Abstract
Sub-arctic environments are undergoing rapid changes. For instance, woody shrubs are encroaching into previously open habitats, and booming goose (Chen caerulescens and Branta canadensis) populations are creating vast areas of bare mud. Across the region, these changes are likely to diminish the amount and quality of breeding habitat for imperiled arctic- and sub-arctic-breeding shorebirds, including the Hudsonian Godwit (Limosa haemastica). We studied godwit nest site selection at two study areas—Churchill, Manitoba, Canada, and Beluga River, Alaska, USA—to identify differences in habitat preferences between the two populations and determine the degree to which each avoided woody vegetation and non-vegetated areas. We used multivariate analyses to evaluate differences in microhabitat between nest sites and random sites within and between each study area. Godwits at both areas selected nest sites characterized by higher amounts of graminoid and shrubby cover with fewer non-vegetated areas than random locations. Habitat attributes preferred by godwits are expected to become less available as the climate changes and as geese continue to degrade arctic and sub-arctic ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two major threats to polar ecosystems are climate change and large-scale human activities (Brown et al. 2001; Saalfeld et al. 2013). Climatic change can alter landscapes through a variety of processes and is likely to profoundly impact physical and ecological attributes (e.g., surface water, vegetation and insect communities) of arctic and sub-arctic habitats (Post et al. 2009; IPCC 2013). For example, higher summer temperatures, changes in precipitation regimes, and longer frost-free seasons are predicted to accelerate permafrost thawing (Schuur et al. 2007), lengthen the growing season (Myneni et al. 1997; Goetz et al. 2005; Bunn and Goetz 2006), dry wetlands (Kaplan and New 2006), and promote the northward expansion of shrubs (Sturm et al. 2001; Stow et al. 2004) and faunal and parasite communities (Parmesan and Yohe 2003; Kutz et al. 2005; Post et al. 2009). Projected climate changes thus suggest that novel communities will ultimately replace arctic and sub-arctic habitats in the future.
In addition to a changing climate, habitats in the arctic and sub-arctic are influenced by human modifications and development. Landscapes can be modified by a wide variety of human activities, including the presence of human-subsidized or facilitated species, such as Common Ravens (Corvus corax, Restani et al. 2001; Liebezeit et al. 2009) and Snow (Chen caerulescens, Abraham et al. 2005a) and Canada Geese (Branta canadensis, Cotter et al. 2013). For example, populations of both Snow and Canada Geese are increasing dramatically and have already altered significant portions of the arctic (Abraham et al. 2005a; Cotter et al. 2013). Foraging by geese (e.g., ‘grubbing’) can profoundly alter soil chemistry and vegetation structure, with overgrazed areas sometimes taking decades or longer to recover (Peterson et al. 2013). In this way, increased goose populations are likely to have lasting impacts on floristic communities and, especially, the extent of suitable habitat for ground-nesting arctic birds, such as shorebirds.
Global climate change is hypothesized to drive declining population trends for many shorebird species (Piersma and Lindström 2004; Bart et al. 2007; Andres et al. 2012), in part because it may limit available habitat for breeding (Wauchope et al. 2016). Most birds, including shorebirds, exhibit strong preferences to nest within or near habitats with specific vegetation characteristics, which are generally thought to be associated with higher nest success (Colwell and Oring 1990; Smith et al. 2007; Skrade and Dinsmore 2013). Habitats lacking nest sites with preferred attributes may even contribute to population declines (Kentie et al. 2015) or compromise population viability (Martin 1993; Newton 1998). Therefore, understanding nest site selection can help us to better interpret drivers of population trends, especially for species of conservation concern.
Habitat alterations caused by changing environments could potentially affect nest site selection, nest survival, and, ultimately, population trends in three ways: First, changing climatic conditions and faunal communities could affect the type of nest that is most favorable. For instance, with longer ice-free seasons, polar bears (Ursus maritimus) depredate the nests of colonial nesting birds more frequently (Smith et al. 2010; Iverson et al. 2014; Prop et al. 2015). Thus, there may be a stronger selective pressure to nest on inaccessible cliffs or in lower density colonies to avoid depredation by polar bears. Second, changing climatic conditions could disrupt the cues used to identify favorable nest sites. For example, in cold environments such as the arctic, microclimates that are amenable to embryonic development and reduce parental energetic costs during incubation are favored as nest sites (Tulp et al. 2012). As temperatures rise, however, warm sites may become less advantageous and potentially create ecological traps if birds continue to rely upon the same cues for nest site choice (Martin 2001; Battin 2004). Third, changing landscapes could limit or eliminate the availability of particular habitat characteristics individual species use to select nesting locations. Shrub encroachment since the 1970s has already eliminated some historic sub-arctic breeding areas for Whimbrel (Numenius phaeopus), reducing local breeding densities (Ballantyne and Nol 2015). In short, changing environments may dramatically influence nest site selection and survival.
One shorebird species that may be vulnerable to environmental change in the arctic and sub-arctic is the Hudsonian Godwit (Limosa haemastica; hereafter ‘godwit’). Godwits are an extreme long-distance migrant (Senner et al. 2014) that breed in three disjunct regions across the Nearctic–Hudson Bay, the northern Northwest Territories and northeastern Alaska, and south-central and western Alaska (Walker et al. 2011). A small population size and reliance upon threatened habitats have garnered godwits a status of high conservation concern (Senner 2010), and there is some indication from counts at stopover and nonbreeding sites that their eastern Canadian breeding population is declining (Bart et al. 2007; Andres et al. 2012). Previous research has shown that godwits breeding in at least some parts of this region have a lower hatching success rate than do godwits breeding elsewhere in the species’ range (Senner et al. 2016). Factors thought to contribute to low nest success include alteration of breeding habitat due to climate change (Tape et al. 2006), creation of nesting structures for Common Ravens, and increased foraging by migrating Snow Geese (Sammler et al. 2008) and rising numbers of breeding Canada Geese. Godwits have traditionally nested in sedge-dominated meadows at the interface of tundra and forest biomes, but the specific characteristics of their nest sites are unknown (Walker et al. 2011).
We studied the nest site selection of Hudsonian Godwits in two disjunct breeding populations, Beluga River, Alaska, USA, and Churchill, Manitoba, Canada, representing the geographic centers of the westernmost and easternmost populations. The Churchill area is experiencing dramatic habitat changes due to woody shrub encroachment and overgrazing by migrating Snow Geese and breeding Canada Geese—shrub and tree cover have increased by a rate of 0.38 and 0.21% annually over the past 35 years, respectively, while goose-related patches of bare ground have increased by 0.16% per year over that same period (Ballantyne and Nol 2015). In contrast, Beluga River has not experienced goose-related habitat degradation and has witnessed generally less climate-related change than other sub-arctic regions (Wolken et al. 2011, Swift pers. obs.). Our study aimed to first describe godwit nest sites and identify potential differences in the nest site microhabitat characteristics selected between the two breeding populations, and to then use these microhabitat characteristics to identify potential future environmental changes that may directly affect godwit population dynamics. We predicted that nesting godwits would choose to nest in areas dominated by non-woody vegetation and avoid non-vegetated areas (Walker et al. 2011), suggesting that the suitability of the current godwit breeding range is expected to decline in the future as climate change continues.
Methods
Study areas
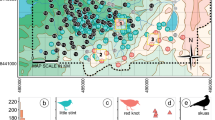
We monitored the breeding biology of godwits at two study areas representing the geographic centers of the south-central Alaska and Hudson Bay breeding populations: Beluga River, Alaska, USA (61.21°N, 151.03°W), and Churchill, Manitoba, Canada (58.93°N, 93.80°W). Research in Churchill was conducted in roughly a 15-km2 study area from 2008 to 2011, while the 8-km2 Beluga River study area was surveyed from 2009 to 2011, and again from 2014 to 2015 (Fig. 1). Both areas support large breeding populations of godwits (5.0 breeding pairs per km2 at Beluga River, and 2.3 pairs per km2 at Churchill; Senner et al. 2016). Both study areas were dominated by sedges, Carex spp., and small shrubs, mostly dwarf birch, Betula glandulosa/nana, yet they appear different—the Churchill study area was located on a large, open fen, while the Beluga River study area was situated in a black spruce, Picea mariana, muskeg bog.
Hudsonian Godwit study areas, 2008–2015. The top panel indicates the location (black boxes) of the study areas—Beluga River, Alaska and Churchill, Manitoba—within the Nearctic; the middle panel their location within the Upper Cook Inlet and Hudson Bay regions; and the bottom panel the distribution of nest (circles) and random sites (squares) within the study areas
Field methods
Nest searching and habitat characteristics
Field teams began nest searching after godwit pair formation and continued through the onset of hatch. We located nests through knowledge of former territories, systematic surveys, and observing behavioral cues of nesting pairs.
After nests had fledged or failed, we measured microhabitat characteristics at the nest site and at associated random points. We defined the microhabitat (nest site) scale as the area within a 1-m diameter circle (0.79 m2) centered on the nest. Around each nest, we placed ten 1-m diameter circular plots at randomly selected points within a 200-m radius of the nest. Two hundred meters was the shortest distance documented between two godwit nests at Churchill and thus represented the presumptive size of the territory of each pair. Random points were generated using a random point generator online at http://www.random.org or in program R (R Development Core Team 2015).
From the center of each circular plot, we measured the distance to the nearest tree (≥2 m tall) and nearest water (≥2 cm deep). For each tree, we measured the diameter at breast height (DBH), and for each water body, we measured the depth at the deepest point. We then identified each species found within the 1-m diameter circle. Following Viereck et al.’s (1992) classification, we also estimated percent cover within each plot comprised by trees (≥2 m tall), saplings (1 m < x < 2 m tall), shrubs (30 cm < x < 1 m tall), and graminoids (sedges, forbs, and herbs <30 cm tall), as well as percent cover for each individual plant species. From our estimates of the percent cover for individual species, we summarized the percentage of the circle comprised of five plant categories—tree, shrub, sedge/grass, forb, and low-mat species—rather than categorizing them by height as in the categories based on Viereck et al.’s (1992). We then summed the number of plant species present in the plot as a metric of species richness. For nests, we also recorded the percent cover of vegetation characteristics for a 10-m diameter circle around the nest; the length, width, and height of the mound on which the nest was placed; and nest cup diameter and depth.
Statistical methods
We used a multiple analysis of variance test (MANOVA) to test for significant microhabitat differences between nest and random sites in program R (R Development Core Team 2015). To avoid multicollinearity, in cases when two variables were highly correlated (Pearson’s r > 0.7), we either combined the two variables, where appropriate, or removed one of the two (Sokal and Rohlf 1995). We grouped the percentage of sedges and grasses categories, and removed tree height. When a significant difference was detected, we followed up with univariate ANOVA tests with a Bonferroni correction. In addition, we compared the characteristics of the nest cup and mound, as well as characteristics of used and random sites, between study populations using separate MANOVA tests. Data were checked for outliers using the ‘mvoutlier’ package (Filzmoser and Gschwandtner 2015), multivariate normality using the ‘mvtnorm’ package (Genz et al. 2015), and homogeneity of variances using the Fligner-Killeen test in program R.
Results
In total, we found 75 godwit nests at Churchill and 107 at Beluga River. Overall, vegetation structure differed significantly between nest sites and random points within each study area (Beluga River: Wilk’s λ = 0.7143, p < 0.0001; Churchill: Wilk’s λ = 0.699, p = 0.001). There was no autocorrelation of habitat variables within either study area (Beluga River: DW = 1.4909, p = 0.7627; Churchill: DW = 0.73469, p = 0.7643). Habitat variables were significantly different between study areas (Wilk’s λ = 0.4165, p < 0.0001) and between nest sites and random points (Wilk’s λ = 0.7563, p < 0.0001) when locations from both study areas were combined.
In general, across study areas, nest sites had higher numbers of species (Beluga River: mean = 13.25, range = 3–29; Churchill: mean = 12.49, range = 5–27) and were closer to shallow water (Beluga River: mean = 31.25 cm, range = 2–97.5 cm; Churchill: mean = 4.5 cm, range = 2–14 cm) than random points. Nests tended to have high proportions of graminoid cover (Beluga River: mean = 27.13%, range = 3–66%; Churchill: mean = 45.84%, range = 11–87%) and low percentages of tall shrubby cover (between 30 cm and 1 m tall; Beluga River: mean = 22.54%, range = 0–75%; Churchill: mean = 9.99%, range = 0–89%). Only 6.5% of located nests had tall trees providing cover, and no nests were found within 0.5 m of a tree >2 m tall. Nest characteristics were largely similar between Beluga River and Churchill (Table 1; Fig. 2). The only inter-study area differences were percentage of shrubs between 30 cm and 1 m tall (13% more in Beluga River then Churchill) and water (23% more in Churchill then in Beluga River) in the 10-m diameter circle surrounding the nest, as well as the size of the nest cup, which was 2-cm narrower and 1.5-cm deeper in Churchill.
At Beluga River, godwits selected nest sites where the closest water was shallower than random sites (Table 2). Nest sites also had 14% more shrubby cover (between 30 cm and 1 m tall), 8% less water, and 9% fewer plants less than 30 cm tall than random sites. In addition, nest sites were disproportionately comprised of graminoids (8% more) and forbs (3% more), and mud (3% more). Only one pair of godwits nested at a site for which >35% of the circular plot was bare ground comprised of mud, water, or rocks. The majority of mud in Beluga River is likely caused by shallow pockets of water that dry by July, when most measurements were taken. Finally, nest sites were more floristically diverse than random sites, with three more species, on average, found at nest sites (Table 2).
At Churchill, godwit nest sites were also closer to water than expected. Nest sites had 6% more shrubby cover (between 30 cm and 1 m tall) and 8% less mud than random points (Table 2). In addition, nest areas had 2% more forbs and low-lying mat species and nearly five more species than random points (Table 2). No godwit nested in an area with >50% cover between 30 cm and 1 m tall, and only two pairs nested where >20% of the circular plot was comprised of mud.
When data were pooled across study areas, the same patterns persisted. Godwit nests were closer to shallow water and were surrounded by greater cover between 30 cm and 1 m tall, more graminoids and forbs, and higher plant species richness than random sites. However, overall habitat differed markedly between study areas when using all sampling locations. Only four habitat variables (out of 17) did not significantly differ between study areas (Table 2).
Discussion
Hudsonian Godwits selected specific vegetative features for nesting and especially favored species-rich areas with high graminoid and moderate shrubby cover and comparatively low amounts of open mud, water, or rocks. These results are consistent with the previous descriptions of breeding habitats of godwits (Walker et al. 2011; Harwood 2014) as well as to reports of nonrandom nest site selection in many other species of arctic and sub-arctic breeding shorebirds (Rodrigues 1994; Smith et al. 2007; Ballantyne and Nol 2011). A number of these sub-arctic breeding shorebirds have already been affected by human-induced habitat changes. For instance, shrub encroachment and treeline advancement have already limited available nest sites and dramatically decreased nesting densities at more southerly breeding sites for Whimbrel (Ballantyne and Nol 2011, 2015), whereas encroachment by Snow Goose colonies has limited use of other portions of their breeding range (Sammler et al. 2008). Although godwits use limited amounts of woody vegetation, their nest site preferences, as a whole, suggest that future environmental changes may reduce habitat availability to similar degrees as projected for other arctic breeding shorebirds (Wauchope et al. 2016).
Projections of global climate change suggest that the availability of suitable nest habitats for Hudsonian godwits will decline in the future, although the interplay between the different habitat characteristics preferred by godwits and the expected responses of these habitat characteristics to future climatic changes introduces a level of uncertainty. For instance, future climate change scenarios predict the drying of arctic ponds (Yoshikawa and Hinzman 2003; Smith et al. 2005), a decline in graminoid habitats (Chapin et al. 1995; Kaplan and New 2006), increased shrub cover (Chapin et al. 1995; Sturm et al. 2005a, b; Tape et al. 2006), and treeline advancement (Caccianiga and Payette 2006; Kaplan and New 2006; Danby and Hik 2007). These predictions suggest that the godwit preferences in our study areas, such as shallow bodies of water and graminoid habitats, will become less common, which may limit the amount of suitable nesting habitat in their current range. Such a reduction in suitable nesting habitat could limit populations via potential increases in competition for nest sites, or heightened rates of nest predation if habitat changes improve search efficiency by predators or otherwise increase the risk of predation (Martin 1993). However, godwits also exhibited a preference for moderate, but not high, densities of shrub cover. Future shrub encroachment caused by climate change may, therefore, provide more suitable nest areas for godwits in the short-term if the shrubs are between 30 cm and 1 m tall. Nonetheless, godwits avoided sites dominated by taller or high densities of shrubs, indicating that the benefits of shrub encroachment may not be permanent. The effect of climate change on the availability of suitable nest sites may thus vary depending upon the successional stage of the site.
Despite several key differences in habitat between the two study areas, godwits overall chose nest sites that were relatively similar compared to random sites. In both study areas, nests were placed in species-rich sites near shallow water with high percentages of graminoids and forbs and moderate levels of shrubby vegetation. Nests at Beluga River were surrounded by more shrubs and less water than at Churchill, perhaps, reflecting the higher temperatures, lack of permafrost, and lower precipitation in south-central Alaska, especially in 2014 and 2015, when most shallow bodies of water dried midseason (RJ Swift pers. obs.). Nest cups were deeper in Churchill and wider in Beluga River, which may reflect differences in hatching success (i.e., and, therefore, chicks trampling nests) between the two populations (Senner et al. 2016).
Both populations of Hudsonian godwits avoided large non-vegetated or barren areas, including those caused by migrating Snow Geese and breeding Canada Geese in the Eastern Canadian Arctic. Driven by agricultural modifications and associated nutrient subsidies at their wintering grounds and migratory stopover areas (Boyd et al. 1982; Abraham et al. 2005a), the mid-continent population of Snow Geese has increased at an annual rate of 5–14% since the 1970s (Alisauskas et al. 2011). These human-subsidized populations of Snow Geese degrade arctic habitats through overgrazing and grubbing of vegetation, which ultimately eliminates plants and alters the soil chemistry, leading to large patches of barren ground/mud and extensive loss of graminoids and shrubs (Jefferies and Rockwell 2002; Abraham et al. 2005b). Such habitat alterations can have lasting impacts on the vegetative community long after the initial vegetation removal occurs. For example, Peterson et al. (2013) documented a 46% reduction in graminoid cover and an 84% reduction in shrub cover 35 years after a Snow Goose colony stopped breeding in their study area. This habitat alteration has been shown to lead to smaller and more isolated patches of the shrubs and graminoids upon which many shorebirds depend for nest sites (Peterson et al. 2013). Though our Churchill study area differs from the study area of Peterson et al. (2013) in that we lacked nearby colonies of breeding Snow Geese, damage from grubbing geese in migratory (Snow Geese) and breeding (Canada Geese) periods was still evident (NR Senner pers. obs.). Indeed, the low hatching success of the Churchill population of godwits, which largely avoided areas of bare mud caused by migrating Snow Goose grubbing (Senner et al. 2016), suggests that overabundant geese may already be having important demographic consequences for godwits.
In contrast, increases in woody vegetation may potentially reduce predation risk for godwits. Other studies have suggested that many shorebirds nest in exposed sites to facilitate early detection of predators, as many adult shorebirds do not use cover in the face of predation, but rather take flight (Metcalfe 1984; Burger 1987; Götmark et al. 1995). However, godwits and some other shorebird species instead use camouflage to decrease the risk of predation (Hagar 1966). Accordingly, godwits selected sites with higher percent cover of graminoids and tall shrubs than random sites, which could obstruct their detection by predators through disruptive camouflage—especially from the Northern Harrier (Circus cyaneus), the main predator of incubating adults (NR Senner pers. obs.). Camouflage provided by shrubs may, therefore, be especially important for godwits. In the near future, then, the contrasting effects of increases in woody vegetation and Snow Goose grubbing may mean that the distribution of successful godwit nests across the landscape becomes patchier and highly context dependent (Swift 2016).
Although our findings suggest that, in general, anticipated changes in climate and habitat will reduce the suitability or availability of nest sites for godwits, there are three important caveats. One, because we could not measure every possible attribute that might be selected by breeding godwits, there remains the possibility that godwits respond to habitat features or cues not included in our study. Two, a wide variety of other factors, not necessarily related to vegetation, also can influence territory and nest site selection. Indeed, other studies have demonstrated that invertebrate and/or predator abundance, conspecific attraction, site fidelity, previous experience, individual specialization, and natal habitat preferences can shape habitat selection in birds (Block and Brennan 1993; Ramsay et al. 1999; Davis and Stamps 2004). Three, as we did not study the direct effects of nest-site attributes on reproduction, survival, or site fidelity, we can only infer the implications of our findings for godwits. Future work should, therefore, address the relationship between habitat, nest placement, and survival of nests or incubating adults to better understand potential fitness consequences.
Nonetheless, our study suggests that habitat changes resulting from grubbing by overabundant geese and a changing climate are likely to reduce the suitability of current sub-arctic habitats for breeding godwits. Additional management of both Canada and Snow Geese may thus prove necessary and fruitful to the maintenance of godwit populations in the Eastern Canadian Arctic. However, the projected encroachment of woody shrubs, advancement in treeline, and drying of ponds due to climate change will not only alter the amount of suitable habitats for nesting godwits across their range, but also be more difficult to actively manage. The complicated interplay among these factors requires additional research to understand and identify management practices that promote survival, health, and reproductive success of godwits.
References
Abraham KF, Jefferies RL, Alisauskas RT (2005a) The dynamics of landscape change and snow geese in mid-continent North America. Global Change Biol 11:841–855
Abraham KF, Jefferies RL, Rockwell RF (2005b) Goose-induced changes in vegetation and land cover between 1976 and 1997 in an Arctic coastal marsh. Arct Antarct Alp Res 37:269–275
Alisauskas RT, Rockwell RF, Dufour KW, Cooch EG, Zimmerman G, Drake KL, Leafloor JO, Moser TJ, Reed ET (2011) Harvest, survival, and abundance of midcontinent lesser snow geese relative to population reduction efforts. Wildlife Monogr 179:1–42
Andres BA, Smith PA, Morrison RG, Gratto-Trevor CL, Brown SC, Friis CA (2012) Population estimates of North American shorebirds, 2012. Wader Study Group Bull 119:178–194
Ballantyne K, Nol E (2011) Nesting habitat selection and hatching success of Whimbrels near Churchill, Manitoba, Canada. Waterbirds 34:151–159
Ballantyne K, Nol E (2015) Localized habitat change near Churchill, Manitoba and the decline of nesting Whimbrels (Numenius phaeopus). Polar Biol 38:529–537
Bart J, Brown S, Harrington B, Morrison RIG (2007) Survey trends of North American shorebirds: population declines or shifting distributions? JAvian Biol 38:73–82
Battin J (2004) When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv Biol 18:1482–1491
Block WM, Brennan LA (1993) The habitat concept in ornithology: theory and applications. In: Power DM (ed) Current Ornithology, vol 11. Plenum Press, NY, pp 35–91
Boyd H, Smith GJ, Cooch FG (1982) The lesser snow goose of the eastern Canadian Arctic: their status during 1964–1979 and their management from 1982 to 1990. Can Wildl Serv Occas Paper 46:1–21
Brown S, Hickey C, Harrington B, Gill RE Jr (2001) United States shorebird conservation plan, 2nd edn, Manomet Center for Conservation Sciences, Manomet
Bunn AG, Goetz SJ (2006) Trends in satellite-observed circumpolar photosynthetic activity from 1982 to 2003: the influence of seasonality, cover type, and vegetation density. Earth Interact 10:1–19
Burger J (1987) Physical and social determinants of nest-site selection in Piping Plover in New Jersey. Condor 89:811–818
Caccianiga M, Payette S (2006) Recent advance of white spruce (Picea glauca) in the coastal tundra of the eastern shore of Hudson Bay (Québec, Canada). J Biogeogr 33:2120–2135
Chapin FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA (1995) Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Colwell MA, Oring LW (1990) Nest-site characteristics of prairie shorebirds. Can J Zoolog 68:297–302
Cotter RC, Hughes RJ, May P, Novalinga P, Johannes J, Hindman LJ, Padding PI (2013) Breeding biology of Atlantic population of Canada Geese in Nunavik, Northern Québec. Arctic 66:301–311
Danby RK, Hik DS (2007) Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. J Ecol 95:352–363
Davis JM, Stamps JA (2004) The effect of natal experience on habitat preferences. Trends Ecol Evol 19:411–416
Filzmoser P, Gschwandtner M (2015) Multivariate Outlier Detection Based on Robust Methods, package ‘mvoutlier’
Genz A, Bretz F, Miwa T, Mi X, Leisch F, Scheipl F, Bornkamp B, Maechler M, Hothorn T (2015) Multivariate Normal and t Distributions: Package ‘mvtnorm’
Goetz SJ, Bunn AG, Fiske GJ, Houghton RA (2005) Satellite-observed photosynthetic trends across boreal North America associated with climate and fire disturbance. P Natl Acad Sci USA 102:13521–13525
Götmark F, Blomqvist D, Johansson OC, Bergkvist J (1995) Nest site selection: a trade-off between concealment and view of the surroundings? J Avian Biol 26:305–312
Hagar JA (1966) Nesting of Hudsonian Godwit at Churchill, Manitoba. Living Bird 5:5–43
Harwood CM (2014) Intraseason re-use of Numenius nest by Limosa. Wader Study Group Bull 121:199–200
IPCC Climate Change (2013) The physical science basis. Available at: http://www.ipcc.ch/ipccreports/ar4-wg1.htm
Iverson SA, Gilchrist HG, Smith PA, Gaston AJ, Forbes MR (2014) Longer ice-free seasons increase the risk of nest depredation by polar bears for colonial breeding birds in the Canadian Arctic. P Roy Soc Lond B Bio 281:20133128
Jefferies RL, Rockwell RF (2002) Foraging geese, vegetation loss and soil degradation in an Arctic salt marsh. Appl Veg Sci 5:7–16
Kaplan JO, New M (2006) Arctic climate change with a 2 °C global warming: timing, climate patterns and vegetation change. Clim Change 79:213–241
Kentie R, Both C, Hooijmeijer JC, Piersma T (2015) Management of modern agricultural landscapes increases nest predation rates in Black-tailed Godwits Limosa limosa. Ibis 157:614–625
Kutz SJ, Hoberg EP, Polley L, Jenkins EJ (2005) Global warming is changing the dynamics of Arctic host–parasite systems. P Roy Soc Lond B Bio 272:2571–2576
Liebezeit JR, Kendall SJ, Brown S, Johnson CB, Martin P, McDonald TL, Payer DC, Rea CL, Streever B, Wildman AM, Zack S (2009) Influence of human development and predators on nest survival of tundra birds, Arctic Coastal Plain, Alaska. Ecol Appl 19:1628–1644
Martin TE (1993) Nest predation and nest sites: new perspectives on old patterns. Bioscience 43:523–532
Martin TE (2001) Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology 82:175–188
Metcalfe NB (1984) The effects of habitat on the vigilance of shorebirds: is visibility important? Anim Behav 32:981–985
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702
Newton I (1998) Population limitation in birds. Academic Press, San Diego
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Peterson SL, Rockwell RF, Witte CR, Koons DN (2013) The legacy of destructive Snow Goose foraging on supratidal marsh habitat in the Hudson Bay lowlands. Arct Antarct Alp Res 45:575–583
Piersma T, Lindström à (2004) Migrating shorebirds as integrative sentinels of global environmental change. Ibis 146:61–69
Post E, Forchhammer MC, Bret-Harte MS, Callaghan TV, Christensen TR, Elberling B, Fox AD, Gilg O, Hik DS, Høye TT, Ims RA, Jeppesen E, Klein DR, Madsen J, McGuire AD, Rysgaard S, Schindler DE, Stirling I, Tamstorf MP, Tyler NJC, van der Wal R, Welker J, Wookey PA, Schmidt NM, Aastrup P (2009) Ecological dynamics across the Arctic associated with recent climate change. Science 325:1355–1358
Prop J, Aars J, Bårdsen BJ, Hanssen SA, Bech C, Bourgeon S, de Fouw J, Gabrielsen GW, Lang J, Noreen E, Oudman T, Sittler B, Stempniewicz L, Tombre I, Wolters E, Moe B (2015) Climate change and the increasing impact of polar bears on bird populations. Front Ecol Evol 3:33
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramsay RJ, Otter K, Ratcliffe LM (1999) Nest-site selection by Black-capped Chickadees: settlement based on conspecific attraction. Auk 116:604–617
Restani M, Marzluff JM, Yates RE (2001) Effects of anthropogenic food sources on movements, survivorship, and sociality of common ravens in the arctic. Condor 103:399–404
Rodrigues R (1994) Microsite variables influencing nest-site selection by tundra birds. Ecol Appl 4:110–116
Saalfeld ST, Lanctot RB, Brown SC, Saalfeld DT, Johnson JA, Andres BA, Bart JR (2013) Predicting breeding shorebird distributions on the Arctic Coastal Plain of Alaska. Ecosphere 4:16
Sammler JE, Andersen DE, Skagen SK (2008) Population trends of tundra-nesting birds at Cape Churchill, Manitoba, in relation to increasing goose populations. Condor 110:325–334
Schuur EA, Crummer KG, Vogel JG, Mack MC (2007) Plant species composition and productivity following permafrost thaw and thermokarst in Alaskan tundra. Ecosystems 10:280–292
Senner NR (2010) Conservation Plan for the Hudsonian Godwit. Version 1.16. Manomet Center for Conservation Science, Manomet
Senner NR, Hochachka WM, Fox JW, Afanasyev V (2014) An exception to the rule: carry-over effects do not accumulate in a long-distance migratory bird. PLoS One 9:e86588
Senner NR, Stager M, Sandercock BK (2016) Ecological mismatches are moderated by local conditions for two populations of a long-distance migratory bird. Oikos 125
Skrade PDB, Dinsmore SJ (2013) Egg crypsis in a ground-nesting shorebird influences nest survival. Ecosphere 4:151
Smith LC, Sheng Y, MacDonald GM, Hinzman LD (2005) Disappearing arctic lakes. Science 308:1429
Smith PA, Gilchrist HG, Smith JNM (2007) Effects of nest habitat, food, and parental behavior on shorebird nest success. Condor 109:15–31
Smith PA, Elliott KH, Gaston AJ, Gilchrist HG (2010) Has early ice clearance increased predation on breeding birds by polar bears? Polar Biol 33:1149–1153
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman, NY
Stow DA, Hope A, McGuire D, Verbyla D, Gamon J, Huemmrich F, Houston S, Racine C, Sturm M, Tape K, Hinzman L, Yoshikawa K, Tweedie C, Noyle B, Silapaswan C, Douglas D, Griffith B, Jia G, Epstein H, Walker D, Daeschner S, Petersen A, Zhou L, Myneni R (2004) Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens Environ 89:281–308
Sturm M, Racine C, Tape K (2001) Climate change - increasing shrub abundance in the Arctic. Nature 411:546–547
Sturm M, Douglas T, Racine C, Liston GE (2005a) Changing snow and shrub conditions affect albedo with global implications. J Geophys Res 110:G01004
Sturm M, Schimel J, Michaelson G, Romanovsky VE, Welker JM, Oberbauer SF, Liston GE, Fahnestock J (2005b) Winter biological processes could help convert arctic tundra to shrubland. Bioscience 55:17–26
Swift RJ (2016) Nest site selection in Hudsonian Godwits: effects of habitat and predation risk. MS Thesis, Cornell University
Tape K, Sturm M, Racine C (2006) The evidence for shrub expansion in northern Alaska and the pan-Arctic. Global Change Biol 12:686–702
Tulp I, Schekkerman H, de Leeuw J (2012) Eggs in the freezer: energetic consequences of nest site and nest design in Arctic breeding shorebirds. PLoS One 7:e38041
Viereck LA, Dyrness CT, Batten AR, Wenzlick KJ (1992) The Alaska vegetation classification. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland
Walker BM, Senner NR, Elphick CS, Klima J (2011) Hudsonian Godwit (Limosa haemastica), The birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/629. Accessed 28 Aug 2016
Wauchope HS, Shaw JD, Varpe Ø, Lappo EG, Boertmann D, Lanctot RB, Fuller RA (2016) Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Global Change Biol 23:1085–1094
Wolken JM, Hollingsworth TN, Rupp TS, Chapin FS, Trainor SF, Barrett TM, Sullivan PF, McGuire AD, Euskirchen ES, Hennon PE, Beever EA, Conn JS, Crone LK, D’Amore DV, Fresco N, Hanley TA, Kielland K, Kruse JJ, Patterson T, Schuur EAG, Verbyla DL, Yarie J (2011) Evidence and implications of recent and projected climate change in Alaska’s forest ecosystems. Ecosphere 2:1–35
Yoshikawa K, Hinzman LD (2003) Shrinking thermokarst ponds and groundwater dynamics in discontinuous permafrost near Council, Alaska. Permafrost Periglac 14:151–160
Acknowledgements
We thank W. Abbott, A. Alstad, H. Batcheller, S. Billerman, B. Davis, J. DeCoste, D. Gochfeld, M. Harvey, J. Heseltine, M. Hilchey, A. Johnson, T. Johnson, J. Karagicheva, J. Klarevas-Irby, B. Lagasse, G. MacDonald, J. Marion, M. McConnell, J. McGowan, K. Parkinson, B. Schultz, G. Seeholzer, H. Specht, and B. Walker for their efforts in the field. J. Fitzpatrick and W. Koenig provided valuable comments on earlier drafts of this manuscript. Funding was provided by the David and Lucile Packard Foundation, U.S. Fish and Wildlife Service, Faucett Family Foundation, National Science Foundation, Cornell Lab of Ornithology, Cornell University, Athena Fund of the Cornell Lab of Ornithology, American Museum of Natural History, Ducks Unlimited Canada, Churchill Northern Studies Centre, and Arctic Audubon Society. All procedures performed in this study involving animals were in accordance with the ethical standards of Cornell University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swift, R.J., Rodewald, A.D. & Senner, N.R. Breeding habitat of a declining shorebird in a changing environment. Polar Biol 40, 1777–1786 (2017). https://doi.org/10.1007/s00300-017-2101-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2101-8