Abstract
Changing environmental conditions in the Arctic have caused widespread but disparate changes in the relative abundances of diatoms in the Cyclotella sensu lato group since 1850 across Arctic lakes. To better understand the mechanisms behind these species changes, we investigated how the nutrient limitation status of a lake alters the responses of three common Cyclotella sensu lato taxa to light. To assess this, we collected source water with the natural phytoplankton assemblages from lakes in southwest Greenland with different nutrient limitation status (phosphorus (P)-limited or nitrogen & phosphorus (N&P) co-limited). The responses of Lindavia bodanica, Lindavia radiosa, and Discostella stelligera to light levels (low, moderate, or high) and nutrients (limited or replete) were tested using a factorial design. The vertical distributions of these taxa across 20 lakes of varying nutrient limitation status and water transparency were also assessed. We found that light affected Cyclotella growth rates, cell densities, and distribution patterns differently depending on lake nutrient limitation status. L. bodanica fared best at low light under P-limitation, and at high light under N&P co-limitation, while the pattern was generally opposite for D. stelligera. For L. radiosa, regardless of nutrient limitation status, moderate-to-high light was important, with this species absent from lakes with lower light conditions. This study reveals that environmental change affects these species via complex interactions between nutrient and light availability, and helps to clarify some of the complex distribution patterns of planktonic diatom taxa found in lakes of many areas around the Arctic as well as at lower latitudes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many lakes in the Arctic are dilute, low productivity systems (Markager et al. 1999; Anderson et al. 2001). Primary production in these lakes is constrained in part by low nutrient concentrations (Miller et al. 1986). While lakes in this region were previously considered limited by phosphorus (P) alone (Schindler 1974; Rigler 1978; Gregory -Eaves et al. 2000), there is now evidence that nutrient limitation patterns vary spatially across Arctic landscapes. In addition to P-limitation, nitrogen (N) limitation as well as N and P co-limitation have been found in lakes across many regions of the Arctic (Levine and Whalen 2001; Ogbebo et al. 2009; Symons et al. 2012; Hogan et al. 2014). Overall, these patterns suggest variation in nutrient limitation among lakes within the same region as well as across the Arctic.
Rapid environmental change occurring in high-latitude regions may alter nutrient availability in lakes. Increases in warming are causing thawing of permafrost, which is rapidly releasing N and P into Arctic surface waters (Frey and McClelland 2009; Reyes and Lougheed 2015). A range of other processes can also influence nutrient availability, including additional catchment processes (e.g., altered hydrology, enhanced weathering) and enhanced atmospheric nitrogen deposition (Holtgrieve et al. 2011; Fritz and Anderson 2013; Reyes and Lougheed 2015). These may lead to changes in the supply of nutrients, which can ultimately alter the balance of nutrients available for phytoplankton communities in lakes.
In addition to nutrient limitation, light is a factor that limits algal production in Arctic lakes. Light availability is also important for determining algal community structure (Reynolds 1984), with fluctuating light availability affecting growth rates and interspecific competition (Litchman 1998). The changing climate in the Arctic can alter light availability in lakes in many ways. Increasing air temperatures have caused changes in the length of the ice-free season (e.g., earlier ice off) for many Arctic lakes (Surdu et al. 2014, 2016). A longer ice-free season can result in longer periods of higher light availability for photosynthesis. Climate can also alter water transparency, particularly via its effects on dissolved organic carbon (DOC) concentrations in lake ecosystems (Williamson et al. 1999; Weyhenmeyer and Karlsson 2009). DOC is a major regulator of water transparency (Williamson et al. 1999), affecting primary production in oligotrophic lakes (Karlsson et al. 2009). When it is present in high concentrations, it plays an important role in limiting whole-lake primary production (Ask et al. 2009; Cole 2009). There have been variable changes in DOC concentrations in Arctic surface waters over recent decades (Striegl et al. 2005; Anderson and Stedmon 2007; Saros et al. 2015), and this has likely altered lake water transparency, which affects the thermal structure of small Arctic lakes (Saros et al. 2016). Such changes in lake mixing and water transparency have important implications for light availability to phytoplankton.
Given the highly variable nutrient and light conditions in Arctic lakes, interactive effects between these resources are likely to shape how phytoplankton in these lakes respond to environmental changes. Varying effects of light and nutrient interaction heavily influence phytoplankton communities, since they have short generation times and respond quickly to environmental change. Experimental studies have demonstrated that under nutrient-rich conditions and low light availability, phytoplankton will compete for light, with species that are superior competitors at low light intensity succeeding (Litchman 2003; Kardinaal et al. 2007). In contrast, in poor nutrient conditions and higher light penetration, phytoplankton will compete for nutrients (Passarge et al. 2006). Nutrient uptake is also often dependent on light, with some nutrients requiring more energy to assimilate than others (Syrett 1981). This dependence often weakens with increasing nutrient limitation. Phytoplankton can synthesize more chlorophyll a to capture more light or adjust photosynthetic machinery and match the prevailing light environment (Falkowski and Raven 2007; Lepetit et al. 2012), but this requires more resources. Phytoplankton can also tune their photosynthetic machinery to maintain relatively high efficiency of photochemistry in photosystem II even when deficient in phosphorus or nitrogen (Silsbe et al. 2015). Clearly, interactions between nutrient and light availability play a key role in determining phytoplankton ecology in lakes.
Broadly, one of the phytoplankton groups most strongly affected by interactive environmental conditions is Cyclotella sensu lato taxa, a group of diatoms. Changing environmental conditions in the Arctic have caused widespread changes in Cyclotella species since 1850 across Arctic lakes (Smol et al. 2005; Perren et al. 2009, 2012). These changes appear to be driven by climate change, but we are only beginning to understand the mechanisms underlying these changes. Several studies have reported that these species distribute along vertical habitat gradients of light and nutrient availability (Winder et al. 2008; Saros et al. 2012), and as a result, the vertical thermal structure of a lake is important as it controls these resources. Changes in lake thermal structure in small lakes (<5 km2) are linked to climate via its effect on mean lake temperatures as well as DOC loading (Fee et al. 1996; Kraemer et al. 2015). DOC strongly affects water transparency, which in turn determines the depth of the thermocline in small lakes (Snucins and Gunn 2000).
Watershed processes affect DOC loading as well as nutrient inputs, and can be highly variable across landscapes (Canham et al. 2004; Seekell et al. 2014). Paleolimnological studies from lakes of southwestern Greenland have reported low coherence across lakes in the timing of response of Cyclotella assemblages to similar climatic forcing (Perren et al. 2009; Law et al. 2015). These studies have attributed variation in catchment processes that influence delivery of nutrients into lakes as the driver of the varied response of Cyclotella assemblages during the mid-Holocene thermal maximum. Experiments in these Arctic lakes of Greenland demonstrated important effects of nutrients (none added or N+P) and light, and sometimes interactive effects between them, on several Cyclotella sensu lato taxa (Saros et al. 2014; Malik and Saros 2016), which may explain variation in species distribution patterns. These experiments did not separate the effects of different nutrients or nutrient limitation status. Given the variable nutrient limitation patterns across Arctic lakes, quantifying the effects of differing nutrient limitation patterns and their potential alterations to light responses of different taxa will help in clarifying the ecology of this group of phytoplankton that is common across Arctic lakes.
Here, we investigated whether the nutrient limitation status of a lake alters the responses of three common Cyclotella sensu lato taxa to light. To assess this, we collected source water with the natural phytoplankton assemblages from two lakes in southwest Greenland with different nutrient limitation status. The responses of these three taxa to differing light levels (low, moderate, or high) were tested under different nutrient limitation conditions. The distributions of these three taxa in relation to nutrient limitation and light availability were also assessed across 20 lakes in the area.
Methods
Taxonomy
The recent reclassification of Cyclotella sensu lato taxo had moved some species into the genera Puncticulata and Handmannia. The character that joins the lineage Lindavia is the presence of one or more rimoportulae on the valve face. We followed the latest classification of Cyclotella into the genus Lindavia (Nakov et al. 2015); hence, we use this genus where appropriate. We also follow the transfer of stelligeroid taxa into the genus Discostella (Houk and Klee 2004). We did not distinguish between Discostella stelligera and Discostella pseudostelligera but rather combined them together into one taxon, referring to them as D. stelligera.
Site description
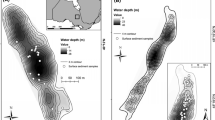
Lakes near Kangerlussuaq, southwest Greenland (67°N, 55°W), were selected for this study (Fig. 1). The lakes around Kangerlussuaq vary in nutrient limitation status (Brutemark et al. 2006; Whiteford et al. 2016) and have abundant Cyclotella sensu lato taxa (Perren et al. 2009). These lakes thermally stratify shortly after ice off, which typically occurs sometime in June; stratification persists until mid-August. Thermal stratification is strongly affected by light attenuation, which in turn is controlled by DOC concentration and quality metrics as well as chlorophyll (Saros et al. 2016). In late June-early July of 2013, 21 lakes were surveyed to assess the distributions of these taxa with respect to nutrient limitation and light availability (Fig. 1).
Map of the study locations near Kangerlussuaq, southwestern Greenland. Lakes studied in the survey are indicated by a different light levels and nutrient limitation. Circle (low light), triangle (moderate light), star (high light). Symbols in white represent P-limitation and in black represents N+P co-limitation. Lakes SS85 and SS67 are experimental lakes
We used the ratio of dissolved inorganic nitrogen (DIN) to total phosphorus (TP) to identify nutrient limitation status, with DIN:TP>3.4 indicating P-limitation, ratios <1.5 indicating N limitation, and values from 1.5 to 3.4 suggesting co-limitation. This metric performs better in oligotrophic lakes than TN:TP (Bergström 2010). Out of these 21 lakes, 14 had DIN:TP indicative of co-limitation, six of P-limitation, and one of N limitation. Two of these 21 lakes were selected for experiments because of their different nutrient limitation status and presence of similar Cyclotella taxa. Lake SS67 had a DIN:TP of 3, suggesting N+P co-limitation, while Lake SS85 had a DIN:TP of six, indicating P-limitation. Both lakes had Lindavia bodanica (Grunow) Håkansson, Lindavia radiosa (Lemmermann) Håkansson and D. stelligera (Cleve and Grunow) Houk and Klee. While we found one lake with DIN:TP indicating N limitation, this lake had very low Cyclotella sensu lato populations, and only one species in common with the other lakes, so we chose to exclude an N-limited lake for those reasons. Both SS67 and SS85 had similar light conditions, with 1% depths of photosynthetically active radiation (PAR) of 8.7 (SS85) and 8.8 (SS67) m in June 2013.
Experiments
For each lake, experiments were established by collecting water samples from different depths of the photic zone using a van Dorn sampling bottle. Source water was filtered through 100-μm Nitex mesh to remove zooplankton grazers, and combined into a single acid-washed 5-L container. Three 50-mL sub-samples of this water were preserved with Lugol’s iodine to determine intial cell densities of diatoms. Initial nutrient concentrations in source water were also determined. TP, nitrate (NO3 −), ammonium (NH4 +) and soluble reactive phosphorus (SRP) were quantified using the persulfate digestion method, cadmium reduction method, phenate method, and ascorbic acid method (APHA 2000), respectively, on a Lachat QuickChem 8500 flow injection analyzer. Limits of quantification were 2 µg L−1 for all nutrients. Concentrations of NO3 − and NH4 + were summed to determine DIN. All nutrient analyses were conducted within four weeks of sample collection, with samples kept refrigerated in the dark until analysis.
Experiments were conducted in a growth chamber in the Kangerlussuaq International Science Station using a 3 × 2 factorial design, in which light (100, 60, or 20% of ambient) and nutrients (limiting nutrient(s) or no addition) were manipulated. Light levels were manipulated by inserting the flask into a window screen mesh pocket (resulting in 20% of ambient PAR), inserting into a mesh bag (resulting in 60% of ambient PAR), or leaving uncovered (resulting in 100% of ambient PAR). These percent transmissions were determined using a spectrophotometer (Varian Cary UV–VIS). We used a BIC radiometer (Biospherical Instruments Inc., San Diego, CA) to measure PAR intensity in the growth chamber. The full light intensity (100%) was 272 μEinsteins m−2 s−1 (μE m−2 s−1), 60% was 163 μE m−2 s−1, and 20% was 54 μE m−2 s−1. In the nutrient addition treatments, nutrients were added in the form of NaNO3 for nitrogen (N = 112 μg L−1) and NaH2PO4 for phosphorus (P = 31 μg L−1). Source water was transferred to 75-mL non-treated culture flasks, with each treatment established in triplicate. Flasks were incubated in the growth chamber for 7 days at a temperature of 15°C on a 14:10 h light:dark cycle. This temperature is representative of mid-summer epilimnetic water temperatures (Saros et al. 2016). After the incubation, all flasks were preserved with Lugol’s iodine.
Phytoplankton cells were enumerated by settling 20-ml sub-samples from each flask in Utermöhl-style chambers for 12 h, and examined with a Nikon TS-100 inverted microscope with ×400 magnification. Empty frustules were present in some treatments and these were not counted; this resulted in negative growth rates in some cases. Counts from each flask were converted to cell densities (cells mL−1) and averaged across the three replicates for each treatment. Three transects were counted per slide for D. stelligera, while the whole slide was counted for L. radiosa and L. bodanica because of their larger cell sizes.
To assess the distribution of species cell densities and growth rates, normal quantile–quantile plots were first examined for univariate non-normality and heterogeneity of variance. Univariate normality was tested by Shapiro-Wilk test and Bartlett Test was used to test homogeneity of variances. Growth rates and cell densities of all three species were log transformed for the N+P co-limited lake to achieve normal distributions. To assess the effects of light and lake nutrient limitation status on each species, two-way analysis of variance (ANOVA) was conducted on growth rate across light levels in only the control treatments (i.e., no nutrients added and therefore limited as indicated by the ambient DIN:TP). We used growth rates for this comparison to normalize for differences in absolute cell densities in the two different lakes. To assess the effects of light and nutrient enrichment on cell densities, two-way analysis of variance (ANOVA) was conducted on the three light by two nutrient (control or added) treatments. All tests were run using R (Version 0.98.501).
Distribution patterns
Distributions of the three Cyclotella sensu lato taxa were assessed across nutrient limitation patterns and light conditions in 20 lakes in the area (the one N-limited lake was excluded from this analysis because of the lack of experimental counterpart). Water was collected with a van Dorn horizontal sampling bottle. Epilimnetic samples were used to determine DIN:TP. DIN and TP were measured as described above. Water from the epi-, meta-, and hypolimnia of each lake were also collected and preserved with Lugol’s iodine for phytoplankton enumeration. These were quantified as described above.
To quantify the light conditions in each lake, water column profiles of PAR were measured with a BIC submersible profiling radiometer coupled with a deck radiometer (Biospherical Instruments Inc., San Diego, California). PAR profiles were used to calculate the depth of 1% PAR attenuation for each lake. We also determined the average depth of each lake from bathymetric maps created as described in Saros et al. (2016). We compared relative light environments across lakes using a ratio of the 1% PAR depth to average depth for each lake. Lakes with ratios of about one or less were placed in the low light category; while low, these lakes all had 1%PAR depths that were about the same as the average lake depth. The calculated ratios ranged from 0.9 to 4.2; we established three light categories across that gradient. Lakes with ratios from 1.2 to 2.0 were categorized as moderate light, while those with ratios >2.0 were considered high light. The high light lakes would have roughly 10% PAR remaining at the average lake depth, while the moderate category would be between 1 and 10% PAR at the average depth. We note that all lakes had sufficient PAR (1% of surface irradiance) for phytoplankton growth at the average depth; lakes in the high light category had 10% PAR or higher at the average depth, suggesting that ecologically relevant light gradients are captured across the lake categories.
Based on DIN:TP and 1%PAR:average depth, each lake had a nutrient limitation (P or co-limitation) and a light (low, moderate, high) status, resulting in six possible categories when these are combined. The vertical distributions of taxa within lakes added a second dimension to light relationships. For each lake, the abundances of each Cyclotella sensu lato taxon were ranked from 1 to 3 across the three lake strata (epi, meta-, and hypolimnia). The layer with the highest abundance received a rank of three, lowest abundance a rank of one. If two layers had the same highest abundance, both received a three and the lower abundance layer received a one (i.e., there was no rank of two in this type of case). For each species, the ranks for each stratum were then averaged across lakes in the same categories. For example, the ranks of L. bodanica in the epilimnia of all low light, N&P co-limited lakes were averaged. Because sample sizes in the different categories varied widely and sometimes only included one lake, statistical analyses were not conducted on these data.
Results
Experiments
Source water from Lake SS85, the P-limited lake, contained 4.2 cells mL−1 of L. bodanica, 68 cells mL−1 of L. radiosa and 47 cells mL−1 of D. stelligera. The source water from SS67, the N+P co-limited lake, contained lower cell densities overall, with 0.1 cells mL−1 of L. bodanica, 6 cells mL−1 of L. radiosa and 23 cells mL−1 of D. stelligera. Initial nutrient concentrations in Lake SS85 were 18 μg L−1 DIN and 3 μg L−1 TP (DIN:TP of 6), while in Lake SS67, DIN was 6 μg L−1 and TP was 2 μg L−1 (DIN:TP of 3).
Light affected Cyclotella growth rates differently depending on nutrient limitation status, with interactive effects of light and limiting nutrient of lakes (p < 0.001) resulting for all species (Table 1). In the P-limited lake, L. radiosa had positive growth rates under all light conditions (Fig. 2b), whereas L. bodanica and D. stelligera had positive growth rates only under low or low and medium light conditions, respectively (Fig. 2a and c). The highest growth rates under P-limitation were observed in medium light for L. radiosa and D. stelligera and in low light for L. bodanica.
In the N+P co-limited lake, responses to light differed for all species. Lindavia bodanica growth rates were positive under all light conditions (Fig. 2a), whereas L. radiosa and D. stelligera had positive growth rates only under high or low light, respectively (Fig. 2b and c). The highest growth rates under N+P co-limitation were observed in high light for L. bodanica and L. radiosa and in low light for D. stelligera.
In both lakes, additions of the limiting nutrient(s) increased cell densities of Cyclotella taxa for most treatments. Final cell densities were higher when nutrient limitation was relaxed in the P-limited versus N+P co-limited assemblages; in general, they were more variable in all of the populations from the N+P co-limited lake (Fig. 3). In some cases, the addition of P changed the response to light compared to P-limited conditions; no change in response to light was apparent with nutrient enrichment of the N+P co-limited lake treatments.
Cell densities of different Cyclotella taxa from P-limited or N+P co-limited lake with manipulated light levels (low (L), medium (M), and high (H)) indicated. Results are shown for treatments without nutrient additions (Control) and with the limiting nutrient(s) added. a and d Lindavia bodanica; b and e Lindavia radiosa; c and f Discostella stelligera. The cells mL−1 for each species are depicted in each case, with bars representing standard error
In populations from the P-limited lake, interactive effects of nutrients and light affected the cell densities of L. bodanica (p = 0.009; Table 2). L. bodanica cell densities were highest under low light for both control and P-addition treatments (Fig. 3a). The addition of P increased their cell densities under high light (approximately doubled) but not in low light treatments, resulting in an interactive effect. L. bodanica cell densities were also affected by independent effects of light (p = 0.001) but not by nutrients (p = 0.85; Table 2). Lindavia radiosa cell densities were highest under medium light in both control and P-addition treatments (Fig. 3b). Addition of P increased cell densities by 1.3 to 1.6 times but did not change the response to light. L. radiosa cell densities were affected by independent effects of nutrients (p = 0.001) and light (p = 0.01) only; there were no interactive effects between nutrients and light (p = 0.85; Table 2). D. stelligera cell densities were affected by interactive effects between nutrients and light (p < 0.001). The addition of P increased cell densities about two to six times compared to controls. Furthermore, P-addition changed their response to light, with highest cell densities under medium light in the control treatment compared to low light with P enrichment (Fig. 3c). D. stelligera cell densities were also affected by independent effects of light (p < 0.001) and nutrients (p < 0.001; Table 2).
In populations from the N+P co-limited lake, there were no interactive effects of nutrients and light for any of Cyclotella species (Table 2). L. bodanica cell densities increased when nutrients were added, but this did not alter their response to light (Fig. 3d). Cell densities of L. bodanica were affected by independent effects of nutrients (p < 0.001) and light (p = 0.02; Table 2), with highest cell densities in the high light treatment. Similarly, L. radiosa cell densities increased about three times when nutrients were added (Fig. 3e). Cell densities of L. radiosa were also affected by independent effects of nutrients and light (p < 0.001 in both cases; Table 2), with highest cell densities in the high light treatment. In contrast, D. stelligera cell densities were affected by nutrients only (p = 0.001; Table 2). Addition of nutrients almost doubled their cell densities, but it did not change their response to light (Fig. 3f).
Distribution patterns
Of the 20 lakes surveyed, 14 had DIN:TP indicative of N+P co-limitation and six of P-limitation (Table 3). The 1% PAR ranged from 5 to 19 m across the lakes. Three lakes were in the low light category, while ten were in moderate and seven in high. There was at least one lake in each of the six defined lake categories (Table 3). For comparison to the light intensities of the growth chamber, the average across all lakes of average PAR intensity of the mixed layer was 568 ± 23 μE m−2 s−1; PAR intensities at the transition between the epi- and metalimnia ranged from 61 to 293 μE m−2 s−1.
L. bodanica was present in 13 of the lakes, L. radiosa in 10 lakes, and D. stelligera in 18 lakes. The distributions of each species varied with nutrient limitation status and light conditions. L. bodanica was generally found in lower light conditions in P-limited lakes (although the moderate light category was not consistent with this observation), and higher light conditions in N+P co-limited lakes (Fig. 4). L. radiosa was absent from the low light lakes in both nutrient limitation categories. Distribution patterns of this species across lake strata varied and did not show a strong trend other than the absence in low light lakes. D. stelligera was generally found in higher light conditions in P-limited lakes, and lower light conditions in N+P co-limited lakes.
Ranked abundances of each taxon in lake strata across the twenty survey lakes, with lakes categorized by nutrient limitation patterns and relative light availability as explained in the text. The number of lakes (n) in each category is indicated. Higher rank values indicate greater abundance of that taxon in that layer
Discussion
Our results demonstrate that variation in the nutrient limitation patterns of lakes has important implications for the response of phytoplankton to changing environmental conditions. Specifically, we found that the nutrient limitation status of a lake altered the responses of three Cyclotella sensu lato taxa to light. This is consistent with previous research that has demonstrated complex, interactive effects of nutrients, and physical variables (e.g., light, temperature) on cell densities of L. bodanica and D. stelligera (Saros et al. 2012, 2014; Malik and Saros 2016). Our results advance understanding further by clarifying how specific types of nutrient limitation (P or both N+P) affect the growth rates and cell densities of three taxa under different light conditions, and reveal how variation in nutrient limitation patterns across Arctic lakes may affect species distribution patterns. With differing limitation patterns and ongoing alterations to Arctic lake nutrient dynamics, our results help to clarify seemingly disparate patterns in Cyclotella sensu lato taxa distributions.
Under P-limitation, two species had their highest growth rates at moderate light and one species at low light. In contrast, under N+P co-limitation, two species had their highest growth rates at high light and one species at low light. There are many physiological mechanisms by which different types of nutrient limitation may affect the response of diatoms to light. The P-limited lake had three times more DIN than the co-limited lake. With a substantial proportion of the light-harvesting apparatus containing nitrogen (Larkum and Barrett 1983), the greater availability of nitrogen in a P-limited lake would enable maintenance of sufficient light harvesting under low-to-moderate light levels. In contrast, phytoplankton under high light conditions will have lower growth rates because P-limitation leads to a decrease in light saturated growth rates and increases susceptibility to photoinhibition (Litchman et al. 2003). Limitations by both N and P can cause a decrease in chlorophyll concentration per cell (Daley and Brown 1973; Porra and Grimme 1974; Litchman et al. 2003), raising light requirements. However, small cells have an advantage under light limiting conditions because light absorption per unit of chlorophyll is higher and internal shading by pigment is lessened (Finkel 2001; Finkel et al. 2004). To maintain photosynthesis in high light conditions, phytoplankton must maintain photoinactivation of PSII with repair. Cell size causes a trade off between PSII photoinactivation and susceptibility. In our experiments, D. stelligera is the smallest diatom; L. bodanica is the largest. In general, small centric diatoms are more susceptible to PSII photoinactivation and depend upon PSII repair in response to high light. They can repair cells faster when photoinactivated whereas large centric diatoms are less susceptible to photoinactivation and therefore incur lower costs to endure short-term exposures to high light (Key et al. 2010).
Nutrient limitation status had a strong effect on the response of L. bodanica to light. The growth rate of this species was highest at low light under P-limitation, and at high light under N&P co-limitation. This suggests that the vertical distribution of this species in a lake will depend on nutrient limitation status, with higher abundances predicted at deeper depths (lower light) in P-limited lakes and at shallower depths (higher light) in N&P co-limited lakes. This is generally consistent with the patterns we observed across these Arctic lakes, with L. bodanica being more abundant in deeper waters of P-limited lakes (although not in the moderate light lake surveyed), whereas in N&P co-limited lakes, it was more abundant in upper layers. This effect weakened slightly in the more transparent lakes, where deeper layers would also be well illuminated. These observations are consistent with distribution patterns at mid-latitudes. L. bodanica had higher cell densities in the hypolimnion of a P-limited alpine lake (Saros et al. 2012), and was most abundant during spring turnover (a period of lower average light intensities) in P-limited Sebago Lake in Maine, USA (Boeff et al. 2016). While we are unaware of results from N&P co-limited lakes, higher abundances of this taxon at shallower depths (high light) have been observed under N-limited conditions in subalpine lakes (Interlandi et al. 1999; Noble et al. 2013). Overall, the relationship of L. bodanica with light appears dependent upon nutrient availability, which will determine its vertical distribution in the water column.
The response of D. stelligera to light was also dependent on nutrient limitation status. The growth rate of this species was highest at moderate light under P-limitation, and at low light under N&P co-limitation. The distribution patterns of this taxon across the twenty lakes generally reflected the experimental results. This species was more abundant in the upper layers of P-limited lakes, and deeper or more evenly distributed across layers in N&P co-limited lakes. These patterns are consistent with those found at mid-latitudes. D. stelligera was more abundant in the epilimnia of P-limited alpine lakes (Saros et al. 2012), and small Cyclotella taxa were more abundant in Lake Tahoe during periods of higher N:P supply ratios and stronger vertical stratification (Winder and Hunter 2008). In Maine (USA), Boeff et al. (2016) found that D. stelligera bloomed in summer in the epilimnion of two P-limited lakes, while it was most abundant during spring turnover (lower light exposure) in Lobster Lake, which was N&P co-limited during spring. As with L. bodanica, the distributions of D. stelligera appear dependent on interactions between nutrient limitation and light conditions.
L. radiosa responded positively to moderate-to-high light under different nutrient limitation treatments, suggesting that light conditions in general, regardless of nutrient limitation status, are important for this species. The growth rate of this species was highest at moderate light under P-limitation, and at high light under N&P co-limitation. The positive effects of moderate-to-high light in both cases suggest that this species should be more abundant in the epi- and metalimnia of lakes. In the twenty survey lakes, we found that this species was absent from lakes in the low light category, while it generally varied in abundance in lake strata of the moderate and high light lakes. Other recent incubation experiments in Arctic lakes confirm these results, indicating that this species had the highest growth rates under high light conditions (Saros et al. 2014; Malik and Saros 2016). In lakes at mid-latitudes, this species was most abundant in the epilimnion of Piburger See, an alpine lake in Austria with high water transparency, and was correlated with the lower range of DIN:TP ratios for this lake, which varied from P to N&P co-limitation (Tolotti et al. 2012). In a eutrophic lake from Northern Ireland and in a German reservoir, L. radiosa disappeared as eutrophication occurred and re-appeared as nutrient enrichment declined (Rippey et al. 1997; Horn et al. 2011), suggesting positive effects of increased water transparency on this species.
The complex effects of nutrient limitation status and light availability on the distributions and abundances of various Cyclotella sensu lato taxa may contribute to the high degree of spatial and temporal variability in Cyclotella patterns in some Arctic lakes. As noted above, paleolimnological studies in southwest Greenland revealed low coherence in the timing of response of Cyclotella assemblages across lakes to similar climatic forcing (Perren et al. 2009; Law et al. 2015), and attributed this to variation in catchment processes that influence delivery of nutrients into lakes. Our results support these conclusions, and further suggest that the role of light and water transparency may also be important. Changes in light exposure and water transparency can occur with variation in the length of the ice-free season as well as in DOC concentrations and quality, all of which are, at least in part, climate-driven alterations to lake ecosystems. Phytoplankton production in southwest Greenland lakes is tightly linked to seasonal changes in nutrients and light availability associated with winter ice cover and open water conditions (Whiteford et al. 2016). With a sub-set of lakes in our study also investigated by Perren et al. (2009) who conducted a “top–bottom” assessment of sedimentary diatom assemblages, we examined whether our results provide additional context for the patterns in sediment records. We compared wherever possible; not all lake categories and taxa are covered, and we further note that sedimentary diatoms are expressed as percent relative abundance, so changes in one taxon affect relative abundances of others. In Perren et al. (2009), the core tops represented recent (~late 1990s) assemblages, while bottoms were estimated to pre-date 1850, hence the tops captured assemblages from a warmer period, and showed diverse responses across lakes of the same taxa. In the two high light, P-limited lakes in common between the two studies (SS56 and SS85 (called SS1478 in Perren et al. 2009), D. stelligera increased by 10–30% from the bottom slice to the top, whereas in the one moderate light, P-limited lake (SS2/SS1616), there was no change in its relative abundance. This is consistent with a higher light association under P-limitation for this taxon. The response in co-limited lakes was always positive: an increase of 30% in the low light lake (SS32), 20% (SS57) or 40% (SS68) increase in the moderate light lakes, and ~2% (SS66), 10% (SS67) or 40% (SS1341) increase in the high light lakes. This variability in the effects of light under N&P co-limitation was also apparent in our experiments. L. radiosa increased in all three high light lakes in common between the two studies (SS56, SS67, SS85/SS1478), whereas in the moderate light lakes, it only increased in one (SS57) and declined in the other two (SS2/SS1616, SS68).
Our results help to clarify some of the complex distribution patterns of planktonic diatom taxa found in lakes of many areas around the Arctic as well as at lower latitudes. They also underscore the conclusion that climate-driven changes in phytoplankton distribution patterns in Arctic lakes are complex, owing at least in part to factors influencing lake nutrient conditions and water transparency. Given the rapid changes occurring in nutrient and organic material delivery by watersheds in Arctic landscapes (Townsend-Small et al. 2011; Schuur et al. 2015), understanding ecological responses to such changes will be critical to assess future changes to species distribution patterns in oligotrophic Arctic freshwaters.
References
Anderson JN, Stedmon CA (2007) The effect of evapoconcentration on dissolved organic carbon concentration and quality in lakes of SW Greenland. Freshw Biol 52:280–289
Anderson NJ, Harriman R, Ryves DB, Patrick ST (2001) Dominant factors controlling variability in the ionic composition of west Greenland lakes. Arct Antarct Alp Res 33:418–425
Ask J, Karlsson J, Persson L, Ask P, Byström P, Jansson M (2009) Terrestrial organic matter and light penetration: effects on bacterial and primary production in lakes. Limnol Oceanogr 54:2034–2040. doi:10.4319/lo.2009.54.6.2034
Bergström AK (2010) The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligo- trophic lakes affected by N deposition. Aquat Sci 72:277–281. doi:10.1007/s00027-010-0132-0
Boeff KA, Strock KE, Saros JE (2016) Evaluating planktonic diatom response to climate change across three lakes with differing morphometry. J Paleolimnol 56:33–47. doi:10.1007/s10933-016-9889-z
Brutemark A, Rengefors K, Anderson NJ (2006) An experimental investigation of phytoplankton nutrient limitation in two contrasting low Arctic lakes. Polar Biol 29:487–494
Canham CD, Pace ML, Papaik MJ, Primack AGB, Roy KM, Maranger RJ, Curran RP, Spada DM (2004) A spatially explicit watershed-scale analysis of dissolved organic carbon in Adirondack lakes. Ecol Appl 14(3):839–854
Cole JJ (2009) Production in pristine lakes. Nature 460:463–464. doi:10.1038/460463a
Daley RJ, Brown SR (1973) Chlorophyll, nitrogen and photosynthetic patterns during growth and senescenceof two blue- green algae. J Phycol 9:395–401. doi:10.1111/j.1529-8817.1973.tb04112.x
Falkowski PG, Raven JA (2007) Light absorption and energy transfer in the photosynthetic apparatus 44. Aquatic photosynthesis, 2nd edn. Princeton University Press, Princeton, pp 44–80
Fee EJ, Hecky RE, Kasian SEM, Cruikshank DR (1996) Effects of lake size, water clarity, and climatic variability on mixing depths in Canadian Shield lakes. Limnol Oceanogr 41(5):912–920. doi:10.4319/lo.1996.41.5.0912
Finkel ZV (2001) Light absorption and size scaling of light-limited metabolism in marine diatoms. Limnol Oceanogr 46:86–94
Finkel ZV, Irwin AJ, Schofield O (2004) Resource limitation alters the 3/4 size scaling of metabolic rates in phytoplankton. Mar Ecol Progr Ser 273:269–279
Frey KE, McClelland JW (2009) Impacts of permafrost degradation on Arctic river biogeo- chemistry. Hydrol Process 23:169–182
Fritz SC, Anderson NJ (2013) The relative influences of climate and catchment processes on Holocene lake development in glaciated regions. J Paleolimnol 49:349–362. doi:10.1007/s10933-013-9684-z
Gregory-Eaves I, Smol JP, Finney BP, Lean DRS, Edwards E (2000) Characteristics and variation in lakes along a north-south transect in Alaska. Fundam Appl Limnol 147:193–223
Hogan EJ, McGowan S, Anderson NJ (2014) Nutrient limitation of periphyton growth in Arctic lakes in south-west Greenland. Polar Biol 37:1331–1342
Holtgrieve GW, Schindler DE, Hobbs WO, Leavitt PR, Ward EJ, Bunting L, Chen G, Finney BP, Gregory-Eaves I, Holmgren S, Lisac MJ, Lisi PJ, Nydick K, Rogers LA, Saros JE, Selbie DT, Shapley MD, Walsh PB, Wolfe AP (2011) A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science 334:1545–1548
Horn H, Paul L, Horn W, Petzolt T (2011) Long-term trends in the diatom composition of the spring bloom of a German reservoir: is Aulacoseira subarctica favored by warm winters? Freshw Biol 56:2483–2499
Houk V, Klee R (2004) The stelligeroid taxa of the genus Cyclotella (Kutz.) Brebisson (Bacillariophyceae) and their transfer to the new genus Discostella gen. nov. Diatom Res 19:203–228
Interlandi SJ, Kilham SS, Theriot EC (1999) Responses of phytoplankton to varied resource availability in large lakes of the Greater Yellowstone Ecosystem. Limnol Oceanogr 44:668–682
Kardinaal WEA, Tonk L, Janse I, Hol S, Huisman J, Visser PM (2007) Competition for light between toxic and non-toxic strains of the harmful cyanobacterium Microcystis. Appl Environ Microbiol 73:2939–2946
Karlsson J, Byström P, Ask J, Ask P, Persson L, Jansson M (2009) Light limitation of nutrient-poor lake ecosystems. Nature 460:506–509. doi:10.1038/nature08179
Key T, McCarthy A, Campbell DA, Six C, Roy S, Finkel ZV (2010) Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environ Microbiol 12:95–104
Kraemer BM, Anneville O, Chandra S, Dix M, Kuusisto E, Livingstone DM, Rimmer A, Schladow SG, Silow E, Sitoki LM, Tamatamah R, Vadeboncoeur Y, Mclntyre PB (2015) Morphometry and average temperature affect lake stratification responses to climate change. Geophys Res Lett 42:4981–4988. doi:10.1002/2015GL064097
Larkum A, Barett J (1983) Light harvesting processes in algae. Adv Bot Res 10:1–219
Law AC, Anderson NJ, McGowan S (2015) Spatial and temporal variability of lake ontogeny in south-western Greenland. Quat Sci Rev 126:1–16
Lepetit B, Goss R, Jakob T, Wilhelm C (2012) Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth Res 111:245–257. doi:10.1007/s11120-011-9633-5 PMID:21327535
Levine MA, Whalen SC (2001) Nutrient limitation of phytoplankton production in Alaskan Arctic foothill lakes. Hydrobiologia 455:189–201
Litchman E (1998) Population and community responses of phytoplankton to fluctuating light. Oecologia 117:247–257
Litchman E (2003) Competition and coexistence of phytoplankton under fluctuation light: experiments with two cyanobacteria. Aquat Microb Ecol 31:241–248
Malik HI, Saros JE (2016) Effects of temperature, light and nutrients on five Cyclotella sensu lato taxa assessed with in situ experiments in Arctic lakes. J Plankton Res 38(3):431–442. doi:10.1093/plankt/fbw002
Markager S, Vincent WF, Tang EPY (1999) Carbon fixation in high Arctic lake: implications of low temperature for photosynthesis. 44:597–607. Limnol Oceanogr 44:597–607
Miller MC, Hatter GR, Spatt P, Westlake P, Yeakel D (1986) Primary production and its control in Toolik Lake. Alaska Fundam Appl Limnol 74:97–131
Nakov T, Guillory WX, Julius ML, Theriot EC, Alverson AJ (2015) Towards a phylogenetic classification of species belonging to the diatom genus Cyclotella (Bacillariophyceae): transfer of species formerly placed in Puncticulata, Handmannia, Pliocaenicus and Cyclotella to the genus Lindavia. Phytotaxa 217:249–264
Noble PJ, Chandra S, Kreamer DK (2013) Dynamics of phytoplankton distribution in relation to stratification and winter precipitation, Fallen Leaf Lake, California. West N Am Nat 73:302–322
Ogbebo E, Evans MS, Waiser MJ, Tumber VP, Keating JJ (2009) Nutrient limitation of phytoplankton growth in Arctic lakes of the lower Mackenzie River Basin, northern Canada. Can J Fish Aquat Sci 66:247–260
Passarge L, Hol S, Escher M, Huisman J (2006) Competition for nutrients and light: stable coexistence, alternative stable states or competitive exclusion? Ecol Monogr 76:57–72
Perren BB, Douglas MSV, Anderson NJ (2009) Diatoms reveal complex spatial and temporal patterns of recent limnological change in West Greenland. J Paleolimnol 42:233–247
Perren BB, Anderson NJ, Douglas MSV, Fritz SC (2012) The influence of temperature, moisture, and eolian activity on Holocene lake development in West Greenland. J Paleolimnol 48:223–239
Porra RJ, Grimme LH (1974) Chlorophyll synthesis and intracellular fluctuations of 5-aminolaevulinate formation during regreening of nitrogen-deficient Chlorella fusca. Arch Biochem Biophys 148:37–43
Reyes FR, Lougheed VL (2015) Rapid nutrient release from permafrost thaw in Arctic aquatic ecosystems. Arct Antarct and Alp Res 47:35–48
Reynolds CS (1984) The ecology of freshwater phytoplankton. Cambridge University Press, Cambridge
Rigler FH (1978) Limnology in the Arctic: a case study of Char Lake. Proc Int Assoc Theor Appl Limnol (SIL) 20:127–140
Rippey B, Anderson NJ, Foy RH (1997) Accuracy of diatom-inferred total phosphorus concentrations and the accelerated eutrophication of a lake due to reduced flushing and increased internal loading. Can J Fish Aquat Sci 54:2637–2646
Saros JE, Stone JR, Pederson GT, Slemmons KEH, Spanbauer T, Schliep A, Cahl D, Williamson CE, Engstrom DR (2012) Climate-induced changes in lake ecosystem structure inferred from coupled neo- and paleo-ecological approaches. Ecology 93:2155–2164
Saros JE, Strock KE, Mccue J, Hogan E, Anderson NJ (2014) Response of Cyclotella species to nutrients and incubation depth in Arctic lakes. J Plankton Res 36(2):450–460
Saros JE, Osburn CL, Northington RM, Birkel SD, Auger JD, Stedmon CA, Anderson NJ (2015) Recent decrease in DOC concentrations in Arctic lakes of southwest Greenland. Geophys Res Lett 42:6703–6709. doi:10.1002/2015GL065075
Saros JE, Northington RM, Osburn CL, Anderson NJ (2016) Thermal stratification in small Arctic lakes of southwest Greenland affected by water transparency and epilimnetic temperatures: thermal Stratification of Arctic Lakes. Limnol Oceanogr 61:1530–1542
Schindler DW, Welch HE, Kalff J, Brunskdl GJ, Kritsch N (1974) Physical and chemical limnology of Char Lake, Cornwallis Island (75°N Lat.). J Fish Res Board Can 31:585–607
Schuur EAG, McGuire AD, Schädel C, Grosse G, Harden JW, Hayes DJ, Hugelius G, Koven CD, Kuhry P, Lawrence DM, Natali SM, Olefeldt D, Romanovsky VE, Schaefer K, Turetsky MR, Treat CC, Vonk JE (2015) Climate change and the permafrost carbon feedback. Nature 520:171–179
Seekell DA, Lapierre JF, Pace ML, Gudasz C, Sobel S, Tranvik LJ (2014) Regional-scale variation of dissolved organic carbon concentrations in Swedish lakes. Limnol Oceanogr 59:1612–1620
Silsbe GM, Smith REH, Twiss MR (2015) Quantum efficiency of phytoplankton photochemistry measured continuously across gradients of nutrients and biomass in Lake Erie (Canada and USA) is strongly regulated by light but not by nutrient deficiency. Can J Fish Aquat Sci 72:651–660
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, Fallu M, Hughes M, Keatley BE, Laing TE, Michelutti N, Nazarova L, Nyman M, Paterson AM, Perren B, Quinlan R, Rautio M, Saulnier-Talbot E, Siitonen S, Solovieva N, Weckström J (2005) Climate-driven regime shifts in the biological communities of Arctic lakes. Proc Natl Acad Sci USA 102:4397–4402
Snucins E, Gunn J (2000) Interannual variation in the thermal structure of clear and colored lakes. Limnol Oceanogr 45:1639–1646
Striegl RG, Aiken GR, Dornblaser MM, Raymond PA, Wickland KP (2005) A decrease in discharge-normalized DOC export by the Yukon River during summer through autumn. Geophys Res Lett 32:L21413. doi:10.1029/2005GL024413
Surdu MC, Duguay CR, Brown LC, Fernández Prieto D (2014) Response of ice cover on shallow lakes of the North Slope of Alaska to contemporary climate conditions (1950–2011): radar remote-sensing and numerical modeling data analysis. The Cryosphere 8:167–180
Surdu MC, Duguay CR, Brown LC, Fernández Prieto D (2016) Evidence of recent changes in the ice regime of lakes in the Canadian High Arctic from spaceborne satellite observations. The Cryosphere 10:941–960
Symons CC, Arnott SE, Sweeetman JN (2012) Nutrient limitation of phytoplankton communities in Subarctic lakes and ponds in Wapusk National Park, Canada. Polar Biol 35(4):481–489
Syrett PJ (1981) Nitrogen metabolism of microalgae. Can Bull Fish Aqua Sci 182–210
Tolotti M, Thies H, Nickus U, Psenner R (2012) Temperature modulated effects of nutrients on phytoplankton changes in a mountain lake. Hydrobiologia 698:61–75
Townsend-Small A, McClelland JW, Holmes RM, Peterson BJ (2011) Seasonal and hydrologic drivers of dissolved organic matter and nutrients in the upper Kuparuk River, Alaskan Arctic. Biogeochemistry 103:109–124. doi:10.1007/s10533-010-9451-4
Weyhenmeyer GA, Karlsson J (2009) Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperatures. Limnol Oceanogr 54(6):2513–2519
Whiteford E, McGowan S, Barry CD, Anderson NJ (2016) Seasonal and regional controls of phytoplankton production along a climate gradient in South-West Greenland during ice-cover and ice-free conditions. Arct Antarct Alp Res 48(1):139–159. doi:10.1657/AAAR0015-003
Williamson CE, Morris DP, Pace ML, Olson OG (1999) Dissolved organic carbon and nutrients as regulators of lake ecosystems: resurrection of a more integrated paradigm. Limnol Oceanogr 44:795–803
Winder M, Hunter DA (2008) Temporal organization of phytoplankton communities linked to physical forcing. Oecologia 156:179–192
Acknowledgements
This research was funded by the Arctic System Science program of the US National Science Foundation (Grant #1203434 to JES). CH2 M Hill Polar Services provided logistical support for this project. We thank Kathryn Warner for field and laboratory assistance, and Nicholas John Anderson for providing the growth chamber and key pilot data for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, H.I., Northington, R.M. & Saros, J.E. Nutrient limitation status of Arctic lakes affects the responses of Cyclotella sensu lato diatom species to light: implications for distribution patterns. Polar Biol 40, 2445–2456 (2017). https://doi.org/10.1007/s00300-017-2156-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2156-6