Abstract
The New Zealand (NZ) sea lion (Phocarctos hookeri) is endemic to NZ and is listed as “Vulnerable” by the International Union for Conservation of Nature. Approximately 99 % of pups are born at the Auckland Islands (a declining population) and Campbell Island (a growing population). The causes of contrasting population trajectory are not well understood, though spatial and temporal variations in prey availability have frequently been implicated for other pinnipeds. This is the first published diet study of the Campbell Island population, located at the species’ southern breeding limit. Prey species were identified and quantified from scats and regurgitate samples collected in March 2013 (n = 159 and 7, respectively). An array of prey taxa was identified, though two species were particularly dominant in terms of reconstituted diet mass (M) of fishes and cephalopods: small-scaled cod (Notothenia microlepidota), which dominated scat samples (50 % M); and yellow octopus (Enteroctopus zealandicus), which dominated a small sample of regurgitates (72 % M). The diet lacked many of the key prey taxa of the Auckland Islands, and we hypothesise that the key prey identified here provides a highly available food source for the growing population of NZ sea lions at Campbell Island. These differences are likely to reflect spatial heterogeneity in prey availability and may be one of the main causes of an increase in NZ sea lion population size at Campbell Island in contrast to a decrease at the Auckland Islands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The New Zealand (NZ) sea lion (Phocarctos hookeri) is currently one of the rarest of all pinniped species and has a concentrated breeding distribution with >99 % of all pups born at the Subantarctic Auckland Islands and Campbell Island to the south of New Zealand (Chilvers 2012; Maloney et al. 2012). A rapid decline in annual pup production at the Auckland Islands (approximately 50 % since the late 1990s; Childerhouse et al. 2014) has led to the species’ classification as “Vulnerable” by the International Union for Conservation of Nature (IUCN 2011) and “Nationally Critical” under the New Zealand threat classification system (Baker et al. 2010). Candidate drivers for this decline include incidental mortalities relating to commercial fishing operations, disease-related mortality, predation, variation in prey abundance caused by changes in climate or commercial fishery catches and others (Robertson and Chilvers 2011). Spatial and temporal variation in prey abundance are thought to significantly affect the productivity of a number of otariid species (e.g. Salazar and Bustamante 2003; Trites and Donnelly 2003). Hence, the characterisation of diet is an essential first step towards the elucidation of mechanisms by which changes in ocean climate and fishing pressure could affect the productivity of NZ sea lions (Meynier et al. 2009; Augé et al. 2012).
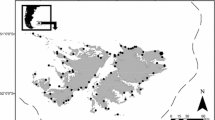
Campbell Island (Fig. 1a) is currently the most southerly breeding location of NZ sea lions (Childerhouse and Gales 1998). NZ sea lion populations are thought to have been decimated by commercial sealing operations in the 1800s (Childerhouse and Gales 1998). Infrequent pup censuses with varying methodologies have indicated that a period of consistent population growth has occurred at Campbell Island in recent years, with 30 pups counted in 1984/85 and 681 in 2009/10 (Taylor and Sadlier 1985; Childerhouse and Gales 1998; Maloney et al. 2012). A number of sea lions (nearly all males) have been captured in the Campbell Island southern blue whiting (Micromesistius australis) trawl fishery (Thompson et al. 2013), but the indirect effects of the fishery remain unknown.
a Location of diet sampling of NZ sea lion in the NZ region [“STF” = approximate position of subtropical front (Smith et al. 2013)]; scale bar 100 km) and b main sampling sites in this study at Campbell Island (B Beeman, C Camp Cove, Ca Capstan Cove, G Garden Cove, L Lookout Cove, M Middle Bay, S Sandy Bay, T Tucker Cove, V Venus Cove, W Whaler Bay Cliffs, Pers. H Perseverance Harbour; scale bar 5 km)
The Campbell Island NZ sea lion population is the largest for which there has been no previous diet study and its biology is poorly understood compared with populations at the Auckland Islands or at the Otago Peninsula, on the NZ South Island (Fig. 1a). Diet assessments have indicated strong seasonal and locational differences that are likely to relate to variation in prey abundance and availability across their range from Otago Peninsula in the north to Macquarie Island, approximately 1000 km to the south (Lalas 1997; McMahon et al. 1999; Meynier et al. 2009; Augé et al. 2012; Lalas and Webster 2014). Here, we present estimates of the autumnal diet of NZ sea lions from prey remains in scats and regurgitates collected at Campbell Island (Fig. 1b). We briefly assess the potential for resource competition with commercial fisheries and, through comparison with estimates of diet composition from other locations, describe geographical variation in NZ sea lion diet throughout their range.
Materials and methods
Scat and regurgitate samples were collected from six sites bordering Perseverance Harbour and four sites bordering Northwest (NW) Bay at Campbell Island (Fig. 1b) from 11 to 29 March 2013. Only fresh samples were collected. Regurgitates and scats were processed in the same way: as much as possible of each scat/regurgitate was transferred to a plastic re-sealable bag in which it was soaked in a 5 % solution of laundry detergent for about 1 week. Samples were then agitated into a paste before separating off undigested remains by passing the contents of each bag through a sieve with mesh size 0.5 mm. Undigested remains were then stored in watertight pots and immersed in a 90 % ethanol solution. Sea lions ashore at each collection site were counted on each sampling day, with counts divided into three categories: (non-pup) males, (non-pup) females and pups.
Diagnostic remains were identified to genus or species level by comparison with specimens in reference collections held by C. Lalas (CL) and the National Institute of Water and Atmospheric Research (NIWA). Teleost fishes were identified from sagittal otoliths (Schwarzhans 1984; Williams and McEldowney 1990; Smale et al. 1995; Furlani et al. 2007) and jaws (Cannon 1987); elasmobranch fishes from jaws, teeth and epidermal thorns; cephalopods from beaks (Clarke 1986; Xavier and Cherel 2009); crustaceans from pieces of exoskeleton (especially carapace, claws and pleopods); birds from feathers and bones; and seals (NZ fur seals, Arctocephalus forsteri) from hair, fur and bones. Scats that lacked any diagnostic remains were excluded from the analyses. Parasitic crustaceans (parasitic copepods and isopods ingested with host fish) were not included as prey items.
Un-eroded diagnostic prey remains were measured to the nearest 0.01 mm. Prey size was estimated from species-specific power equations applied to key measures on diagnostic remains. These included previously unpublished equations for three species of cephalopod calculated from beaks and 11 species of teleost fishes calculated from otoliths (Online Resource 1). Published equations to estimate prey size from otoliths were used for red cod (Pseudophycis bachus) and deepwater triplefin (Matanui bathytaton) from Lalas and McConnell (2012), and for opalfish (Hemerocoetes 2 spp.) from Lalas et al. (2014). Lengths of some fish were estimated by interpolation from specimens in the CL reference collection: jaw bones of small-scaled cod (Notothenia microlepidota); opercular spine of giant stargazer (Kathetostoma gigantum); and orbital, tail and alar thorns of rough skate (Zearaja nasuta).
Otoliths and other diagnostic fish bones were sorted into left and right, and cephalopod beaks were sorted into upper and lower. Teleost fishes were enumerated after accounting for paired otoliths, and other diagnostic bones and cephalopods were enumerated after accounting for paired beaks. Fish and cephalopod prey represented only by unmeasurable diagnostic remains were excluded from estimates of prey size. However, they were included in counts of prey, and each was assigned a nominal mass estimated either from a qualitative assessment of the size of prey remains or as the average mass of other conspecifics represented in the same sample. The occurrence and number of swimming crabs (cf. Nectocarcinus bennetti) in prey remains were estimated from diagnostic remains (claws and carapaces). However, none of these diagnostic remains of swimming crabs were measurable, and so each crab was assigned a nominal mass of 70 g, the mean for this species as prey of NZ sea lions at the Auckland Islands (CL unpublished data).
The occurrence of feathers in a scat or regurgitation was considered to represent one depredated seabird, with species identified from feather morphology. Feathers of predated penguins could not be identified to species level (A. Tennyson and C. Tinkler, personal communication), and so a nominal mass of 4 kg was assigned—equal to the average mass of the two most abundant penguin species at Campbell Island: 5.5 kg for yellow-eyed penguins (Megadyptes antipodes) and 2.5 kg for rockhopper penguins (Eudyptes chrysocome). A single non-spheniscid avian prey item, also identified from feathers, was assigned a mass of 2 kg, approximating to that of the Campbell Island shag (Phalacrocorax campbelli)—the most commonly sighted seabird observed in proximity to NZ sea lions during sampling. The occurrence of fur seal fur or hair in a scat or regurgitation was considered to represent one depredated NZ fur seal. The approximate size of NZ fur seal prey was determined from the colouration of guard hairs found in scats and regurgitates: wholly dark in young pups but pale tipped in older individuals (Lalas and Webster 2014), and 40 kg was assigned as the nominal mass for NZ fur seals older than pups.
Diet composition was estimated separately for each sample type (scats and regurgitates). Three indices were calculated: frequency of occurrence (% O), the proportion of samples containing each prey species; numerical frequency (% N), the number for each prey species as a proportion of the total number of prey items; and mass frequency (% M), the estimated original mass contributed by each prey species as a proportion of the estimated total mass of prey items.
The sampling location (Perseverance Harbour or NW Bay) effect on diet composition of fish and cephalopod prey taxa was assessed using one-way permutational multivariate analysis of variance (PERMANOVA) with 999 permutations. Permutational analysis of multivariate dispersions (PERMDISP) was used to assess the multivariate homogeneity of group dispersions. Both PERMANOVA and PERMDISP were conducted using the “vegan” package (Oksanen et al. 2013) in R Core Team (2014).
Results
A total of 159 scats and seven regurgitates were collected: 107 scats and four regurgitates from Perseverance Harbour, and 52 scats and three regurgitates from NW Bay (Table 1). All seven regurgitates contained diagnostic prey remains, though 55 scats lacked any including six with no solid remains.
A minimum total of 593 prey items (estimated total mass 118 kg) were represented in scats: 562 fish (15 spp.); 20 cephalopods (three spp.); seven crabs (one sp.); three seabirds (2–3 spp.); and one NZ fur seal. The corresponding total from regurgitates was 85 prey items (estimated total mass 91 kg): 56 fish (seven spp.); 28 cephalopods (one sp.); and one NZ fur seal. All species identified from regurgitates were also found in scat samples. The two NZ fur seals were both older than pups, each assigned a nominal mass of 40 kg. The three seabirds were two penguins (yellow-eyed penguin and/or rockhopper penguin) and one non-penguin seabird (cf. Campbell Island shag) assigned a combined reconstituted mass of 10 kg. The combination of NZ fur seals and seabirds accounted for <1 % of the 593 prey items but 42 % of the total mass from scats, and 1 % of the 85 prey items but 44 % of the total mass from regurgitates. They were excluded from further analyses in order to deduce the relative importance among fish, cephalopod and crustacean prey.
The combination of fishes, cephalopods and crustaceans accounted for 589 prey items totalling 68.5 kg from scats and 84 prey items totalling 50.8 kg from regurgitates (Table 2). Small-scaled cod was the most important species represented in scats, accounting for half of the total mass and occurring in one-third of samples (37 % O, 50 % M). No other species contributed ≥10 % towards total mass from scats. Opalfish was the most numerous species in scats, but its small size (mean mass 8 g, range 1–31 g) resulted in only a small contribution towards total mass (55 % N, 4 % M). Yellow octopus (Enteroctopus zealandicus) was the main prey species represented in a very small sample of regurgitates, accounting for 72 % of the total mass. Rough skate was the only other species that contributed ≥10 % towards total mass (11 % M). Oblique-banded rattail (Coelorinchus aspercephalus) was most numerous in regurgitates, though comprised only a small proportion of reconstituted mass (45 % N, 5 % M).
Measurements of diagnostic remains were used to calculate the size of over three-quarters (518 of 673) of prey items obtained (Table 2). The proportion of each species’ diagnostic remains that were measurable ranged from 60 % (three species in Table 3) to 100 %, with the exception of all swimming crabs for which all remains were too fragmented to make reliable measurements. The largest individual prey among fish and cephalopods was yellow octopus, ling and rough skate (mean mass of 4.0, 3.7 and 3.5 kg, respectively; Table 3). Three of the most numerous species (small-scaled cod, oblique-banded rattail and yellow octopus) each exhibited a broad range in individual mass (approximately 2 orders of magnitude; Table 3).
Counts of individual sea lions during sampling suggested that females were more abundant than males at sampling sites in Perseverance Harbour (male/female ratio = 0.65) and males were more likely to use haul-out sites in the NW Bay area (male/female ratio = 2.61) (Table 1). However, there was no significant variation in diet composition comparing scat samples collected at Perseverance Harbour and NW Bay (one-way PERMANOVA: pseudo F = 1.34, p = 0.184). The PERMDISP indicated multivariate homogeneity of group dispersions for both sample type (deviations from centroid: F = 0.129, p = 0.720) and sampling area (F = 0.226, p = 0.636).
Discussion
Limitations of study
This is the first study to describe the diet of the NZ sea lions at Campbell Island, at the southern limit of their breeding distribution. However, the sample size was small and limited to 1 month, and it is likely that some prey species were not detected in this study. Also, there are likely to be strong biases associated with scat and regurgitate sampling: scat sampling will underestimate the importance of species that do not leave indigestible hard parts, or of teleost or cephalopod species with small or less robust otoliths or beaks (Tollit et al. 2006). As yet there have been no captive feeding studies on NZ sea lions from which to derive correction factors for the estimation of diet composition from the two sources of samples, and so results from scats and regurgitates were analysed separately. As such, it was not possible to identify the complete diet from the analysis of hard parts recovered from scats and regurgitates. However, it was possible to make comparisons based on the species that were identified. Methods used to estimate diet at the other locations were similar, and so biases associated with differential retention of prey remains should have a minimal effect on between-population comparisons of diet composition.
Key prey species
From the small sample of regurgitates, it was evident that yellow octopus is an important prey species of NZ sea lions at Campbell Island, comprising more than two-thirds of the reconstituted mass of fish, cephalopods and crustaceans from this sample type. The ecology of the yellow octopus is poorly understood, but it has previously been identified as a key prey item for NZ sea lions at the Auckland Islands (Childerhouse et al. 2001; Meynier et al. 2009). Yellow octopuses are distributed from the shoreline down to at least 500 m depth and may be abundant at shallow depths around Campbell Island (O’Shea 1999). The nototheniid small-scaled cod, which dominated scat samples, is also known to be a minor prey species in the diet of NZ sea lions at the Auckland Islands (Childerhouse et al. 2001) where it is the most abundant fish species of inshore rocky reefs (Kingsford et al. 1989). The preliminary results of a biotelemetry study of sub-adult NZ sea lions tagged at Campbell Island indicate that some individuals are primarily foraging close to the island (Leigh Torres personal communication 2014) and small-scaled cod may be one of the main prey of these individuals.
Oblique-banded rattails and opalfish sp. were present in a large proportion of the scat samples though contributed only low proportions of the reconstituted prey mass. These species have been identified as frequently occurring in the diet of NZ sea lions at the Auckland Islands (Childerhouse et al. 2001; Meynier et al. 2009), but not in the diet of populations further north (Augé et al. 2012; Lalas and Webster 2014). Many of the key prey species of NZ sea lion populations to the north were lacking from the diet of the Campbell Island population, e.g. southern arrow squid (Nototodarus sloanii) and red cod at the Auckland Islands (Childerhouse et al. 2001; Meynier et al. 2009), and barracouta (Thyrsites atun) and jack mackerel (Trachurus sp.) at Otago Peninsula (Meynier et al. 2009; Augé et al. 2012) (Table 4). These species may have low abundance or availability around Campbell Island relative to yellow octopus and small-scaled cod. However, sampling was conducted during a limited time period, and they may have greater importance during other seasons.
NZ fur seal have been reported as prey of NZ sea lions at Macquarie Island (Robinson et al. 1999), the Auckland Islands (Childerhouse et al. 2001), Snares Island (Lalas and Webster 2014) and Otago Peninsula. NZ sea lions at Otago Peninsula eat not only NZ fur seal pups, with the remains of up to three pups found in one regurgitation (Bradshaw et al. 1998), but also older individuals including large males (Lalas et al. 2007). Fur seals tend to be omitted from reconstituted prey mass calculations, because the age and therefore mass of an individual cannot be accurately estimated from hair and fur. In this study, NZ fur seal remains were found in one scat and one regurgitate sample. Assigning a mass of 40 kg for each individual older than a pup, NZ fur seals would have comprised approximately 29 and 43 % of the reconstituted prey mass from scats and regurgitates, respectively, and may also be a major prey species at Campbell Island, particularly for large male NZ sea lions. Penguins are likely to provide another energy-rich addition to the diet, with yellow-eyed penguins (Moore and Moffat 1992) and rockhopper penguins (Morrison et al. 2015) recorded as prey. However, predation by the increasing number of NZ sea lions at Campbell Island has been implicated as a major cause of population decrease in yellow-eyed penguins (Moore et al. 2001) and a minor cause of population decrease in rockhopper penguins (Morrison et al. 2015).
The analysis of differences between sampling locations at Campbell Island indicated that diet composition was similar comparing NW Bay and Perseverance Harbour sampling sites. However, hoki and ling (both mid-depth species over the Campbell Plateau; Bagley et al. 2013) were only found in samples collected at NW Bay, where males were more abundant (Table 1). As such, there may be sex differences in habitat use, with males foraging further from the island where hoki and ling are more abundant. This is consistent with the strong male bias in incidental mortalities associated with the Campbell Rise southern blue whiting trawl fishery (Thompson et al. 2013).
Potential fishery interactions
The southern blue whiting trawl fishery is the largest currently operating around Campbell Island, and a number of sea lions have died as a result of direct interactions with fishing gear, with a strong male bias in captures (Thompson et al. 2013). The fishery targets dense spawning aggregations to the east of Campbell Island in August and September (Gautier et al. 2011) and has low rates of finfish by-catch. As such, the greatest potential for resource competition with NZ sea lions is likely to be for the target species, though this study indicated that southern blue whiting is only a minor constituent of the diet in March (Table 2). However, little is known of the spatial distribution of southern blue whiting outside of their spawning period when they may have reduced availability to NZ sea lions. Further diet studies would be required for a thorough assessment of potential resource overlap, including sampling in months when southern blue whiting aggregate for spawning and the trawl fishery is most active. Seasonal variation in the diet of NZ sea lions has previously been observed at Otago Peninsula, which is likely to reflect temporal variation in local prey abundance (Lalas 1997), and this should also be expected at Campbell Island where a number of prey species may be close to their cold water range limits.
Location and climate effects on diet
Many otariid species are considered to be generalist predators, with variation in foraging strategy and diet occurring in response to spatial and temporal gradients in prey abundance or availability (e.g. Lalas 1997; Sinclair and Zeppelin 2002). A comparison with previous studies of the diet of NZ sea lions indicates major locational differences in composition (Table 4). Sampling for these studies was conducted in different years and months such that locational effects will be confounded with seasonal and inter-annual variation in diet, and it was not possible to distinguish spatial and temporal variation. However, very strong north-to-south gradients in prey occurrence suggest that these trends may at least partly relate to spatial variation. For instance, jack mackerel and barracouta dominated the autumn diet of female NZ sea lions at Otago Peninsula (Augé et al. 2012) at their northern breeding limit. However, these species comprised only a small component of summer diet at the Auckland Islands (Childerhouse et al. 2001; Meynier et al. 2009) and were absent from the diet at Campbell Island in Autumn and Macquarie Island during year-round sampling (McMahon et al. 1999). Jack mackerel and barracouta are most abundant to the north of the subtropical front (STF; Fig. 1a) in the relatively warm waters surrounding the NZ mainland, though are infrequently captured in survey trawls to the south of the Auckland Islands (Anderson et al. 1998). This compares with the Subantarctic nototheniid small-scaled cod, which appeared to be the most important teleost prey at Campbell Island though was only a minor prey species at the Auckland Islands (Childerhouse et al. 2001; Meynier et al. 2009) and was absent from the diet of the Otago Peninsula population (Augé et al. 2012). Two Subantarctic species, Patagonian toothfish (Dissostichus eleginoides) and Antarctic horsefish (Zanchlorynchus spinifer), dominated the diet of males at Macquarie Island to the southwest of the Campbell Plateau (McMahon et al. 1999) and neither has been recorded as prey of NZ sea lions of the Campbell Plateau.
The observed north-to-south variation in diet composition (Table 4) suggests that temperature gradients may be a key factor mediating the relative prey mix available to NZ sea lion populations across the species’ range. Warmer water species were less frequently occurring in the diet of southern populations and Subantarctic species were lacking from diet samples collected from northern populations. As such, regional warming/cooling events may affect the relative productivity of different NZ sea lion populations via alteration of prey availability. Robertson and Chilvers (2011) argued that climate variability could not explain the contrasting pup production trajectories of NZ sea lions at the Auckland Islands and Campbell Island. However, there appear to be differences in the diet composition of these two populations, which may largely be driven by north-to-south gradients in ocean climate. Many of the prey species of the Otago Peninsula and Auckland Islands populations are rarely observed in research trawl survey tows located far to the south of the STF (Anderson et al. 1998; Table 4), though southward redistributions of these species may potentially occur during warmer climate regimes, such that they would be intermittently available to the Campbell Island population. Thus, oceanic warming and cooling events may have different effects on the nutrition of the Auckland Islands and Campbell Island populations.
Prey quality
The contrasting energetic costs and benefits associated with foraging for different prey assemblages are likely to be an important factor in the regulation of population growth of NZ sea lions across their distribution. The Campbell Island population has grown significantly in recent years, and a large dietary contribution of high-energy prey may be expected. In fact, the main prey species identified in this study—yellow octopus (3.8 kJ g−1; Meynier et al. 2008) and small-scaled cod (no available proximate composition data for this species, though Paranotothenia magellanica 4.8 kJ g−1; Fernàndez et al. 2009)—are both of low energy density. However, small-scaled cod are an abundant fish species of Subantarctic shallow rocky reefs, and low energetic returns may be offset by comparatively low energetic costs of foraging where these are captured during shallow dives. An alternative explanation for the apparent discrepancy between increased population size and low energy content of prey is that the longer-term diet at Campbell Island has not been adequately represented by the brief duration of this study and the availability of higher-quality prey may vary seasonally. Indeed, some of the higher-energy prey species predated at locations to the north including hoki (7.1 kJ g−1) and red cod (6.7 kJ g−1) (Meynier et al. 2008) have sporadically appeared in the catch of fisheries of the Campbell Rise since the late 1970s (JR unpublished data), and they may have been more abundant over the southern Campbell Plateau in years preceding this study.
Further research recommendations
This study has provided an indication of the main prey species of NZ sea lions at Campbell Island, though further studies are required to assess the degree of seasonal and annual variation in diet and may consider adopting a similar sampling protocol to facilitate comparison. In addition, DNA-based analysis of scats may allow a more complete assessment of the diet spectrum than is possible from hard parts analysis alone. This technique would also facilitate an assessment of the relative importance of avian and mammalian prey to the nutrition of NZ sea lions. A diet study in August or September would be particularly valuable, given that strong seasonal variation in local abundance of prey species is expected and southern blue whiting will have formed into the spawning aggregations targeted by the commercial trawl fishery. In addition, the continued monitoring of foraging distributions will provide some of the key information requirements for assessments aimed at explaining the contrasting population trajectories of NZ sea lion populations.
References
Anderson OF, Bagley NW, Hurst RJ, Francis MP, Clark MR, McMillan PJ (1998) Atlas of New Zealand fish and squid distributions from research bottom trawls. NIWA Technical Report, Wellington
Augé AA, Lalas C, Davis LS, Chilvers BL (2012) Autumn diet of recolonising female New Zealand sea lions based at Otago Peninsula, South Island, New Zealand. N Z J Mar Freshw Res 46:97–110
Bagley NW, Ballara S, O’Driscoll RL, Fu D, Lyon W (2013) A review of hoki and middle-depth summer trawl surveys of the Sub-Antarctic, November December 1991–1993 and 2000–2009. N Z Fisheries Assessment Report 2013/41, Ministry for Primary Industries, Wellington, New Zealand
Baker CS, Chilvers BL, Constantine R, Dufresne S, Mattlin R, Van Helden A, Hitchmough R (2010) Conservation status of New Zealand marine mammals (suborders Cetacea and Pinnipedia), 2009. N Z J Mar Freshw Res 44:101–115
Bradshaw CJA, Lalas C, McConkey S (1998) New Zealand sea lion predation on New Zealand fur seals. N Z J Mar Freshw Res 32:101–104
Cannon DY (1987) Marine fish osteology: a manual for archaeologists. Department of Archaeology, Simon Fraser University, British Columbia
Childerhouse S, Gales N (1998) Historical and modern distribution and abundance of the New Zealand sea lion Phocarctos hookeri. N Z J Zool 25:1–16
Childerhouse S, Dix B, Gayles N (2001) Diet of New Zealand sea lions (Phocarctos hookeri) at the Auckland Islands. Wildl Res 28:291–298
Childerhouse S, Hamer D, Maloney A, Michael S, Donnelly D, Schmitt N (2014) Final report: CSP project 4522 New Zealand sea lion ground component 2013/14. Blue Planet Mar, Nelson
Chilvers BL (2012) Using life-history traits of New Zealand sea lions, Auckland Islands to clarify potential causes of decline. J Zool 287:240–249
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Fernàndez DA, Lattuca ME, Boy CC, Pérez AF, Ceballos SG, Vanella FA, Morriconi ER, Malanga GF, Aureliano DR, Rimbau S, Calvo J (2009) Energy density of sub-Antarctic fishes from the Beagle Channel. Fish Physiol Biochem 35:181–188
Furlani D, Gales R, Pemberton D (2007) Otoliths of common Australian temperate fish: a photographic guide. CSIRO Publishing, Collingwood
Gauldie RW, Coote G, Mulligan KP, West IF, Merrett NR (1991) Otoliths of deep water fishes: structure, chemistry and chemically-coded life histories. Comp Biochem Physiol Part A Physiol 100:1–31
Gautier S, Fu D, O’Driscoll RL, Dunford A (2011) Acoustic estimates of southern blue whiting from the Campbell Island Rise, August–September 2009. New Zealand Fisheries Assessment Report 2011/9
Hecht T (1987) A guide to the otoliths of Southern Ocean fishes. S Afr J Antarct Res 17:1–87
IUCN (2011) IUCN red list of threatened species. Version 2011.1
Kingsford MJ, Schiel DR, Battershill CN (1989) Distribution and abundance of fish in a rocky reef environment at the subantarctic Auckland Islands, New Zealand. Polar Biol 9:179–186
Lalas C (1997) Prey of Hooker’s sea lions Phocarctos hookeri at Otago Peninsula New Zealand. In: Hindell M, Kemper C (eds) Marine mammal research in the southern hemisphere. Surrey Beatty and Sons, Chipping Norton
Lalas C, McConnell HM (2012) Prey of Auckland Island shags (Leucocarbo colensoi) in winter. Notornis 59:130–137
Lalas C, Webster T (2014) Contrast in the importance of arrow squid as prey of male New Zealand sea lions and New Zealand fur seals at The Snares, subantarctic New Zealand. Mar Biol 161:631–643
Lalas C, Ratz H, McEwan K, McConkey SD (2007) Predation by New Zealand sea lions (Phocarctos hookeri) as a threat to the viability of yellow-eyed penguins (Megadyptes antipodes) at Otago Peninsula, New Zealand. Biol Conserv 135:235–246
Lalas C, McConnell HM, Meynier L (2014) Estimating size of opalfish from otoliths: implications for analyses of New Zealand sea lions diet. N Z J Mar Freshw Res 48:1–14
Maloney A, Chilvers BL, Muller CG, Haley M (2012) Increasing pup production of New Zealand sea lions at Campbell Island/Motu Ihupuku: can it continue? N Z J Zool 39:19–29
McMahon CR, Holley D, Robinson S (1999) The diet of itinerant male Hooker’s sea lions, Phocarctos hookeri, at sub-Antarctic Macquarie Island. Wildl Res 26:839–846
Meynier L, Morel PCH, Mackenzie DDS, Macgibbon A, Chilvers BL, Duignan PJ (2008) Proximate composition, energy content, and fatty acid composition of marine species from Campbell Plateau, New Zealand. N Z J Mar Freshw Res 42:425–437
Meynier L, Mackenzie DDS, Duignan PJ, Chilvers BL, Morel PCH (2009) Variability in the diet of New Zealand sea lion (Phocarctos hookeri) at the Auckland Islands, New Zealand. Mar Mamm Sci 25:302–326
Moore PJ, Moffat RD (1992) Predation of yellow-eyed penguin by Hooker’s sea lion. Notornis 39:68–69
Moore PJ, Fletcher D, Amey J (2001) Population estimates of yellow-eyed Penguins, Megadyptes antipodes, on Campbell Island, 1987–1998. Emu 101:225–236
Morrison KW, Battley PF, Sagar PM, Thompson DR (2015) Population dynamics of Eastern Rockhopper Penguins on Campbell Island in relation to sea surface temperature 1942–2012: current warming hiatus pauses a long-term decline. Polar Biol 38:163–177
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Vegan: community ecology package. R package version 2.0-10. http://CRAN.R-project.org/package=vegan
O’Shea S (1999) The marine fauna of New Zealand: octopoda (Mollusca: Cephalopoda). NIWA Biodiversity Memoir 112, Wellington
Paulin CD (1983) A revision of the family Moridae (Pisces: Anacanthini) within the New Zealand region. Natl Mus N Z Rec 2:81–126
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL:http://www.R-project.org/
Robertson BC, Chilvers BL (2011) The population decline of the New Zealand sea lion Phocarctos hookeri: a review of possible causes. Mamm Rev 41:253–275
Robinson S, Wynen L, Goldsworthy S (1999) Predation by a Hooker’s sea lion (Phocarctos hookeri) on a small population of fur seals (Arctocephalus spp.) at Macquarie Island. Mar Mamm Sci 15:888–893
Salazar PS, Bustamante RH (2003) Effects of the 1997–1998 El Niño on population size and diet of the Galapagos sea lion (Zalophus wollebaeki). Not de Galapagos 62:40–45
Schwarzhans W (1984) Fish otoliths from the New Zealand Tertiary. Report New Zealand Geological Survey, Department of Scientific and Industrial Research, Wellington
Schwarzhans W (1999) A comparative morphological treatise of recent and fossil otoliths of the order Pleuronectiformes. Verlag F. Pfeil, München
Sinclair EH, Zeppelin TK (2002) Seasonal and spatial differences in diet in the western stock of steller sea lions (Eumetopias jubatus). J Mamm 83:973–990
Smale MJ, Watson G, Hecht T (1995) Otolith atlas of southern African fishes. Ichthyol Monogr 1:1–253
Smith RO, Vennell R, Bostock HC, Williams MJM (2013) Interaction of the Subtropical Front with topography around southern New Zealand. Deep Sea Res I 76:13–26
Taylor RH, Sadlier RM (1985) Report on work of ecology division, DSIR, during the sub-Antarctic cruise of HMNZS Monowai, 7 February-13 March 1985. Unpublished report, Ecology Division, New Zealand Department Scientific and Industrial Research, Lower Hutt
Thompson FN, Berkenbusch K, Abraham ER (2013) Marine mammal bycatch in New Zealand trawl fisheries, 1995–1996 to 2010–2011. New Zealand aquatic environment and biodiversity report. New Zealand Ministry for Primary Industry, Wellington
Tollit D, Heaslip S, Deagle B, Iverson S, Joy R, Rosen D, Trites A (2006) Estimating diet composition in sea lions: which technique to choose? Pages 293–307. In: Trites AW, Atkinson SK, DeMaster DP, Fritz LW, Gelatt TS, Rea LD, Wynne KM (eds) Sea lions of the world. Alaska Sea Grant College Program, University of Alaska Fairbanks
Trites AW, Donnelly CP (2003) The decline of Steller sea lions Eumetopias jubatus in Alaska: a review of the nutritional stress hypothesis. Mamm Rev 33:3–28
Williams R, McEldowney A (1990) A guide to the fish otoliths from waters off the Australian Antarctic Territory, Heard and Macquarie Island. ANARE Research Notes 75, Australian National Antarctic Research Expeditions, Kingston
Xavier JC, Cherel Y (2009) Cephalopod beak guide for the Southern Ocean. British Antarctic Survey, Cambridge
Acknowledgments
This research was supported by the NIWA Core Funding Program and the NZ Department of Conservation. We would like to thank Mary-Anne Lea, Mark Hindell (both University of Tasmania, Australia), Kimberley Vinette Herrin (Sydney Zoo, Australia) and Robert Mattlin (Marine Wildlife Research, NZ) for assistance with the collection of samples. Also Jeff Forman for the identification of invertebrate prey items and Darren Stevens (both NIWA, NZ) for assistance with identification from cephalopod beaks and Alan Tennyson and Colin Tinkler (both Te Papa Museum, NZ) for tentative identifications from bird feathers. All samples were collected in accordance with ethical conditions specified in the NZ Department of Conservation Research Permit Number 35879-FAU. CL thanks Sanford Ltd for permission to collect specimen fish, cephalopod and crustaceans aboard their chartered trawlers. Finally, we thank the four reviewers who provided appreciated suggestions for improving this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roberts, J., Lalas, C. Diet of New Zealand sea lions (Phocarctos hookeri) at their southern breeding limits. Polar Biol 38, 1483–1491 (2015). https://doi.org/10.1007/s00300-015-1710-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1710-3