich vegetation to the other nesting points (Fig. 7) sometimes poor in vegetation.
In the 2015/16 breeding season, we observed a preference for certain locations for nest material collection by gulls. Some preferred locations coincided with gull social aggregations, known as ‘clubs’. During the 2015/16 season, we found a number of such clubs, located on Skua Island (one of its northern cliffs), in the northern part of Indicator Island, on the northern shore of Galindez Island near Krapla Rock (65°14.827′S, 64°14.609′W), on Larus Island, in the northern and central parts of the largest of Shelter Islands and in a bay with rock islets in a strait between the Barchans Islands. The material transfer distance between these locations was 0.5–3 km. The Grotto Islands and southern part of Uruguay Island were also used by gulls for collection of plant nest material.
Discussion
The composition of nest materials found in this study is generally consistent with our previous findings in a single season study on the Argentine Islands area (Parnikoza et al. 2012) and that of Quintana et al. (2001) from Cierva Point further north on the Antarctic Peninsula. Hairgrass while widely present in nests did not dominate the nest material with, rather, a greater diversity of plant components particularly in the Argentine Islands, and variation in the proportions of these components between seasons in both regions. On the Argentine Islands, the bryophyte fraction dominated consistently (Fig. 3), while the fraction of hairgrass varied, generally being substantially lower compared to the 1992/1993 report from the Cierva Point oasis, Danco Coast (64º09′S, 60º57′W), where D. antarctica contributed on average 60.6% of dry mass (Quintana et al. 2001). This annual variability in the general nest composition might be related to the use of different nesting spots by the same gull pairs within the oasis, or differences in vegetation material availability due to seasonal variability in snow cover.
The fractional presence of hairgrass on Fildes is likely to reflect its distribution and growth in this area, where it is not the most abundant vegetation formation. The species is mostly concentrated near shores and glacier margins, whereas on rocks near gull nesting spots it occurs irregularly. Although kelp gull nests are also primarily located on rocks close to shores, usually between several and 50 m from the coastline (in one case 119 m), their locations only partially overlap with hairgrass populations. In the 2013/2014 season, as much as 57% of nests that contained hairgrass were located within Deschampsia stands, as was also the case at Cierva Point (Quintana et al. 2001). All the other nests on Fildes were located 160–600 m from hairgrass stands and did not contain the D. antarctica. The composition of nest materials at Fildes indicated that the plant fractions were collected from local surrounding areas, with no suggestion of more distant transportation.
The presence of hairgrass in most nests on the Argentine Islands can be explained in the same way as on Fildes and at Cierva Point. On the Argentine Islands, most kelp gull nests were located from several to 30 m from the coastline. Consistent with our previous observations (Parnikoza et al. 2014), most nests on Galindez Island were located close to or directly within D. antarctica stands. Nonetheless, not all nests containing substantial fractions of hairgrass (Indicator and other similar small islands, such as Three Little Pigs, Grotto, Moot, the islands of the Shelter group, and Sterna Point on Galindez Island) were located close to hairgrass populations. Some of them have very small D. antarctica populations. Others like Indicator Island have had rapidly declining D. antarctica populations over the last decade (Parnikoza et al. pers. obs.) and do not provide sufficient nest grass material for the gulls. However, the overall high presence of hairgrass in nests from these locations is consistent with active transport of the plants from more distant areas of high abundance. Overall, 17 plant, lichen and algae taxa were found in nests on Galindez Island, and 15 on Indicator Island, including species that are locally scarce such as Polytrichum strictum and Usnea antarctica (Fig. 5).
The transfer of hairgrass from more distant high abundance areas is also supported by our direct observations of the birds collecting and transferring the vegetation material in nesting period (Fig. 7) but additionally by our previous analysis of nest material dropped by gulls on the Argentine Islands during transportation (Parnikoza et al. 2012).
Our data give evidence for the successful rooting and establishment of transferred grass material in or near to gull nests, in the form of the presence of living tillers, some of which had already gone on to produce seeds. Experiments modeling transfer of Antarctic vascular plants on the Argentine Islands and at Point Thomas (King George Island) have demonstrated that in favorable conditions experimentally transferred plants rooted successfully at new locations (Parnikoza et al. 2009a, 2015a).
The nesting and feeding activity of gulls in the Argentine Islands promotes the development of a specific biotope in this area, which we term “gull rocks,” characterized by a range of different vegetation components that have the potential to be transferred by gulls (Parnikoza et al. 2014, 2015a). In this study we have confirmed that similar biotopes are present on Fildes. However, these pioneer populations may only have a precarious hold in worsening climatic conditions and can easily disappear.
The domination of the genus Sanionia in the bryophyte fraction is directly linked to the moss carpet sub-formation being primarily formed by Sanionia species. This formation is one the most common types of vegetation near gull nesting areas both on Fildes and the Argentine Islands (Smith and Corner 1973; Peter et al. 2008). The dominant contribution of these mosses in kelp gull nests may not be simply a matter of availability, but also stem from the moss mechanical properties, with shoots that are easily detachable from the ground. Similar extensive use of S. uncinata by the glaucous gull (Larus hyperboreus Gunnerus, 1767) has been noted in the Arctic (Parnikoza et al. 2015b).
On Fildes, we also recorded Chorisodontium aciphyllum, common species of the moss turf sub-formation. However, we did not detect the other major component of this sub-formation, Polytrichum strictum, in the nest material. While the moss turf sub-formation is common in the South Shetland Islands in general (Lindsay 1971; Furmańczyk and Ochyra 1982), P. strictum is rare on Fildes and grows mainly on Ardley Island (Ochyra 1998). Shallow and small banks of C. aciphyllum do occur as rims around S. uncinata beds (Peter et al. 2008), which may suggest that this species is collected by gulls together with Sanionia. Chorisodontium aciphyllum was only recorded as a very small component of the bryophyte fraction in gull nests on the Argentine Islands area in some seasons, which is consistent with its scarcity in the moss turf sub-formation at this location. These observations are again consistent with local sources being the primary driver of nest material composition on Fildes.
In contrast to the Fildes area, on the Argentine Islands area P. strictum is the main component of the moss turf sub-formation. Gulls do not nest directly on the moss banks, however large moss banks are widespread in the vicinity of nesting sites. The share of P. strictum in the bryophyte fraction of nests typically ranged from 1 to 20% (exceptionally reaching 30–50%). Additionally, there was substantial annual variability in its presence in kelp gull nests. This is consistent with data from Cierva Point (Quintana et al. 2001), where this moss is not dominant in gull nests, despite its abundance. The physical characteristics of this moss species, in particular its firm attachment to the ground through rhizoids, might make it less attractive to gulls.
On the Argentine Islands, the presence of some moss species in nests suggests that some materials are transferred from more distant locations. For example, in 2009/2010 on Wild Rock (65°14.969′S, 64°16.349′W) and 2011/2012 on Winter Island, Bryum pallescens Schwägr. was present in some nests, a species that has only been reported from the local mainland (Data of British Antarctic Service at Cambridge; Smith and Corner 1973). In 2009/10 in one studied nest from Winter Island, and in 29% of the studied nests on Indicator Island, we recorded Polytrichum piliferum Hedw., which has similarly only been reported from mainland oases (Data of British Antarctic Service at Cambridge; Smith and Corner 1973).
The presence of Usnea species in nests in both regions again reflects the relative abundance of these species in the local areas. The abundance of these lichens in nests indicates the exploitation of the fruticose lichen and moss cushion sub-formations (Smith and Corner 1973; described by Gremmen et al. (1994) as the Usnea complex). Such communities are more abundant at Fildes, consistent with the higher proportion of this material in nests there compared to the Argentine Islands. On Fildes, kelp gulls also collect Ramalina terebrata, another component of the fruticose lichen and moss cushion sub-formation that is also abundant on rocks.
The differences we have identified in this study in the use of nesting materials by kelp gulls in the two study regions likely reflect differences in local ecological conditions in different parts of the maritime Antarctic. On Fildes, the earlier emergence of vegetation from under the snow cover in the spring may allow the gulls to start nesting earlier and use a wider vegetation spectrum that is already available in the immediate vicinity. On the Argentine Islands, nesting starts when only small pinnacles of terrain elevations are exposed from under the winter snow cover, forcing the gulls to collect and transfer material from more distant locations.
Transfer of nest building materials by kelp gulls likely provides a passive dispersal opportunity for a number of other Antarctic biota, including limnoterrestrial invertebrates, algae, bacteria, and fungi. In this way, the gulls may play an important role in the dispersal and gene pool exchange for a wide spectrum of local biota, especially between highly isolated populations from small islands and ice-free areas. This is a topic that clearly requires further investigation.
References
Barsch D, Blümel W-D, Flügel W-A, Mäusbacher R, Stäblein G, Zick W (1985) Untersuchungen zum Periglazial auf der König-Georg-Insel Südshetlandinseln/Antarctica. Ber Polarforsch 24:75
Burger J, Gochfield M (1981) Nest site selection by kelp gulls in southern Africa. Condor 83:243–251
Data of British Antarctic Service at Cambridge. http://apex.nerc-bas.ac.uk/f?p=207:2:2462625742825624::NO::: Accessed 03 March 2017
de Albuquerque MP, de Carvalho VF, Schünemann AL, Putzke J, Gunski RJ, Seibert S, Petry MV, Pereira AB (2012) Plant composition of skuas nests at Hennequin Point, King George Island, Antarctica. Am J Plant Sci 3:688–692. https://doi.org/10.4236/ajps.2012.35082
Fowbert JA, Smith RIL (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296. https://doi.org/10.2307/1551941
Furmańczyk K, Ochyra R (1982) Plant communities of the Admiralty Bay region (King George Island, South Shetland Islands, Antarctic) I. Jasnorzewski Gardens. Pol Polar Res 3:25–39
Govoruha LS (1997) Glaciographic and glacioclimatologic characteristics of the Pacific shores of Graham Land. Bull Ukrainian Antar Center 1:60–66
Gozhyk PF, Greku RKH, Usenko VP, Vernigorov VP, Greku TR, Ostrecov GA, Gonchar AI, Klochan YA, Moc VN (2002) A map of the bottom relief of the shallow zone of the Argentine Islands Archipelago in the vicinity of the Ukrainian Antarctic station “Akademik Vernadsky”. Geolog Zhurn 1:128–131
Gremmen NJM, Huiskes AHL, Francke JW (1994) Epilithic macrolichen vegetataion of the Argentine Islands, Antarctic Peninsula. Antarct Sci 6:463–471
Hołdyński C, Loro PM, Pisarek W (2003) Wind dispersal of Deschampsia antarctica diaspores at the vicinity of the Arctowski polar station. 29th Intern. Polar symposium: the functioning of polar ecosystems as viewed against global environmental changes. Kraków, pp 57–60
Hughes K, Ott S, Bölter M, Convey P (2006) Colonisation processes. Trends in Antarctic terrestrial and limnetic ecosystems. In: Bergstrom DM, Convey P, Huiskes A (eds) Antarctica as a global indicator. Springer, Dordrecht
Lindsay DC (1971) Vegetation of the South Shetland Islands. Br Antarct Surv Bull 25:59–83
Martazinova VF, Timofeyev VE, Ivanova EK (2010) Present-day regional climate of the Antarctic Peninsula and Academic Vernadsky station. UAJ 9:231–248
Ochyra R (1998) The moss flora of King George Island, Antarctica. Polish Academy of Sciences, W. Szafer Institute of Botany, Kraków
Ochyra R, Smith RIL, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Øvstedal DO, Smith RIL (2001) Lichens of Antarctica and South Georgia: a guide to their identification. Cambridge University Press, Cambridge
Ozheredova IP, Parnikoza IYu, Poronnik OO, Kozeretska IA, Demidov SV, Kunakh VA (2015) Mechanisms of Antarctic vascular plant adaptation to abiotic environmental factors. Cytol Genet 2:139–145. https://doi.org/10.3103/S0095452715020085
Parnikoza IYu, Maidanuk DN, Kozeretska IA (2007) Are Deschampsia antarctica Desv. and Colobanthus quitensis (Kunth) Bartl. migratory relicts? Cytol Genet 4:36–40. https://doi.org/10.3103/S0095452707040068
Parnikoza I, Kozeretska O, Kozeretska I (2009a) Is a translocation of indigenous plant material successful in the Maritime Antarctic? Polarforschung 78:25–27
Parnikoza I, Convey P, Dykyy I, Trokhymets V, Milinevsky G, Inozemtseva D, Kozeretska I (2009b) Current status of the Antarctic herb tundra formation in the central Argentine Islands. Global Change Biol 15:1685–1693. https://doi.org/10.1111/j.1365-2486.2009.01906.x
Parnikoza I, Dykyy I, Ivanets V, Kozeretska I, Kunakh V, Rozhok A, Ochyra R, Convey P (2012) Use of Deschampsia antarctica for nest building by the kelp gull in the Argentine Island area (maritime Antarctica) and its possible role in plant dispersal. Polar Biol 11:1753–1758. https://doi.org/10.1007/s00300-012-1212-5
Parnikoza IY, Abakumov EV, Dykyy IV, Pilipenko DV, Shvydun PP, Kozeretska IA, Kunakh VA (2014) Influence of birds on the spatial distribution of Deschampsia antarctica (Desv.) on Galindez Island (Argentine Islands, maritime Antarctic). Russ Ornithol J 23:3095–3107
Parnikoza IYu, Abakumov EV, Dykyy IV, Pilipenko DV, Shvydun PP, Kozeretska IA, Kunakh VA (2015a) Influence of birds on the spatial distribution of Deschampsia antarctica E. Desv. on Galindez Island (Argentine Islands, maritime Antarctic). Vest Sankt-Peterburg. Univ. 1:78–97
Parnikoza I, Hadwiczak M, Barcikowski M, Stempniewicz L (2015b) Arctic and Antarctic large white-headed gull species nest materials—similarity across the globe. 26th International Congress on Polar Research: High latitudes and high mountains: driver of or driven by global change? German Society for Polar Research, Munich, pp 117–118
Parnikoza I, Miryuta N, Ozheredova I, Kozeretska I, Smykla J, Kunakh V, Convey P (2015c) Comparative analysis of Deschampsia antarctica Desv. population adaptability in the natural environment of the Admiralty Bay region (King George Island, maritime Antarctic). Polar Biol 38:1401–1411. https://doi.org/10.1007/s00300-015-1704-1
Parnikoza I, Abakumov E, Korsun S, Klymenko I, Netsyk M, Kudinova A, Kozeretska I (2016) Soils of the Argentine Islands, Antarctica: diversity and characteristics. Polarforschung 86:83–96. https://doi.org/10.2312/polarforschung.86.2.83
Peklo AM (2007) The birds of Argentine Islands and Petermann Island. Mineral Publishers, Kryvyy Rih
Peter H-U, Buesser C, Mustafa O, Pfeiffer S (2008) Risk assessment for the Fildes Peninsula and Ardley Island, and development of management plans for their designation as Specially Protected or Specially Managed Areas. Federal Environment Agency Dessau-Rosslau, May 2008 http://www.umweltbundesamt.de/publikationen/risk-assessment-for-fildes-peninsula-ardley-island. Accessed 03 March 2017
Quintana RD, Cirelli V, Benitez O (2001) Nest materials of skuas (Catharacta spp.) and kelp gulls (Larus dominicanus) at Cierva Point. Antarctic Peninsula. Notornis 48:235–241
Smith RIL, Corner RWM (1973) Vegetation of the Arthur Harbour—Argentine Islands region of the Antarctic Peninsula. Br Antarct Surv Bull 33–34:89–122
Yorio P, Borboroglu P (2002) Breeding biology of kelp gulls (Larus dominicanus) at Golfo San Jorge, Patagonia, Argentina. Emu 102:257–263. https://doi.org/10.1071/MU00077
Acknowledgements
The fieldwork was supported by the State Institution National Antarctic Scientific Center, Ministry of Education and Science of Ukraine during the the 18th and 20th Ukrainian Antarctic expeditions. We thank I. Dyyky, D. Pilipenko, V. Smagol, P. Khoetsky, V. Omelyanovich, O. Salgansky, A. Dzhulai, V. Papitashvili, Z. Yu., D. Beilman for help in expedition preparation and sample collection. This study was carried out as part of the State Priority Scientific and Technical Research Program in the Antarctic during 2011–2020 within the NASU and PAS joint 2015–2017 project “Adaptive strategies of mutual survival of organisms in extreme environments.” J. Smykla was supported by the Polish Ministry of Science and Higher Education within the program “Supporting International Mobility of Scientists” and grant no. NN305376438. P. Convey was supported by NERC core funding to the British Antarctic Survey’s ‘Biodiversity, Evolution and Adaptation’ and R. Ochyra gained financial support through the statutory fund of the W. Szafer Institute of Botany of the Polish Academy of Sciences. This paper also contributes to the Scientific Committee on Antarctic Research ‘State of the Antarctic Ecosystem’ international research program. We thank the Editor and anonymous referees for constructive comments on an earlier version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This study was not accompanied by the emergence of potential conflicts of interest and did not include Human Participants or Animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parnikoza, I., Rozhok, A., Convey, P. et al. Spread of Antarctic vegetation by the kelp gull: comparison of two maritime Antarctic regions. Polar Biol 41, 1143–1155 (2018). https://doi.org/10.1007/s00300-018-2274-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2274-9